Abstract
We describe a procedure for producing transgenic bottle gourd plants by inoculating cotyledon explants with Agrobacterium tumefaciens strain AGL1 that carries the binary vector pCAMBIA3301 containing a glufosinate ammonium-resistance (bar) gene and the β-d-glucuronidase (GUS) reporter gene. The most effective bacterial infection was observed when cotyledon explants of 4-day-old seedlings were co-cultivated with Agrobacterium for 6–8 days on co-cultivation medium supplemented with 0.1–0.001 mg/l l-α-(2-aminoethoxyvinyl) glycine (AVG). The putatively transformed shoots directly emerged at the proximal end of cotyledon explants after 2–3 weeks of culturing on selection medium containing 2 mg/l dl-phosphinothricin. These shoots were rooted after 3 weeks of culturing on half-strength MS medium containing 0.1 mg/l indole acetic acid and 1 mg/l dl-phosphinothricin. Transgenic plants were obtained at frequencies of 1.9%. Stable integration and transmission of the transgenes in T1 generation plants were confirmed by a histochemical GUS assay, polymerase chain reaction and Southern blot analyses. Genetic segregation analysis of T1 progenies showed that transgenes were inherited in a Mendelian fashion. To our knowledge, this study is the first to show Agrobacterium-mediated transformation in bottle gourd.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bottle gourd (Lagenaria siceraria Standl.) has been used routinely as a source of rootstock for watermelon and other cucurbit crops in both Korea and Japan as a means to reduce the incidence of soil-borne diseases and to promote the vigor of the root system of the crop under conditions of low temperature (Lee and Oda 2003). Recently the potential of Lagenaria rootstock to confer resistance to the carmine spider mite has also been reported (Edelstein et al. 2000). Most of the damage that arises from the continuous cropping of vegetables grown for commercial purposes in the greenhouse is caused by soil-borne diseases and salinization of the soil. To overcome these problems, more than 95% of the commercially grown watermelons are grafted onto bottle gourd or squash (an interspecific hybrid between Cucurbita maxima × C. moschata) (Lee et al. 1998). In addition, watermelon plants that are grafted onto bottle gourd rootstock generally have fruits with a better taste, a higher sugar content and less fiber than do those grafted onto squash rootstock.
To date, genetic improvement of bottle gourd has been achieved mainly by conventional plant breeding methods, but recent advances in gene transformation techniques have opened new avenues for crop improvement. As the latter necessitate efficient procedures for the routine transfer of foreign functional genes into plant genomes, the development of a bottle gourd transformation system is crucial for producing improved watermelon or other cucurbit crops. Agrobacterium-mediated transformation in Cucurbitaceae has been reported to be successful with Cucumis melo (Fang and Grumet 1990; Ayub et al. 1996) and Cucumis sativus (Chee 1990; Nishibayashi et al. 1996). These studies have facilitated targeted gene transfer into useful crop members of this family. Coat protein-mediated protection of transgenic plants against a virus has been demonstrated via the stable transfer of the coat protein gene of cucumber mosaic virus-white leaf strain into the plant genome by biolistic transformation of C. melo L. (Gonsalves et al. 1994). As another example, three transgenic cucumber lines harboring a rice chitinase gene exhibited enhanced resistance against Botryris cinerea (Tabei et al. 1998). However, to our knowledge, no report exists to date on the production of a transgenic bottle gourd.
Ethylene is a factor involved in plant-microbe interactions (Spanu and Boller 1989) and is released from wounded tissues in plants. Wounded tissues have been used as explants in most of the transformation experiments involving inoculation of Agrobacterium. One hypothesis is that the efficiency of Agrobacterium infection is affected by the ethylene from wounded tissues. Ezura et al. (2000) reported that ethylene production was promoted by Agrobacterium inoculation and that increased levels of ethylene resulted in a reduction in the efficiency of gene transfer. They also reported that the addition of l-α-(2-aminoethoxyvinyl) glycine (AVG) to a co-cultivation medium reduced ethylene production.
In many cases, the lack of an efficient regeneration system is a major factor preventing the development of gene transfer technologies. We have recently developed an efficient shoot regeneration system using cotyledon explants in bottle gourd (Han et al., in press). The aim of the study reported here was to establish an efficient Agrobacterium-mediated transformation method for bottle gourd and to investigate the effect of ethylene action and biosynthesis inhibitors on the infection of Agrobacterium.
Materials and methods
Plant materials
The bottle gourd (Lagenaria siceraria Standl.) inbred line G5 (National Horticultural Research Institute, Korea) was used in this study. Following removal of the seed coats, the seeds were surface sterilized by submergence first in 70% (v/v) ethanol for 3 min, then in 0.2% (w/v) sodium dodecyl sulfate (SDS, Sigma-Aldrich, St. Louis, Mo.) for 25 min, 25% (v/v) YUHANROX (commercial bleach containing 4% sodium hypochlorite; Yuhan-Clorox, Korea) for 40 min and finally in 12.5% YUHANROX for 20 min. After each step, the seeds were rinsed three times with sterile distilled water. The surface sterilized-seeds were blot dried on sterile filter paper for 1–2 min. Ten seeds were then placed on 15×87-mm2 petri dishes, each containing 25 ml of hormone-free MS medium (Murashige and Skoog 1962) solidified with 30 g/l sucrose and 8 g/l plant agar (Duchefa Biochemie, The Netherlands). The pH of the medium was adjusted to 5.8 prior to autoclaving at 121°C/18 psi for 20 min. Each culture plate was incubated at 25°C under a 16/8-h (day/night) photoperiod (50 μmol/m2 per second). For the preparation of explants, the radicals and plumules of the 4-day-old seedlings were cut off, then a pair of cotyledons was split open. Each cotyledon was cut in half across its width, and the proximal half of each cotyledon explant was used as a source of co-cultivation material.
Agrobacterium strain and plasmid
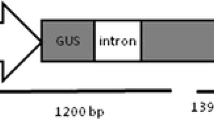
The supervirulent Agrobacterium tumefaciens strain AGL1 (Lazo et al. 1991) carrying the binary vector pCAMBIA3301 (Curtis and Nam 2000; kindly supplied by Dr. H.G. Nam, Pohang University, Korea) was used. The binary vector contained the cauliflower mosaic virus (CaMV) 35S promoter-bar (bialaphos resistance gene)-35S terminator and the 35S promoter-gus first exon-catalase intron-gus second exon-nos (nopaline synthase ) terminator located between the left and right borders of the T-DNA. Agrobacterium was maintained on YEP medium (An 1987) supplemented with 50 mg/l rifampicin (Sigma-Aldrich) and 50 mg/l kanamycin sulphate (Sigma-Aldrich) using standard procedures (Curtis et al. 1994).
Sensitivity test of cotyledon explants to dl-phosphinothricin
To determine an appropriate concentration of dl-phosphinothricin (PPT; Duchefa Biochemie) for the selection of transgenic shoots, cotyledon explants were cultured in 20×95-mm2 petri dishes containing 30 ml of a shoot induction medium (MS medium with 3.0 mg/l BA, 0.5 mg/l AgNO3, 500 mg/l cefotaxime sodium, 3% sucrose and 0.8% plant agar, pH 5.8) supplemented with different concentrations of PPT (0, 0.5, 1, 2, 5 and 10 mg/l). Seventy-seven explants were used per treatment, and the response of explants and the number of regenerated shoots were recorded after 4 weeks of culture.
Transformation, selection and plant regeneration
To determine the optimum conditions for Agrobacterium infection, we tested the effect of different durations of co-cultivation with Agrobacterium and the use of an ethylene action inhibitor (AVG; Sigma-Aldrich) and a biosynthesis inhibitor (AgNO3; Sigma-Aldrich) in the co-cultivation medium. A 50-μl aliquot of bacterial culture (O.D.600=1.0) was centrifuged at 3,179g for 10 min (4°C). Following removal of the supernatant, the pellet was suspended in the same volume of inoculation medium [MS medium containing 0.5 mg/l 2-morpholinoethanesulfonic acid monohydrate (MES) and 3% sucrose, pH 5.2], and this suspension was used as an inoculum for infection. The cotyledon explants were immersed in the bacterial inoculum for 20 min and then washed with the same medium for about 10 s. Following washing, they were cultured in 20×95-mm2 petri dishes with 30 ml of co-cultivation medium (MS medium containing 0.5 mg/l MES, 3% sucrose, 3.0 mg/l BA and 0.8% plant agar, pH 5.2). The Agrobacterium infection frequency (number of GUS-positive explants/number of explants examined) was determined after co-cultivation for 0, 2, 4, 6 and 8 days by performing a histochemical GUS assay.
To investigate the effect of ethylene biosynthesis and action inhibitors and 3′,5′-dimethoxy-4′hydroxyacetophenone (acetosyringone; Sigma-Aldrich) on transformation efficiency, we supplemented the inoculation and co-cultivation media with AVG (0.001 mg/l or 0.1 mg/l) or AgNO3 (0.5 mg/l), with or without 50 μM acetosyringone. The intensity of gus transient expression was categorized into five classes, as described by Ezura et al. (2000), with some modifications, based on the degree of blue staining in the section: index 0, explants without a blue-stained area; index 1, explants with ≤10% blue-stained areas; index 2, explants with ≤40% but more than 10% blue-stained areas; index 3, explants with ≤70% but more than 40% blue-stained areas; index 4, explants with more than 70% blue-stained areas (Fig. 1b).
Effects of AgNO3, AVG and acetosyringone in the co-cultivation medium on the efficiency of Agrobacterium infection of bottle gourd (Lagenaria siceraria Standl.) cotyledon explants. a Effect of ethylene action and biosynthesis inhibitors in the co-cultivation medium on GUS transient expression of co-cultivated cotyledon explants. Infection index (0–4) represents the degree of GUS transient expression. Bars represent the standard error of means and values, with the same letter within each frame indicating a non-significant different according to Duncan’s multiple range test at the 5% level. b Degree of GUS transient expression in co-cultivated cotyledon explants. Index: 0–4. c Explants treated with 0.5 mg/l AgNO3 (upper) and 0.001 mg/l AVG (lower) 6 days after Agrobacterium tumefaciens inoculation. Blue staining indicates gus transient expression
Cotyledon explants co-cultivated with bacteria on the co-cultivation medium were washed with liquid selection medium (MS medium containing 3.0 mg/l BA, 3% sucrose, 0.5 mg/l AgNO3, 500 mg/l cefotaxime sodium, 2 mg/l PPT, pH 5.8), blot-dried on sterile filter paper and then placed on the selection medium solidified with 0.8% plant agar. After 4 weeks, shoots having expanded leaves were independently isolated and then transplanted in a rooting medium (half-strength MS medium containing 0.1 mg/l IAA, 3% sucrose, 1 mg/l PPT, 500 mg/l cefotaxime sodium and 0.8% plant agar, pH 5.8).
Histochemical GUS assay
The assay for β-d-glucuronidase (GUS) activity using 5-bromo-4-chloro-3-indoyl-β-d-glucuronic acid (Duchefa Biochemie) as the substrate was carried out as described by Jefferson et al. (1987). The assay culture was incubated overnight at 37°C, and the explants were subsequently soaked overnight in 95% methanol.
Basta test for putative transformants
Leaves from acclimated putative transformants and non-transformed regenerants of a similar age were thoroughly painted with various concentrations (0%, 0.01%, 0.05%, 0.1%, 0.5%, 1.0%, v/v) of a Basta solution (18% glufosinate ammonium; Kyungnoog Korea). Three putative transformants were used per treatment. Each leaf was divided into two parts along the midrib, and then the right section of the upper surface of each individual leave was gently rubbed with a cotton swab bathed in Basta. The opposite side of leaves was left untreated as a control to investigate any systemic pervasion of Basta. Resistance to the herbicide was confirmed on whole plants by spraying with a 0.1% Basta solution. Plants were initially sprayed at the 12-leaf stage of development, and then once again 3 days after the first application.
DNA isolation and polymerase chain reaction
Genomic DNA was isolated from newly developing young leaves of acclimated plants in the greenhouse using the DNeasy Plant kit (QIAGEN, Germany). The PCR analysis was performed in a 25-μl volume containing 20 ng genomic DNA from each putative transformed and non-transformed regenerant. Two primers were used for both the bar gene (primer 1, 5′-TCAAATCTCGGTGACGGGCA-3′; primer 2, 5′-GGTCTGCACCATCGTCAACC-3′) and gus gene (primer 1, 5′-AACTGGACAAGGCACTAGCG-3′; primer 2, 5′-CACCGAAGTTCATGCCAGTC-3′). Amplification of the bar and gus gene consisted of 35 cycles of 95°C (30 s), 62°C (45 s) and 72°C (1 min) in the iCycler (Bio-Rad, Hercules, Calif.).
Southern blot analysis
A 10-μg aliquot of genomic DNA from each randomly selected transformant and un-transformed regenerant was digested overnight with HindIII (which makes only one cut in the T-DNA region and another cut elsewhere in the plant DNA) and then electrophoresed on a 0.8% agarose gel. The DNA was then transferred to a Hybond N+ nylon membrane (Amersham-Pharmacia Biotech, Piscataway, N.J.) using capillary blotting. A 1.1-kb fragment of the gus gene from pCAMBIA3301 amplified by PCR was extracted from the gel, labeled with [32P] and used as a probe for Southern hybridization. Blotting, labeling, hybridization and washing were carried out according to the manufacturer’s instructions.
Progeny segregation test
Progenies were screened for resistance to Basta by applying a 0.1% solution (v/v). Plants were initially sprayed at the two- or three-leaf stage of germination and then once again 3 days after the first application. The number of seedlings that were resistant or susceptible to Basta was counted, and the analysis of gus expression was performed histochemically in the surviving plants 3 days following the second application of the Basta solution.
Results and discussion
Effect of PPT on shoot regeneration
After a 4-week culture period on shoot induction medium with 0.5 mg/l PPT, 71.4% of the cotyledon explants with partial necrosis survived, and some cotyledon explants (3.9%) formed adventitious shoots. Concentrations of 1.0 mg/l and 2.0 mg/1 PPT caused serious necrosis in cultured cotyledon explants, and no shoots developed on explants cultured at these concentrations (data not shown). To prevent any escapes, we chose 2.0 mg/l PPT as the selective concentration to be used in the transformation experiments.
Factors influencing Agrobacterium infection
To determine the optimum conditions for Agrobacterium infection of bottle gourd cotyledon explants, we examined the infection frequency, based on transient GUS expression. The duration of the co-cultivation period with bacteria affected the infection frequency (Table 1). The optimum length of the co-cultivation period was 6–8 days, resulting in an infection frequency of 96.8–100%. The blue area indicating the transient expression of the gus gene was initially observed after 2 days of co-cultivation; after 4 days of co-cultivation the number of GUS positive explants dramatically increased. However, when the cotyledon explants were co-cultivated for 8 days, we could not easily eliminate the Agrobacterium through the addition of cefotaxime sodium and, consequently, we chose 6 days as the optimum co-cultivation period for transformation.
The extent of Agrobacterium infection on explants was categorized into five classes according to Ezura et al. (2000) with some modifications, and the infection efficiency was compared among the AVG, AgNO3 and acetosyringone treatments. The percentage of blue-stained areas on the cut surface of co-cultivated cotyledon explants was significantly increased following addition of AVG to the co-cultivation medium (Fig. 1a,c lower). The addition of 0.5 mg/l AgNO3 did not effectively increase the infection index (Fig. 1a,c upper), although the presence of AgNO3 at this concentration does promote the induction of adventitious shoots (Han et al. in submitted). The addition of 50 μM acetosyringone to the co-cultivation medium also did not increase the infection efficiency compared with its total absence (Fig. 1a). Previous studies have shown that AVG blocks the activity of aminocyclopropane carboxylic acid (ACC) synthase (Rando 1974), a key enzymatic step in regulating ethylene production (Yang and Hoffman 1984). In more recent studies, Ezura et al. (2000) reported that Agrobacterium inoculation increased the ethylene production of explants excised from the melon cotyledon and that the application of AVG at that time resulted in a reduction of ethylene production. Consequently, they concluded that the gene transfer into explants was increased by an elevation of Agrobacterium infection. Our findings using AVG also suggest that ethylene production inhibits transformation efficiency.
Regeneration of transformants
Agrobacterium-mediated transformation of bottle gourd was performed by co-cultivating cotyledon explants with the bacteria for 6 days on co-cultivation medium supplemented with 0.001 mg/l AVG. In total, 1,629 cotyledon explants co-cultivated with Agrobacterium were transferred to solid selection medium following washing with liquid selection medium. Within 2–3 weeks, some cotyledon explants showed adventitious shoot formation, with a range of 1.5–3.1 shoots per explant. To remove any non-transformed shoots, we transferred only those shoots having expanded leaves to rooting medium containing 1 mg/l PPT. A total of 194 shoots elongated and rooted, and of the these plantlets, 36 were successfully acclimated in the greenhouse (Table 2, Fig. 2a). Phenotypically these plantlets were indistinguishable from non-transformed regenerants or control seedlings. In our transformation experiments, non-transgenic shoots (about 69% of the total developing shoots) developed under the conditions described above, and these shoots did not root on rooting medium. This result indicates that our method did eliminate “escapes.” “Escapes” are particularly common in melon, which belongs to Cucurbitaceae (Dong et al. 1991; Galperin et al. 2003). The transformation frequency of bottle gourd inbred line G5 was 1.9% based on the GUS histochemical assay and PCR analyses of the gus and bar genes (Table 2, Fig. 3).
a Acclimated putative transgenic bottle gourd (T0) in the greenhouse. b Resistance test to Basta solution (0.05%, v/v) for putative transgenic bottle gourd plant (upper) and non-transformed regenerant (lower) 3 days after application. c Spray test of whole T0 transgenic (left) and non-transformed regenerant (right) with 0.1% Basta solution. d Fruit setting from transgenic plants. e T1 progenies from a transgenic T0 by selfing were screened for resistance to the Basta using a 0.1% solution (v/v). The progenies were divided into susceptible and resistant plants based on the symptoms of the herbicide effects
PCR detection of the bar (top) and gus (middle) genes and expression of GUS activity (bottom) in some putative T0 transgenic plants. The top and middle PCR analyses of some putative transgenic plants show the presence of the expected 0.5-kb and 1.1-kb DNA fragments of the bar and gus genes, respectively, except for one plant (lane 6). Lane C Non-transformed regenerant, lanes 1–6 putative transgenic plants
In various Cucurbitacea transformation studies, cotyledons have often been used as explants for Agrobacterium infection. This may be due to easy handling and the particular form of regeneration in this family (Dabauza et al. 1997). Since organogenesis is not restricted to one small area of the explant but to many small independent areas, the probability of the cells being competitive for both regeneration and transformation is high.
When the same transformants were gently wetted by painting with various diluted Basta solutions, the leaves from the transformants showed resistance to the 0.05% (v/v) Basta solution, whereas non-transformed regenerants and control seedlings showed severe necrosis 3 days after a single painting with the 0.05% (v/v) Basta solution (Fig. 2b). Resistance to the herbicide was also confirmed on whole plants at the 12-leaf stage by spraying with a 0.1% Basta solution (Fig. 2c). The transformants showed no symptoms of herbicidal damage, grew normally to maturity and set fruits (Fig. 2d), whereas the leaves of non-transformed regenerants and control seedling plants became necrotic and fell off (Fig. 2c). The bialaphos resistance gene (bar) derived from Streptomyces hygroscopicus (Thompson et al. 1987) has been shown to be a very effective selectable marker gene in the production of transgenic plants in several crops, such as cotton (Keller et al. 1997), lettuce (Mohapatra et al. 1999) and soybean (Zeng et al. 2004). The use of this herbicide resistance gene for selecting putative transformed plants may be carried out cheaply with minimal expertise compared to the use of antibiotic resistance genes (D’Halluin et al. 1992). In our study, the bar gene was a very effective selectable marker gene for bottle gourd transformation. Bottle gourd seems to be more sensitive to glufosinate than other plants, such as pakchoi (Qing et al. 2000), enabling the selection for non-transgenic regenerants to be undertaken efficiently with a minimal use of herbicide.
Genomic DNA was extracted from the leaf tissues of acclimated plants. The putative transformants were verified for the presence of the transgenes by PCR analysis or histochemical GUS assay (Fig. 3) and Southern blot analysis (Fig. 4). PCR analysis revealed the presence of the expected 492-bp and 1,105-bp amplified products of the bar and gus genes, respectively, in all of the putative transformed T0 plants except one (Fig. 3). When the same transformants were subjected to the histochemical GUS assay, all of the putative transformants tested also showed GUS activity. Of the 31 transformants tested, 30 possessed both the bar and gus genes, and one possessed only the gus gene. We are unsure how the single line completely lost the bar gene. It is possible that an incomplete insertion of T-DNA occurred; alternatively, a loss of the bar gene may have occurred during plant development. Another scenario to explain its absence in the single line is inadequate selective pressure using 1 mg/l PPT (Park et al. 1998; Srivatanakul et al. 2000). Future work will be needed to establish proper regimes that will achieve effective selection but at the same time minimize escapes and incomplete insertions of T-DNA. A Southern blot analysis re-confirmed the presence of the gus gene in transformants, with one or two copies of that gene integrated (Fig. 4).
Progeny segregation test
The seeds (T1 generation) obtained from six T0 randomly selected plants by selfing were sown in the greenhouse in order to study the transgene segregation. The progenies showed no symptoms of herbicidal damage and grew normally, whereas the leaves of non-transformed plants became necrotic and fell off following spraying with a 0.1% Basta solution (Fig. 2e). Live T1 plants were assayed for GUS activity after the herbicide treatment. Clearly, chimeras or non-germ-line events were not a concern in this study. Our statistical analysis confirmed the segregation of foreign gene transmission among all of the progenies of five T0 plants, which fits an expected segregation ratio of 3:1 or 15:1 (Table 3).
We are currently developing transgenic bottle gourd lines expressing an Arabidopsis H+/Ca+ transporter gene (Hirschi et al. 2001) and testing its effectiveness in conferring tolerance to biotic and/or abiotic stresses.
Abbreviations
- Acetosyringone:
-
3′,5′-Dimethoxy-4′hydroxyacetophenone
- AVG:
-
l-α-(2-Aminoethoxyvinyl) glycine
- BA:
-
6-Benzylaminopurine
- GUS:
-
β-d-Glucuronidase
- IAA:
-
Indole acetic acid
- PPT:
-
dl-Phosphinothricin
References
An G (1987) Binary Ti vectors for plant transformation and promoter analysis. Methods Enzymol 153:292–305
Ayub R, Guis M, Ben Amor M, Gillot L, Roustan JP, Latche A, Bouzayen M, Pech JC (1996) Expression of ACC oxidase antisense gene inhibits ripening of cantaloupe melon fruits. Nat Biotechnol 14:862–866
Chee PP (1990) Transformation of Cucumis sativus tissue by Agrobacterium tumefaciens and the regeneration of transformed plants. Plant Cell Rep 9:245–258
Curtis IS, Nam HG (2000) Transgenic radish (Raphanus sativus L. longipinnatus Bailey) by floral-dip method—plant development and surfactant are important in optimizing transformation efficiency. Transgenic Res 10:363–371
Curtis IS, Power JB, Blackhall NW, de Laat AMM, Davey MR (1994) Genotype-independent transformation of lettuce using Agrobacterium tumefaciens. J Exp Bot 45:1441–1449
Dabauza M, Bordas M, Salvador A, Roig LA, Moreno V (1997) Plant regeneration and Agrobacterium-mediated transformation of cotyledon explants of Citrullus colocynthis (L.) Schrad. Plant Cell Rep 16:888–892
D’Halluin K, De Block M, Deneoke J, Janssens J, Leemans J, Reynaerts A, Botterman J (1992) The bar gene as selectable and screenable marker in plant engineering. Methods Enzymol 216:415–426
Dong JZ, Yang MZ, Jia SR, Chua NH (1991) Transformation of melon (Cucumis melo L.) and expression from the cauliflower mosaic virus 35S promoter in transgenic melon plants. Biotechnology 9:858–863
Edelstein M, Tadmor Y, Abo-Moch F, Karchi Z, Mansour F (2000) The potential of Lagenaria rootstock to confer resistance to the carmine spider mite, Tetranychus cinnabarinus (Acari: Tetranychidae) in Cucurbitaceae. Bull Entomol Res 90:113–117
Ezura H, Yuhashi KI, Yasuta T, Minamisawa K (2000) Effect of ethylene on Agrobacterium tumefaciens-mediated gene transfer to melon. Plant Breed 119:75–79
Fang G, Grumet R (1990) Agrobacterium tumefaciens-mediated transformation and regeneration of muskmelon plants. Plant Cell Rep 9:160–164
Galperin M, Patlis L, Ovadia A, Wolf D, Zelcer A, Kenigsbuch D (2003) A melon genotype with superior competence for regeneration and transformation. Plant Breed 122:66–69
Gonsalves C, Xue B, Yepes M, Fuchs M, Ling K, Namba S, Chee P, Slightom JL, Gonsalves D (1994) Transferring cucumber mosaic virus-white leaf strain coat protein gene into Cucumis melo L. and evaluating transgenic plants for protection against infections. J Am Soc Hortic Sci 119:345–355
Hirschi KD (2001) Vacuolar H+/Ca+ transporter: Who’s directing the traffic? Trends Plant Sci 6:100–104
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Keller G, Spatola L, McCabe D, Martinell B, Swain W, John ME (1997) Transgenic cotton resistant to herbicide bialaphos. Transgenic Res 6:385–392
Lazo GR, Stein PA, Ludwig RA (1991) A DNA transformation competent Arabidopsis genomic library in Agrobacterium. Biotechnology 9:963–967
Lee JM, Oda M (2003) Grafting of herbaceous vegetable and ornamental crops. Hortic Rev 28:61–124
Lee JM, Bang HJ, Han HS (1998) Grafting of vegetables. J Jpn Soc Hortic Sci 67:1098–1114
Mohapatra U, McCabe MS, Power JB, Schepers F, Van der Arend A, Davey MR (1999) Expression of the bar gene confers herbicide resistance in transgenic lettuce. Transgenic Res 8:33–44
Murashige T, Skoog FA (1962) Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nishibayashi S, Kaneko H, Hayakawa T (1996) Transformation of cucumber (Cucumis sativus L.) plants using Agrobacterium tumefaciens and regeneration from hypocotyl explants. Plant Cell Rep 15:809–814
Park SH, Rose SC, Zapata C, Srivatanakul M, Smith RH (1998) Cross-protection and selectable marker genes in plant transformation. In Vitro Cell Dev Biol Plant 4:117–121
Qing CM, Fan L, Lei Y, Bouchez D, Tourneur C, Yan L, Robaglia C (2000) Transformation of Pakchoi (Brassica rapa L. ssp. chinensis) by Agrobacterium infiltration. Mol Breed 6:67–72
Rando RR (1974) Chemistry and enzymology of kcat inhibitors. Science 185:320–324
Spanu P, Boller T (1989) Ethylene biosynthesis in tomato plants infected by Phytophthora infestans. J Plant Physiol 134:533–537
Srivatanakul M, Park SH, Salas MG, Smith RH (2000) Additional virulence genes influence transgene expression: transgene copy number, integration pattern and expression. J Plant Physiol 157:685–690
Tabei Y, Kitade S, Nishizawa Y, Kikuchi N, Kayano T, Hibi T, Akutsu K (1998) Transgenic cucumber plants harboring a rice chitinase gene exhibit enhanced resistance to gray mold (Botryris cinerea). Plant Cell Rep 17:159–164
Thompson CJ, Rao Movva N, Tizard R, Crameri R, Davies JE, Lauwereys M, Botterman J (1987) Characterization of the herbicide-resistance gene bar from Streptomyces hygroscopicus. EMBO J 6:2519–2523
Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35:155–189
Zeng P, Vadnais DA, Zhang Z, Polacco JC (2004) Refined glufosinate selection in Agrobacterium-mediated transformation of soybean [Glycine max (L.) Merrill]. Plant Cell Rep 22:478–482
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K.K. Kamo
Rights and permissions
About this article
Cite this article
Han, JS., Kim, C.K., Park, S.H. et al. Agrobacterium-mediated transformation of bottle gourd (Lagenaria siceraria Standl.). Plant Cell Rep 23, 692–698 (2005). https://doi.org/10.1007/s00299-004-0874-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0874-z