Abstract
AtFPF1 (FLOWERING PROMOTING FACTOR 1) is a gene that promotes flowering in Arabidopsis. An expression vector containing AtFPF1 driven by a Ubi-1 promoter was constructed. The gene was introduced into rice callus by Agrobacterium-mediated transformation and fertile plants were obtained. The presence of AtFPF1 in rice plants was confirmed by PCR, Southern and Northern blot analyses, as well as by β-glucuronidase assay. The results showed that, as in Arabidopsis, AtFPF1 reduced flowering time in rice. Furthermore, introduction of AtFPF1 enhanced adventitious root formation but inhibited root growth in rice during the seedling stage. The results suggest that AtFPF1 promotes flowering time in both dicots and monocots, and plays a role in the initiation of adventitious roots in rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the ABC genetic model of floral development was proposed (Coen and Meyerowitz 1991; Ge et al. 2001), molecular and physiological characterization of the mechanism that modulates flowering time has become an important issue. It is now well established that developmental genes in plants have conservative functions in diverse species. LFY, a floral homeotic gene in Arabidopsis, is able to promote flowering in heterogeneous species such as tobacco and poplar (Populus tremula × tremuloides) (Yong et al. 2000). Flower development in rice, a monocotyledonous model plant, shares a similar pattern of genetic control to that of Arabidopsis (Ge et al. 2001). A number of genes that affect floral development in rice have been discovered that also have a conserved function in other species. For example, constitutive expression of the rice gene OsMADS1 involved in floral development also modulates flowering in tobacco (Chung et al. 1994).
Flowering and floral development are very important traits for cultivars in agriculture since they impact crop yield. AtFPF1 (FLOWERING PROMOTING FACTOR 1) is one of the important genes involved in the genetic control of flowering time in Arabidopsis. It is expressed in apical meristems immediately after photoperiodic induction of flowering in long-day plants, which flower only when exposed to long days (Kania et al. 1997). During the transition to flowering, the FPF1 gene is expressed at the same time as LFY and earlier than AP1. FPF1 modulates the acquisition of competence to flower in the apical meristem. Overexpression of FPF1 leads to early flowering in Arabidopsis (Kania et al. 1997; Melzer et al. 1999). Plants doubly transgenic for the genes AP1 and FPF1 showed a synergistic effect in the shortening of flowering time. The co-overexpression of FPF1 and LFY in Arabidopsis showed an additive reduction in flowering time. FPF1 regulates flowering in Arabidopsis through an independent pathway that is parallel to that of LFY and AP1 (Melzer et al. 1999). So far, few functions of AtFPF1 other than floral development have been reported.
OsRAA1, the protein produced by another rice gene, OsRAA1 (Oryza sativa root architecture associated 1), shares 58% amino-acid sequence identity with AtFPF1, and modulates root architecture and development (Ge et al. 2004). In Arabidopsis, it has been reported that many genes involved in auxin signal transduction control the development of roots (Marchant et al. 2002). The AUX/IAA family, the SAUR (small auxin up-regulated RNA) family, and the GH3 family, all involved in root development, are regulated by auxin in Arabidopsis. Previous studies have confirmed that auxin controls its own response through a feedback regulation by inducing SCFTIR1-dependent degradation of AUX/IAA proteins (Gray et al. 2001). Moreover, the auxin signal transduction pathway may be conserved between monocot and dicot plants. Unlike in Arabidopsis, the molecular mechanisms regulating the development of root system in monocots remain largely unknown. There are several root development mutants in maize and rice, but the genes corresponding to these mutants have not been cloned (Hochholdinger et al. 2001; Hao et al. 2002). Recently OsRAA1, a homologue of FPF1, was reported by our group (Ge et al. 2004). Overexpression of OsRAA1 in rice leads to pleiotropic phenotypes including altered leaf, flower and root development and root response to gravity, which are mediated by auxin.
Little is known about the molecular mechanisms that control root development in rice (Ge et al. 2004). Similarly, the molecular genetic network of flowering in rice is also unclear. In this context, studying the function of the AtFPF1 gene in rice will help us to understand the functional conservation of this dicot gene in a monocot species. The specific aim of this study was to identify the developmental role of AtFPF1 gene in rice. Constitutive expression of AtFPF1 shows that it regulates flowering and root development in rice.
Materials and methods
Rice (Oryza sativa L. ssp japonica, cv Zhonghua 10) obtained from the Chinese Agricultural Academy of Sciences at Beijing, China, was used in all the experiments. Plants were grown in a plant growth chamber or a greenhouse at 30°C under a 12 h photoperiod. The cDNA clone of AtFPF1 was a gift from Siegbert Melzer of the Institute for Plant Sciences, Switzerland.
Construction of overexpression vector
A full-length cDNA of AtFPF1 gene from Arabidopsis was inserted into SmaI and KpnI sites in the plasmid pUN130 to create an expression vector. The pUN1301 vector is derived from the pCAMBIA1301 series of binary vectors for Agrobacterium -mediated plant transformation (Roberts et al. 1997). AtFPF1 was driven by a maize Ubi-1 promoter (Christensen et al. 1992) (Fig. 1). The β-glucuronidase (GUS) reporter gene, and the selectable marker gene for hygromycin resistance (HYG) were driven by the cauliflower mosaic virus (CaMV) 35S promoter. The integrity of expression vector was confirmed by restriction analysis and DNA sequencing.
Agrobacterium-mediated transformation and analysis of transgenic plants
Mature seeds were dehulled and surface sterilized in 10% (v/v) Clorox (6% sodium hypochlorite) plus 0.2% (v/v) Tween 20 (Polysorbate 20) with vigorous shaking for 10 min. Following rinsing with sterile distilled water, seeds were plated on callus induction medium [MS medium (Murashige and Skoog 1962) plus 3.0 mg/l 2,4-dichlorophenoxyacetic acid (2,4-D), 500 mg/l glutamine, 500 mg/l proline, 3% sucrose, 0.25% phytagel, pH 5.8] for callus induction. Cultures were transferred at 25°C in the dark.
Embryogenic calli were visually selected and subcultured on fresh callus-induction medium and kept in the dark at 25°C for 1 week before co-cultivation. The embryonic calli were infected for 3 days in the dark at 25°C with Agrobacterium tumefaciens EHA105 containing a binary vector, as described by Huang et al. (2000). Subculturing of transgenic calli was conducted every 2 weeks using MS medium containing 30 g/l sucrose, 1 mg/l α-naphthaleneacetic acid (NAA), 5 mg/l kinetin (KT), 5 g/l gelrite and 50 mg/l hygromycin (Hyg). Transgenic calli were selected in a nutrient broth (NB) medium containing Hyg (50 mg/l). NB medium contains N6 major salts (Zhu et al. 1975) and minor salts, vitamins, 300 mg/l casamino acid, 500 mg/l proline, 500 mg/l glutamine, 30 mg/l sucrose and 7 mg/l agar, pH 5.8 (Wu and Chen 1987). Antibiotic treatment and the entire selection process were performed at 25°C in the dark. At 6 weeks after infection, Hyg-resistant calli were regenerated into plants by cultivating in RE1-CH differentiation medium (MS, 30 g/l sucrose, 25 g/l sorbitol, 500 mg/l casamino acid, 300 mg/l cefotaxime, 50 mg/l hygromycin, and 2.5 g/l gelrite, pH 5.8) containing 3 mg/l 6-benzyladenine (6-BA), 2.5 mg/l KT, 0.2 mg/l zeatin (ZT), and subsequently in RE2-H medium containing MS vitamins, one-quarter strength MS salts, 1 mg/l paclobutrazol, 0.5 mg/l NAA, 50 mg/l Hyg and 6.5 g/l agar (pH 5.8). Plants were rooted on one-half strength MS medium (1/2 MS) containing 75 mg/l Hyg in the light (2,000 lx, 16 h light/8 h dark) at 25°C for 14 days. GUS activity in transformed plants was assayed using the method of Jefferson (1989). Transgenic plants were grown in a greenhouse for collecting seeds.
Seeds of the T1 generation were germinated in 1/2 MS containing 75 mg/l Hyg and GUS gene expression in roots was assayed. As a control, non-transgenic seeds were germinated under the same conditions except for the absence of Hyg in the medium. The number of roots at the seedling stage was counted. At the flowering stage, the numbers of secondary branches on spikes, denoted as the number of spikelets per plant, were monitored. The number of days before floral emergence was monitored in each transgenic line, as well as in non-transgenic plants. Data were subject to t-test using the software Origin 6.1 (http://www.originlab.com).
PCR identification
Seeds for molecular analysis were placed on 1/2 MS plus 75 mg/l Hyg for germination. About 3 weeks after germination, fresh leaf tissue was collected and genomic DNA was isolated according to the method described by Dellaporta et al. (1983). Both primers in PCR were designed based on the cDNA sequence of FPF1 open reading frame (ORF). The forward primer was 5′-GCACGA GTC ATG TCA GGC GTG T3′ and the reverse primer was 5′-AAT GGG AGT CTC GGA CAT GGA A-3′. PCR was carried out for 30 cycles under the following conditions: initial incubation at 94°C for 2 min, 94°C for 1 min for denaturation, 58°C for 1 min for annealing and 72°C for 1 min for extension, with an additional extension step at 72°C for 10 min in the last cycle. The expected size of the PCR product was 300 bp.
Southern blot analysis
Genomic DNA was extracted from tissues as described by Dellaporta et al. (1983). Genomic DNA extracted from 10 mg material was digested with restriction enzymes (EcoRI and HindIII) and fractionated on a 1.0% agarose gel. After the DNA was blotted onto positively charged nylon membranes (Boehringer Mannheim, Germany), the blots were pre-hybridized and hybridized as previously described (Ge et al. 2000). cDNA of AtFPF1 was labelled by [32P]CTP for the hybridization. Pre-hybridization (6× SSC, 5× Denhardt’s, 0.5%SDS, 10 mg/ml salmon sperm DNA) and hybridization (6× SSC, 0.5%SDS, 10 mg/ml salmon sperm DNA) were carried out at 65°C for 3 and 17 h, respectively. Blots were washed once with 2× SSC buffer plus 0.5% sodium dodecyl sulphate (SDS) at room temperature for 5 min followed with 2× SSC buffer plus 0.1% SDS at room temperature for 15 min, and then washed in 0.1× SSC plus 0.1% SDS at 65°C for 20 min. An autoradiograph of the DNA blots was obtained by exposing the blot to X-ray film at −80°C.
Northern blot analysis
Total RNA was isolated from root and leaf tissues using the RNeasy plant mini kit (Qiagen, Hilden, Germany). Total RNA (15 μg) was electrophoretically separated and transferred onto a nylon membrane (Hybond N+) as described by Ge et al. (2000). AtFPF1 cDNA from Arabidopsis labeled with [32P]dCTP was used as the probe for hybridization. After hybridization for 20 h at 68°C, the membrane was washed once with 2× SSC plus 0.1% SDS at 68°C for 20 min, then washed with 1× SSC plus 0.1% SDS at 37°C for 30 min and exposed to X-ray film at −70°C for 3–7 days.
Histochemical analysis of GUS activity
Analysis of GUS activity was performed according to the method described by Jefferson (1989). Hyg-resistant calli and shoots of transgenic plant seedlings were incubated in a GUS staining solution [containing 100 mM Na-phosphate buffer at pH 7.0, 10 mM EDTA, 0.5 mM K3Fe(CN)6, 0.5 mM K4Fe(CN)6, 0.1% Triton X-100, and 1 mM X-Gluc] at 37°C for 12 h. All samples were vacuum-infiltrated for 5 min prior to incubation.
Results and discussion
Integration of the AtFPF1 gene into the rice genome
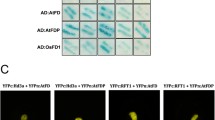
The AtPFP1 gene driven by a maize Ubi-1 promoter was successfully introduced into the rice genome by Agrobacterium-mediated transformation. The transformation efficiencies with the control vector pUN1301 and the FPF1-expressing vector were 52.9% and 32.9%, respectively, based on the number of calli growing on selective medium. Nearly 57% of the selected calli showed positive GUS staining. Among the 129 AtFPF1 transgenic plants regenerated, 84% (105 plants) were GUS positive in the experiment. Plants from calli were defined as the T0 generation. The T1 transgenic lines were termed fot-1 (FPF1 overexpression transgenic line), fot-2, fot-3, and so on. Transgenic T1 seeds were selected by germinating on Hyg-containing medium and then staining for GUS activity (data not shown). In Hyg-resistant plants, typical GUS staining always appeared at outer tissues of the shoot while there was no staining in the non-transformed plants (Fig. 2). Eight T1 transgenic lines (fot-1 to fot-8) were obtained. The T1 plants were further confirmed by PCR (Fig. 3a) and Southern blot assays (Fig. 3b). The expected PCR product of 300 bp was sequenced to confirm integration of FPF1 transgene. This PCR product was present in all the transgenic plant lines tested (Fig. 3a). Southern blots probed with the AtFPF1 cDNA showed the presence of 2–4 bands in the range of 2–20 kb in different transgenic lines (fot-1, fot-2, fot-3, fot-4). No detectable signal was observed in non-transformed plants (Fig. 3b). Sequence analysis showed that EcoRI and HindIII restriction sites were absent in the AtFPF1 coding region (data not shown), suggesting that multiple copies of AtFPF1 were integrated into different genomic loci of the transgenic plants tested. All transgenic plants were further identified by either Northern blot or RT-PCR (data not shown). Results of Northern blot analysis showed that a strong hybridization signal was detected in both roots and leaves in all transgenic lines, whereas no signal was observed in the same tissues of wild type plants under the same growth conditions (Fig. 4).
Identification of T1 transgenic plants. a PCR analysis. Lanes: 1 Molecular weight markers; 2 single 5′end primer; 3 single 3′ end primer; 4 negative control (water); 5 positive control (plasmid DNA); 6 non-transgenic plant, 7–9 transgenic lines fot-1, fot-2, fot-3, respectively. b Southern blot analysis. Lanes: 1 Molecular weight markers; 2, 7 non-transgenic plants; 3, 8 fot-1; 4, 9 fot-2; 5, 10 fot-3; 6, 11 fot-4 transgenic lines. Lanes 2–6, genomic DNA digested by EcoRI; lanes 7–11, genomic DNA digested by HindIII. Arrows AtFPF1 hybridizing fragments
Effect of AtFPF1 on flowering and root development in rice
AtFPF1 transgenic plants showed a clear phenotype in flowering time (Table 1, Fig. 5). The transgenic lines fot-1, fot-2, fot-3, fot-4 flowered 15–21 days earlier than the control plants under the same growth conditions. Statistical analysis showed that the average number of secondary branches in spikes (spikelets) in the transgenic plants (5.6–7.8) was significantly less than that in the control (8.8) (Table 1). Moreover, flowering duration of transgenic plants (5 days) was shorter than that of non-transformed plants (9 days) under the same conditions (Table 1). On average, transgenic plants ripened 13 days earlier than non-transgenic plants (Table 1). The reduction in flowering time of the transgenic rice plants was similar to that observed in transgenic Arabidopsis (Kania et al. 1997).
Root developmental changes were observed in AtFTP1 transgenic rice plants (Fig. 6). Initiation of adventitious roots was affected more than root growth (Tables 2, 3). The inhibition of growth (length) was noticed in the seedling stage but was not discernible in the mature phase. In the seedling stage, inhibition was more prominent for fourth adventitious roots than with first, second and third (Table 3). The percentage of long roots (>1 cm) in the transgenic lines (68.1%) was much lower than that in the control (81.7%) in 9-day-old seedlings (Table 2). In both seedlings and mature plants, more adventitious roots grew in transgenic plants (Fig. 6), which indicated a promoting effect of AtFPF1 on initiation and development of adventitious roots in rice. Statistical analysis showed that numbers of total and adventitious roots in transgenic plants were significantly higher than in non-transformed plants (P<0.05).
FPF1 protein was first studied as a promoter of flowering in mustard (Sinapis alba) (Kania et al. 1997; Melzer et al. 1990). It was reported that FPF1 is involved in a GA-dependent signaling pathway (Kania et al. 1997), and that it may work synergistically with AP1 and LFY to regulate shoot apical meristem competence for flowering (Melzer et al. 1999). Constitutive expression of FPF1 in Arabidopsis leads to early flowering under both long-day and short-day conditions, and leads to a shortened juvenile phase as measured by the trichome distribution on the abaxial leaf surface. Overexpression of AtFPF1 in rice caused an early flowering phenotype. Meanwhile, transgenic rice plants displayed a shortened flowering phase and ripened faster (Table 2), suggesting that the function of AtFPF1 in promoting flowering is conserved in both monocots and dicots.
Flower and root development are traits that directly impact crop yield. Information on genes involved in the control of both root development and flowering are limited (Ge et al. 2004). MADS box genes are involved in the control of flower development and at least one such gene controls root development (Zhang and Forde 1998). When the AtFPF1 gene was introduced into rice, transgenic plants headed earlier than non-transformed control plants. The model of FPF1 modulating flowering time in Arabidopsis may explain the altered flowering phenotype in transgenic rice. FPF1 modulates competence for flowering through the pathway mediated by LFY and/or some other alternate pathway (Melzer et al. 1999). Root initiation was promoted but root growth was suppressed in the transgenic plants during the seedling stage, which totally matched the behavior of OsRAA1 transgenic rice plants (Ge et al. 2004). Notably, constitutive expression of OsRAA1 also modulates flowering time in rice (Ge et al. 2004).
In conclusion, the AtFPF1 transgene leads to altered flowering time and root development in rice, showing that its function is conserved in both dicots (Arabidopsis) and monocots (rice). It is likely that FPF1 could be exploited for agricultural and horticultural crop improvement. As in Arabidopsis (Kania et al. 1997), the modulating effects of FPF1 in floral development in rice may include the GA signal transduction pathway. However, nothing is known about the biochemical mechanism controlling AtFPF1 action in root development,.
Abbreviations
- 6-BA:
-
6-Benzyladenine
- GUS:
-
β-Glucuronidase
- Hyg:
-
Hygromycin
- KT:
-
Kinetin
- NAA:
-
α-Napthylacetic acid
- ZT:
-
Zeatin
References
Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18:675–689
Chung YY, Kim SR, Finkel D, Yanofsky MF, An G (1994) Early flowering and redunced apical dominance result from ectopic expression of a rice MADS box gene. Plant Mol Biol 26:657–665
Coen ES, Meyerowitz EM (1991) The war of the whorls: genetic interactions controlling flower development. Nature 353:31–37
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Ge L, Liu JZ, Wong WS, Chong K, Hsiao WLW, Xu ZK, Li N (2000) Identification of a novel multiple environmental factor responsive 1-aminocyclopropane-1-carboxylate synthase gene, NT-ACS2, from tobacco. Plant Cell Environ 23:1169–1182
Ge L, Tan KH, Chong K, Xu ZH (2001) Advances on genetic control of rice floral development. Chin Sci Bull 46:705–712
Ge L, Chen H, Jiang JF, Zhao Y, Xu ML, Xu YY, Tan KH, Xu ZH, Chong K (2004) Overexpression of OsRAA1 causes pleiotropic phenotypes in transgenic rice plants including altered leaf, flower and root development and root response to gravity. Plant Physiol 135:1502–1513
Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414:271–276
Hao ZB, Cang J, Sun X (2002) The abnormal root gravitropism in a no-lateral-root mutant of rice. Acta Phytophysiol Sin 28:205–210
Hochholdinger F, Park WJ, Feix GH (2001) Cooperative action of SLR1 and SLR2 is required for lateral root-specific cell elongation in maize. Plant Physiol 125:1529–1539
Huang JQ, Wei ZM, An HL, Xu SP, Zhang B (2000) High efficiency of genetic transformation of rice using Agrobacterium mediated procedure. Acta Bot Sin 42:1172–1178
Jefferson RA (1989) The GUS reporter gene system. Nature 342:837–838
Kania T, Russenberger D, Peng S, Apel K, Melzer S (1997) FPF1 promotes flowering in Arabidopsis. Plant Cell 9:1327–1338
Marchant A, Bhalerao R, Casimiro I, Eklof J, Casero PJ, Bennett M, Sandberg G (2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14:589–597
Melzer S, Kampmann G, Chandler J, Apel K (1999) FPF1 modulates the competence to flowering in Arabidopsis. Plant J 18:395–405
Melzer S, Majewski DM, Apel K (1990) Early changes in gene expression during the transition from vegetative to generative growth in the long-day plant Sinapis alba. Plant Cell 2:953–961
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–479
Roberts CS, Rajagopal S, Yang W, Nugroho S, Smith L, Nguyent T, Ravi KS, Dransfield L, Harcourt R, Vijayachandra K, Patell V, Salland C, Desamero N, Slamet I, Keese P, Kilian A, Jefferson RA (1997) A comprehensive new set of modular vectors to allow both routine and advanced manipulations and efficient transformation of rice by both Agrobacterium and direct gene-transfer methods. Rockefeller Foundation Meeting of the International Program on Rice Biotechnology, September 15–19 (1997) Malacca, Malaysia
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning, a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
Wu CY, Chen Y (1987) A study on the genotypical difference in anther culture of keng rice (Oryza sativa ssp. keng) (in Chinese with English abstract). Acta Genetica Sin 14:168–174
Yong WD, Chong K, Xu ZH, Tan KH, Zhu ZQ (2000) Gene control of flowering time in higher plants. Chin Sci Bull 45:455–466
Zhang H, Forde BG (1998) An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279:407–409
Zhu ZQ, Wang JJ, Sun JS, Xu Z, Yin GC, Zhu ZY, Bi FY (1975) Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources (in Chinese with English abstract). Sci Sin 18:659–668
Acknowledgements
The authors are grateful to Dr. S. Melzer, Swiss Federal Institute of Technology, Institute for Plant Sciences, Zurich, Switzerland, for his kind gift of the plasmid with AtFPF1. We also thank Dr. C.B. Chen, Penn State University, Dr. L.N. Tian, Agriculture & Agri-food Canada, and Dr. C Larue, University of Missouri for their critical reading of the manuscript. This project was supported by the Major State Basic Research Program of P.R. China (G19990116), partially by the National Nature Science Foundation of China (NSFC, 30270143), and the Innovation Grant of CAS, as well as the State High-Tech Project (2001AA222281).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Lakshmanan
Ming-Li Xu and Jia-Fu Jiang contributed equally to this work
Rights and permissions
About this article
Cite this article
Xu, ML., Jiang, JF., Ge, L. et al. FPF1 transgene leads to altered flowering time and root development in rice. Plant Cell Rep 24, 79–85 (2005). https://doi.org/10.1007/s00299-004-0906-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0906-8