Abstract
Intergeneric somatic hybrids between embryogenic callus-derived protoplasts of round kumquat (Fortunella japonica Swingle) and ‘Morita navel’ orange (Citrus sinensis Osbeck) were produced by electrofusion. Among the eight different fusion strains obtained, six showed normal morphology, whereas the remaining two showed malformation. All the regenerated plants were intermediate in leaf morphology and had thick and round leaves, which are typical characteristics of polyploids. Ploidy analyses by flow cytometry and chromosome counting in root-tip cells revealed that these plants are amphidiploid (2n=4×=36). Hybridity of the fusion products was confirmed by random amplified polymorphic DNA and cleaved amplified polymorphic sequence (CAPS) analyses. Furthermore, analyses of chloroplast (cp) and mitochondrial (mt) DNA by CAPS showed that these somatic hybrids contained cp- and mt-DNA of round kumquat without recombination in the regions analyzed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The kumquat (Fortunella), a close relative of Citrus, consists of a few species and is primarily grown in China and Japan. As well as being an ornamental plant, kumquat fruit is used for preserve, candy, syrup and fresh consumption because of its special flavor and thick edible rind (Hodgson 1967). In the southern part of Japan, kumquat is one of the most important fruit trees and is a regional specialty product crop. However, breeding of kumquat has lagged far behind that of Citrus species, and only one cultivar—Meiwa kumquat (Fortunella crassifolia Swingle)—is under cultivation. Production of new cultivars with unique characteristics such as seedlessness and attractive flavor is desired.
Somatic hybridization can overcome barriers of sexual incompatibility to allow production of hybrids between distantly related species, offering the chance to generate novel cultivars possessing traits from both parents. In Citrus and related species, somatic hybridization techniques are well developed and have been employed to facilitate improvement of Citrus scion and root stock (Grosser and Chandler 2000; Grosser et al. 2000). To date, more than 150 citrus somatic hybrids have been produced throughout the world, including intergeneric combinations (Moreira et al. 2000; Guo et al. 2002; Liu et al. 2002). However, only a few somatic hybrids have been produced between Fortunella and Citrus species, i.e., Meiwa kumquat with sweet orange (Deng et al. 1992; Shi et al. 1998; Cheng et al. 2003), Meiwa kumquat with ‘Succari’ sweet orange (Grosser et al. 1996), round kumquat with Mediterranean mandarin (Ollitrault et al. 1996), and Kinzu kumquat with trifoliate orange (Miranda et al. 1997). In these examples of intergeneric somatic hybrids between Fortunella and Citrus species, abnormal growth was reported, the reasons for which are still unclear due to the limited number of examples. Cheng et al. (2003) reported that nuclear-cytoplasmic incompatibility might be the cause of such abnormal growth.
In the present study, we describe regeneration of intergeneric somatic hybrids produced by electrofusion between embryogenic callus-derived protoplasts of round kumquat (Fortunella japonica Swingle) and ‘Morita navel’ orange (Citrus sinensis Osbeck) with male and female sterility, and discuss possible reasons for abnormal growth of these intergeneric somatic hybrids.
Materials and methods
Plant materials
Round kumquat (F. japonica Swingle) and ‘Morita navel’ orange (C. sinensis Osbeck) were used in this study. To obtain embryogenic calli from these species, mature fruits were surface-sterilized and then cut open aseptically. Unfertilized ovules were carefully dissected and placed on MT (Murashige and Tucker 1969) medium containing 5 mg l−1 adenine, 500 mg l−1 malt extract, 30 g l−1 sucrose and 2 g l−1 gellan gum (Kunitake et al. 1991). White, friable nucellar calli, with high embryogenic ability, were induced after approximately 3 months of culture. Embryogenic calli of both species were maintained for 3 years by subculturing on MT medium containing 10 mg l−1 6-benzylaminopurine (BA), 30 g l−1 sucrose and 2 g l−1 gellan gum at 25°C under continuous illumination (38 μmol m−2 s−1).
Protoplast isolation
Protoplasts were isolated from embryogenic calli of ‘Morita navel’ and round kumquat according to the method of Kunitake et al. (2002). Prior to protoplast isolation, embryogenic calli of both species were pretreated for 10 days by transferring to MT medium containing 50 g l−1 lactose and 2 g l−1 gellan gum to prevent bursting of protoplasts. The pretreated calli were gently squashed and incubated with an enzyme solution containing 0.3% (w/v) Cellulase Onozuka R-10 (Yakult, Tokyo, Japan), 0.3% (w/v) Macerozyme R-10 (Yakult), 0.1% Driselase (Kyowa, Tokyo, Japan), one-half strength MT macro elements and 0.7 M sorbitol, on a rotary shaker (70 rpm min−1) for 16 h at 25°C to liberate protoplasts. Protoplasts were purified by filtration through Miracloth (Calbiochem, San Diego, Calif.) and washed twice with 0.6 M mannitol solution after centrifugation (800 rpm, 3 min).
Electrofusion and protoplast culture
Isolated protoplasts were fused by electrofusion according to Kunitake et al. (2002). After fusion, protoplasts were cultured in MT medium containing 0.6 M sucrose and 2 g l−1 gellan gum, according to Ohgawara et al. (1985). After 5 months of culture, several green somatic embryos were transferred onto MT medium containing 500 mg l−1 malt extract, 30 g l−1 sucrose and 2 g l−1 gellan gum. After a further 1 month, mature somatic embryos were transferred onto one-half strength MT medium containing 1 mg l−1 gibberellic acid (GA3), 10 g l−1 sucrose and 3 g l−1 gellan gum for germination.
Flow cytometry
Young leaves of approximately 1 cm2 were collected from each of the parents as well as the regenerated plants, and chopped with a razor blade. The leaf samples were treated for 5 min in 1 ml buffer solution containing 1.0% (v/v) Triton X-100, 140 mM mercaptoethanol, 50 mM Na2SO3 and 50 mM Tris-HCl at pH 7.5, according to the method of Harusaki et al. (2000). Crude samples were filtered through Miracloth and stained with 25 μg l−1 propidium iodide. The relative fluorescence of total DNA was measured for each nucleus with an EPICS XL flow cytometry system (Beckman-Coulter, Munich, Germany) equipped with an argon laser (488 nm, 15 mW).
Chromosome number
Radicular tips of about 1.5–2.0 cm long were excised from the putative somatic hybrid plants, immersed in 2 mM 8-hydroxyquinoline for 15 h at 4°C and fixed in a mixed solution of ethanol:acetic acid (3:1) for 24 h at 4°C.
Enzymatic maceration and air-drying were performed according to Fukui (1996) with some modifications. The root tips were washed in distilled water to remove the fixative and macerated in an enzyme mixture containing 2% (w/v) Cellulase Onozuka RS (Yakult), 1%(w/v) Macerozyme R-200 (Yakult), 0.3% Pectolyase (w/v) (Kikkoman, Tokyo, Japan), and 200 mM EDTA at 37°C for 10 min.
Chromosomes were stained with 2% Giemsa solution (Merck, Darmstadt, Germany) in 1/30 phosphate buffer (pH 6.8) for 30 min, rinsed with distilled water, air-dried and observed under an optical microscope.
Extraction of total DNA
Total DNA was extracted from plants and cotyledonary tissues that failed to germinate normally, according to the method of Doyle and Doyle (1987). The total DNA was used for analyses of nuclear and cytoplasmic DNA by random amplified polymorphic DNA (RAPD) and cleaved amplified polymorphic sequence (CAPS) analyses.
RAPD analysis of nuclear DNA
RAPD analysis of nuclear DNA was performed by a method modified from that of Williams et al. (1990). The reaction mixtures (25 μl) contained 10 mM Tris-HCl pH 8.9, 80 mM KCl, 1.5 mM MgCl2, 100 μM dNTPs, 0.3 μM primer, 2.5 U Tth Taq DNA polymerase and 10 ng genomic DNA. Reactions were carried out by repeating 45 cycles of the following thermal treatments: 94°C for 30 s, 37°C for 2 min and 72°C for 3 min, in an ASTEC Program Control System PC-700. Random 10-mer primers OPA-6, OPA-9 and OPA-10 (Operon Technology, Alameda, Calif.) were used for PCR. Reaction products were resolved by electrophoresis on 1.3% agarose gels containing 0.5 μg ml−1 ethidium bromide and subsequently photographed under UV light (360 nm). For each combination of samples and primers, PCR was carried out twice, and only stable polymorphism was taken into account.
CAPS analysis of nuclear DNA
The internal transcribed spacer (ITS) region in nuclear ribosomal RNA (rRNA) was used for nuclear DNA analysis using primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (Yasui and Ohnishi 1998). The reaction mixture (50 μl) contained 10 mM KCl, 2.0 mM MgCl2, 0.2 μM dNTPs, 0.2 μM of each primer, 1 U Ex Taq DNA polymerase and 10 ng genomic DNA. Amplification was conducted by the following thermal treatments: pre-denature at 95°C for 5 min and 30 cycles of 1 min at 95°C, 1 min annealing at 60°C, 2 min at 72°C and further extension at 72°C for 10 min in a TaKaRa PCR thermal cycler MP TP3000. PCR products were digested with ten restriction endonucleases (EcoRI, XbaI, MspI, HaeIII, HinfI, MboI, XhoI, PvuI, PvuII and KpnI) for 15 h at 37°C. The digested DNA samples were resolved by electrophoresis on a 3.0% agarose gel containing 0.1 μg ml−1 ethidium bromide and visualized under UV light.
CAPS analysis of cytoplasmic DNA
Amplification of chloroplast (cp) and mitochondrial (mt) DNA using cp and mt universal primer pairs (Table 1) was performed in a TaKaRa PCR thermal cycler MP TP3000. For analysis of cp-DNA, three primer pairs (RbcL-PSAI, TrnD-TrnT and trnK3914F-trnK2R) were used for amplification according to the methods of Cheng et al. (2002) and Ureshino and Mayajima (2002). For analysis of mt-DNA, three primer pairs of 18SrRNA-5SrRNA, nad5/1-nad5/2r and nad7/1-nad7/2r were used for amplification according to the method of Cheng et al. (2002) and Dumolin-Lapegue et al. (1997). PCR products were digested with ten restriction endonucleases, as described above, resolved by electrophoresis on a 3.0% agarose gel, and visualized under UV light as described above.
Results and discussion
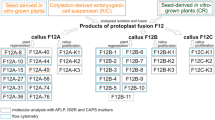
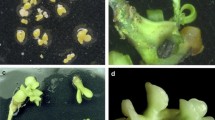
The first cell division occurred 1 week after electrofusion, and visible colonies were observed after 3 months of culture. Some of these colonies formed between one and five green somatic embryos per plastic dish after a further 2 months. These embryos, approximately 2 mm in diameter, were transferred individually to a hormone-free medium as independent strains. One month after transfer, most of these embryos formed several secondary somatic embryos. The 13 independent strains thus obtained (R1–R13) were transferred to one-half strength MT medium containing 1 mg l−1 GA3, 10 g l−1 sucrose and 3 g l−1 gellan gum to promote normal germination. The germination rate of these somatic embryos varied from 0 to 60% (Table 2). Finally, 32 regenerated plants were obtained from eight strains 2 months after transfer (Fig. 1A). In other strains, somatic embryos showed only enlargement of cotyledonary tissue, and normal germination was not observed (Fig. 1B). The regenerated plantlets were transferred to pots and grown under greenhouse conditions. Six of the eight strains (R1, R3–R7) had normal morphological characteristics, but the remaining two (R2 and R8) showed malformation (Fig. 2). Normally, regenerated plants exhibited leaf size intermediate between that of both parents. They also had thick and round leaves, which are typical characters of somatic hybrids and polyploids.
Flow cytometry analysis of all regenerated plants showed that their fluorescence intensity coincided with that of the 4× control (Fig. 2), while fluorescent intensity of cotyledonary tissues of five strains that did not germinate also corresponded to that of the 4× control. Chromosome counts of root tip cells revealed that the chromosome number of R1–R8 plants was 36 (Fig. 3), which is the sum of chromosome numbers of round kumquat (2n=18) and ‘Morita navel’ orange (2n=18).
To confirm the hybridity of regenerated plants, we employed RAPD analysis in R1, R3, R5–R7 and their parents. As shown in Fig. 4, these strains yielded bands specific to both parents. Hybridity of these strains was further confirmed using CAPS in the regenerated plants (R5, R6 and R7) and their parents. Amplification of the ITS region of nuclear DNA resulted in a fragment of the same size from somatic hybrids and both parents. After digestion of the fragment with MspI, the regenerated plants had specific bands derived from both parents (Fig. 5). Furthermore, hybridity of R10 and R12 somatic embryos, which failed to germinate normally, was confirmed using RAPD analysis. Primers OPA-6, OPA-9 and OPA-10 amplified specific bands from both parents (data not shown). These results indicate that seven strains, including R10 and R12, were somatic hybrids.
Cp- and mt-DNA amplifications were performed on three somatic hybrids (R5, R6 and R7) and both parents using three cp- and mt-DNA universal primer pairs. While every primer pair amplified bands satisfactorily, they did not reveal any polymorphism on agarose gels. When the PCR products were digested with ten restriction endonucleases, cp-DNA polymorphism was observed in nine primer/enzyme combinations, RbcL-PSAI/MspI, HinfI (Fig. 6A), HaeIII and MboI, TrnD-TrnT/MspI, HinfI, HaeIII and MboI(Fig. 6B), and trnK3914F-trnK2R/HinfI(Fig. 6C). All of the three somatic hybrids had uniform bands identical to those of round kumquat. On the other hand, mt-DNA polymorphism was seen in only one combination of 18SrRNA-5SrRNA/MspI (Fig. 7), and the remaining combinations showed no polymorphism. With this combination, these somatic hybrids showed the same band patterns as round kumquat. These results showed that three somatic hybrids had cp- and mt-DNA of round kumquat.
The cytoplasmic composition of somatic hybrids has been investigated mostly through Southern analysis of cytoplasmic or total DNA with cp- and mt-DNA probes, or through cp- and mt-DNA restriction-profile analysis (Earle 1995; Cardi et al. 1999; Bastia et al. 2000). These methods are usually expensive and/or time-consuming. Moreover, a considerable amount of DNA is required. Recently, new types of PCR-based molecular markers for cp- and mt-DNA—CAPS markers—have been developed. CAPS is simple, more rapid and less expensive when compared to the direct analysis of restricted cp- and mt-DNA. In citrus, CAPS markers were employed to characterize the cp- and mt-DNA of somatic hybrids or cybrids between Microcitrus papuana and Citrus jambhiri (Liu et al. 2002), Citrus reticulata and Poncirus trifoliata (Guo et al. 2002), and C. sinensis and F. crassifolia (Cheng et al. 2003). In the present study, cp- and mt-DNA analyses using CAPS markers revealed that cp- and mt-DNA of the somatic hybrids were derived from round kumquat without recombination in the regions analyzed.
Deng et al. (1992) produced intergeneric somatic hybrids between ‘Valencia’ sweet orange and Meiwa kumquat. These somatic hybrids grew less vigorously and showed dieback of their shoots annually (Shi et al. 1998). Cheng et al. (2003) described that both the cp- and mt-DNA in these somatic hybrids were derived from ‘Valencia’ sweet orange. Furthermore, they also suggested that the mt-DNA pattern is correlated with the phenotypic abnormality of these somatic hybrids and that nuclear-cytoplasmic incompatibility may be the cause of dieback. In the present study, such phenotypic abnormality was also observed in the somatic hybrids between round kumquat and ‘Morita navel’ orange, which had both the cp- and mt-DNA of round kumquat. In our preliminary studies, we carried out hybridization between the monoembryonic cultivar ‘Kiyomi’ tangor (Citrus unshiu × C. sinensis) and several Fortunella species. ‘Kiyomi’ tangor yielded a lot of hybrid seed in crosses with Changshou kumquat (Fortunella obovata) and Malayan kumquat (Fortunella polyandra) (data not shown). However, when 30 flowers of ‘Kiyomi’ tangor were crossed with Meiwa kumquat and oval kumquat (Fortunella margarita), only two hybrid seeds were obtained in each case. The two seeds obtained in each cross germinated but grew very slowly. Furthermore, we could not obtain normal seeds from hybridization between ‘Kiyomi’ tangor and round kumquat. These results suggest that the reason for this phenotypic abnormality may not be nuclear-cytoplasmic incompatibility, but nuclear-nuclear incompatibility between some Citrus species and round kumquat.
In conclusion, we obtained intergeneric somatic hybrids between round kumquat and ‘Morita navel’ orange with cp- and mt-DNA of round kumquat. This is the first report of successful somatic hybridization of kumquat and navel orange with male and female sterility.
To date, three normal strains have already been grafted onto trifoliate orange and planted in our experimental field. These amphidiploid somatic hybrids could be useful parents for producing triploid seedless kumquat cultivars with flavor of navel orange. These plants have already been propagated and are under cultivation for evaluating male or female fertility and fruit characteristics.
Abbreviations
- BA :
-
6-Benzylaminopurine
- CAPS :
-
Cleaved amplified polymorphic sequence
- RAPD :
-
Random amplified polymorphic DNA
References
Bastia T, Carotenuto N, Basile B, Zoina A, Cardi T (2000) Induction of novel organelle DNA variation and transfer of resistance to frost and Verticillium wilt in Solanum tuberosum through somatic hybridization with 1EBN S. commersonii. Euphytica 116:1–10
Cardi T, Bastia T, Monti L, Earle ED (1999) Organelle DNA and male fertility variation in Solanum spp. and interspecfic somatic hybrids. Theor Appl Genet 99:819–828
Cheng YJ, Guo WW, Deng XX (2002) Inheritance of organelle genomes of the somatic hybrid between Cleopatra mandarin (Citrus reticulata) and Flying dragon (Poncirus trifoliata). Yi Chuan Xue Bao 29:364–369
Cheng YJ, Guo WW, Deng XX (2003) Molecular characterization of cytoplasmic and nuclear genomes in phenotypically abnormal Valencia orange (Citrus sinensis) + Meiwa kumquat (Fortunella crassifolia) intergeneric somatic hybrids. Plant Cell Rep 21:445–451
Deng XX, Grosser JW, Gmitter FJ Jr (1992) Intergeneric somatic hybrid plants from protoplast fusion of Fortunella crassifolia cultivar ‘Meiwa’ with Citrus sinensis cultivar ‘Valencia’. Sci Hortic 49:55–62
Doyle J, Doyle JL (1987) A rapid DNA isolation procedure for small quantities fresh leaf tissue. Phytochem Bull 19:11–15
Dumolin-Lapegue S, Pemonge MH, Petit RJ (1997) An enlarged set of consensus primers for the study of organelle DNA in plants. Mol Ecol 6:393–397
Earle ED (1995) Mitochondrial DNA in somatic hybrids and cybrids. In: Levings CS III, Vasil I (eds) The molecular biology of plant mitochondria. Kluwer, Dordrecht, pp 557–584
Fukui K (1996) Plant chromosome at mitosis. In: Fukui K, Nakayama S (eds) Plant chromosome. Laboratory methods. CRC Press, Boca Raton, Fla. pp 1–17
Grosser JW, Chandler JL (2000) Somatic hybridization of high yield, cold-hardy and disease resistant parents for citrus rootstock improvement. J Hortic Sci Biotechnol 75:641–644
Grosser JW, Mourao-Fo FAA, Gmitter FG Jr, Louzada ES, Jiang J, Baergen K, Ouiros A, Cabasson C, Schell JL, Chandler JL (1996) Allotetraploid hybrids between Citrus and seven related genera produced by somatic hybridization. Theor Appl Genet 92:577–582
Grosser JW, Ollitrault P, Olivares-Fuster O (2000) Somatic hybridization in Citrus: an effective tool to facilitate variety improvement. In Vitro Cell Dev Biol Plant 36:434–449
Guo WW, Cheng YJ, Deng XX (2002) Regeneration and molecular characterization of intergeneric somatic hybrids between Citrus reticulata and Poncirus trifoliata. Plant Cell Rep 20:829–834
Harusaki K, Kokuryo D, Kunitake H, Komatsu H (2000) Determination of ploidy level of Citrus species using flow cytometry. Proc School Agric Kyushu Tokai Univ 19:45–52
Hodgson RW (1967) Horticultural varieties of citrus. In: Reuther W, Webber HJ, Batchelor LD (eds) Citrus index, vol 1. University of California, Riverside, Calif. pp 431–592
Kunitake H, Kagami H, Mii M (1991) Somatic embryogenesis and plant regeneration from protoplasts of ‘Satsuma’ mandarin (Citrus unshu Marc.). Sci Hortic 47:27–33
Kunitake H, Nagasawa K, Takami K, Komatsu H (2002) Molecular and cytogenetic characterization of triploid somatic hybrids between ‘Shougun’ mandarin and grapefruit. Plant Biotechnol 19:345–352
Liu JH, Pang XM, Cheng YJ, Meng HJ (2002) Molecular characterization of the nuclear and cytoplasmic genomes of intergeneric diploid plants from cell fusion between Microcitrus papuana and Rough lemon. Plant Cell Rep 21:327–332
Miranda M, Motomura T, Ikeda F, Ohgawara T, Saito W, Endo T, Omura M, Moriguchi T (1997) Somatic hybrids obtained by fusion between Poncirus trifoliata (2×) and Fortunella hindsii (4×) protoplasts. Plant Cell Rep 16:401–405
Moreira CD, Chase CD, Gmitter FG Jr, Grosser JW (2000) Transmission of organelle genomes in citrus somatic hybrids. Plant Cell Tissue Organ Cult 61:165–168
Murashige T, Tucker DPH (1969) Growth factor requirement of citrus tissue culture. Proceedings 1st International Citrus Symposium 3:1155–1161
Ohgawara T, Kobayashi S, Ohgawara E, Uchimiya H, Ishii S (1985) Somatic hybrid plants obtained by protoplast fusion between Citrus sinensis and Poncirus trifoliata. Theor Appl Genet 71:1–4
Ollitrault P, Dambier D, Sudanhono (1996) Somatic hybridization in Citrus: some new hybrid and alloplasmic plants. In: Proceedings, VIII Congress of the International Society of Citriculture. 12–17 May 1996, Sun City, South Africa, pp 907–912
Shi YZ, Deng XX, Yi HL (1998) Variation of intergeneric somatic hybrids between Citrus sinensis cv ‘Valencia’ and Fortunella crassifolia cv ‘Meiwa’. Acta Bot Sin 40:1060–1066
Ureshino K, Miyajima I (2002) The study on the relationship between leaf colors and ptDNA inheritance in intersectional cross of Rhododendron kiusianum × R. japonicum f. flavum, resulting in an unexpected triploid progeny. J Jpn Soc Hortic Sci 71:214–219
Williams JGK, Kubelik AR, Lival KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Yasui Y, Ohnishi O (1998) Phylogenetic relationships among Fagopyrum species revealed by the nucleotide sequences of the ITS region of the nuclear rRNA gene. Genes Genet Syst 73:201–210
Acknowledgements
The authors are grateful to Dr. Masahiro Mii of the Faculty of Horticulture, Chiba University, for his advice and critical reading of this manuscript. This work was supported in part by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Ebinuma
Rights and permissions
About this article
Cite this article
Takami, K., Matsumara, A., Yahata, M. et al. Production of intergeneric somatic hybrids between round kumquat (Fortunella japonica Swingle) and ‘Morita navel’ orange (Citrus sinensis Osbeck). Plant Cell Rep 23, 39–45 (2004). https://doi.org/10.1007/s00299-004-0777-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0777-z