Abstract
Anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) is a primary small-vessel vasculitis group with three distinct clinical entities, including GPA, MPA, and EGPA. The incidence of AAV has increased since the 1980s and remained stable since the early 2000s. Distinct phenotypes of AAV may also differ in various geographical regions. Elderly people are susceptible to developing AAV, and AAV is used to be a fatal disease before the introduction of glucocorticoids and immunosuppressants. Different treatment protocols should be employed for patients with different disease severity levels. Several randomized controlled trials evaluated the efficacy and safety of treatment protocols for remission induction and maintenance. Glucocorticoid and cyclophosphamide therapies remain the mainstay for treating AAV. Rituximab is non-inferior to cyclophosphamide for inducing remission, and it is more effective for relapsing and refractory disease. The combination of low-dose glucocorticoids with less toxic immunosuppressants, such as azathioprine, rituximab, and methotrexate, is suggested in place of cyclophosphamide. The prognosis of patients with AAV is another crucial issue due to the accumulating damage caused by both the disease activity and treatment toxicity. This review focused on recent progress on the prevalence, treatment, and outcomes of patients with AAV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
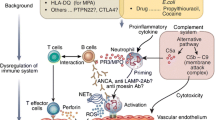
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of primary small-vessel vasculitis with three distinct clinical entities, including granulomatosis with polyangiitis (GPA, formerly Wegener’s granulomatosis), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA, formerly Churg–Strauss syndrome) [1]. The vast majority of small-vessel vasculitis diseases have similar clinical and histopathological features. Kidneys and lungs are the most commonly involved organs in AAV. ANCA is the serological marker of AAV. By indirect immunofluorescence (IIF), two fluorescence patterns of ANCA are distinguished: the cytoplasmic staining pattern (cANCA) and perinuclear staining pattern (pANCA). Most patients with a cANCA pattern obtained by IIF have ANCA directed against proteinase-3 (PR3). Patients with pANCA mostly have ANCA directed against one of a variety of antigens, and myeloperoxidase (MPO) is the most important one.
This review focuses on the prevalence in different regions and the current therapeutic approaches for AAV. The outcomes of AAV, such as infection, relapse, treatment resistance, and renal prognosis, are also discussed.
Prevalence and incidence
AAV is a common autoimmune disease. Most epidemiology studies of AAV have been conducted in Europe, Japan, USA, and Oceania in past decades. The overall annual incidence rates of AAV in Europe are 10–20/million [2], and there is a prevalence of 46–184 cases per million [3]. A comparison study from three regions in Europe showed that the overall incidence rates of AAV are similar at approximately 19/million [2]. The incidence rate has increased in the European population since the 1980s [4, 5] and remained stable since the early 2000s [3], which may be a consequence of the increased awareness among physicians following the introduction of routine serologic tests for ANCA. Japan has a similar overall annual incidence rate as the UK (22.6/million in Japan and 21.8/million in UK) [6], while the incidence rate in USA is estimated as 10.35/million, as reported by Zeftet al [7].
Although AAV can affect people of all ages, elderly people are more susceptible, with a peak age of 65–74 years [8]. In most studies, the incidence of AAV was similar between males and females.
The incidence of the distinct phenotypes of AAV may differ in various geographical regions. GPA is more common in northern Europe, while MPA is more prevalent in southern Europe. The annual incidence of GPA in three regions of Europe was as follows: Tromsø (Norway, latitude 70°N), Norwich (UK, latitude 52°N), and Lugo (Spain, latitude 43°N)—10.5, 10.6, and 4.9/million, while the annual incidence of MPA in these three regions 2.7, 8.4, and 11.6/million, respectively [2]. New Zealand and Australia have similar incidence rates of GPA to northern Norway. The incidence of GPA in Australia and northern Norway was 6–7 and 8/million/year, respectively [9, 10]. The point prevalence for GPA in Canterbury, New Zealand in 2003 (93.5/million) was similar to that in northern Norway (95/million) [11]. The overall annual incidence levels of GPA and MPA are reported to be 11.3 and 5.9/million in the UK, respectively [12]. In a comparative study, MPA is the predominant subtype in Japan (18.2/million, 83%), while GPA is more frequent in the UK (14.3/million, 66%) [6]. There is little, if any, epidemiological data of AAV in China yet. Data from a single-center study in China demonstrated that MPA is also more common than GPA in China, which accounts for about 70–80% of patients with AAV [13]. The annual incidence of GPA in Taiwan was 0.37/million patient-years; the ratio of the number of patients with GPA to that with MPA was 0.57 [14], which is similar to the study from the Mainland [15]. EGPA is much rarer than GPA and MPA, and it has an annual incidence of 0.5–2.0/million [16].
Treatment
The treatment for AAV includes induction and maintenance of remission. Patients with different levels of disease severity respond to different treatment protocols. The European Vasculitis Study Group (EUVAS) proposed categories to classify the clinical AAV subtypes and assign different treatment regimens (Table 1) [17]. In the past few decades, substantial progress has been made in the treatment, and patient survival has dramatically improved. Major clinical trials for AAV are listed in Table 2.
Induction of remission
Glucocorticoids, in combination with immunosuppressants, especially cyclophosphamide or rituximab, are the most commonly used treatment to induce remission. High-dose pulse methylprednisolone and plasma exchange are also considered for use in patients with severe disease.
Cyclophosphamide
Currently, the combination of cyclophosphamide (CYC) with corticosteroids is the standard treatment for AAV and has transformed AAV from a uniformly fatal disease to a chronic relapsing condition in recent decades [18]. This regimen is effective in 70–90% of patients with AAV [19]. In general, patients are treated with oral CYC at doses of 2 mg/kg/day, lasting for 3–6 months, with doses adjusted based on the patients’ age and renal function [20, 21]. For continuous oral low-dose cyclophosphamide, the dose is reduced by 25% for > 60 years of age and by 50% for > 75 years of age, while the dose adjustment for pulsed high-dose cyclophosphamide is shown in Table 3 [17, 20]. However, adverse effects, including haemorrhagic cystitis, infertility, infection, bone marrow suppression, and malignancy, are associated with the cumulative dose of CYC.
To minimize the drug toxicity, pulsed intravenous CYC (IV-CYC) was designed and tested in several randomized trials [22,23,24]. In the CYCLOPS (randomized trial of daily oral versus pulse Cyclophosphamide as therapy for ANCA-associated Systemic Vasculitis) study, newly diagnosed AAV cases with renal involvement and without life-threatening disease were treated with prednisolone in combination with either oral CYC (2 mg/kg/day, a maximum of 200 mg/day) or IV-CYC (15 mg/kg, a maximum of 1200 mg/pulse, every 2–3 weeks). The results showed that IV-CYC therapy was non-inferior to oral CYC for inducing AAV remission in the time to remission (HR, 1.098; P = 0.59) and remission rates (88.1% in the pulse group versus 87.7% in the daily oral group), decreasing the cumulative CYC doses by half and treatment-related leukopenia by one-third [25].
However, a lower cumulative CYC dose seems to be associated with a higher relapse rate. A long-term follow-up study (median 4.3 years) showed that continuous oral CYC for induction has a lower subsequent relapse rate compared with a pulsed IV-CYC regimen (20 and 40%, respectively) [26]. Nevertheless, some patients who do not respond well and/or rapidly to IV-CYC induction could also achieve remission when switched to oral CYC [25]. Physicians need to balance the possibly higher relapse rates in IV-CYC with higher rates of side effects in daily oral regimens [27] and establish individually tailored treatment approaches based on the disease stage and activity.
All patients treated with CYC are encouraged to be well hydrated during treatment, and patients with the IV-CYC regimen are encouraged to take oral or intravenous Mesna (2-mercaptoethane sulfonate sodium) to reduce the bladder toxicity. Cortimoxazole is also recommended as a prophylaxis against Pneumocystis jirovecii infection for all patients during treatment if there are no intolerance reactions or contraindications. Strict monitoring of complete blood count tests should be routinely performed for leukopenia and lymphocytopenia, especially for patients with oral CYC regimen (usually twice a week). In both administration modalities, dose changes or discontinuation of CYC may be necessary in the event of acute leukopenia or a gradual decrease over time [28].
Rituximab
Rituximab (RTX), a chimeric anti-CD20 IgG monoclonal antibody, was reported as effective for patients with severe, refractory relapsing AAV in the previous case reports, and retrospective and prospective clinical trials [29,30,31,32,33,34,35]. Furthermore, two randomized controlled trials were conducted to evaluate its efficacy and safety in introducing remission for AAV. The RITUXVAS study (Rituximab versus Cyclophosphamide in ANCA-Associated Renal Vasculitis) [36] recruited 44 patients with newly diagnosed GPA or MPA and renal involvement (including patients requiring dialysis and undergoing plasma exchange). The rituximab group (n = 33) received high-dose oral corticosteroids (with the initial dose of 1 mg/kg/day, tapering to 5 mg/day at the end of 6 months) plus 4 consecutive infusions of rituximab (375 mg/m2 weekly) and 2 infusions of intravenous cyclophosphamide (15 mg/kg, with the first and third rituximab infusions), while the cyclophosphamide arm received high-dose oral corticosteroids plus intravenous cyclophosphamide (15 mg/kg) for 3–6 months, which was followed by maintenance by azathioprine (AZA). The primary endpoint, sustained remission, was achieved by 76% in the rituximab arm compared to 82% in the CYC arm at 12 months, indicating that rituximab is as efficient as cyclophosphamide for inducing remission in AAV [36].
In the RAVE study (Rituximab versus Cyclophosphamide for ANCA-associated Vasculitis) [37], in addition to tapering the corticosteroid dose, 197 ANCA-positive GPA or MPA patients, including newly diagnosed and relapsing AAV patients, were randomly assigned in a 1:1 ratio according to clinical site and ANCA type, to receive 4 infusions of rituximab (375 mg/m2 weekly plus daily placebo-cyclophosphamide, n = 99) without maintenance therapy, or oral cyclophosphamide (2 mg/kg per day adjusted for renal insufficiency plus placebo–rituximab infusions, n = 98), which was followed by maintaining remission with AZA (2 mg/kg/day). The complete remission rates at 6 months, in the absence of corticosteroids, were not significantly different between the two arms (64% in the rituximab group and 53% in cyclophosphamide group, P < 0.001) [37]. This study also demonstrated that rituximab is comparable to oral cyclophosphamide for remission induction. In the subset of patients with relapsing disease (n = 101), rituximab showed superior efficacy compared to cyclophosphamide, with remission rates of 67% in the rituximab group versus 42% in the cyclophosphamide group [37]. Furthermore, patients with PR3–ANCA were more likely to achieve disease remission than those with MPO–ANCA in the RTX arm (50% patients in the RTX arm became negative for PR3–ANCA, as compared with 40% for MPO–ANCA) [37]. Regarding remission on long-term follow-up, rituximab was likely to be more effective than cyclophosphamide at 12 months (48 versus 39%), which was not the case at 18 months (39 versus 33%) [37].
Adverse events were similar for both the rituximab and cyclophosphamide regimens in the two trials, indicating that those side effects were probably caused by high-dose glucocorticoids and the disease itself and current cyclophosphamide regimen would be reasonably safe.
These studies suggest that rituximab is non-inferior to cyclophosphamide for AAV remission induction and is more effective than cyclophosphamide in patients with relapsing disease. However, rituximab treatment does not result in earlier remission or a lower incidence of severe adverse events. As there was no maintenance following the RTX regimen in both trials, further refinement and evaluation of treatment strategies following RTX-based induction therapy are still required.
Methotrexate
Methotrexate (MTX) may be an option for remission induction when dosed at 20–25 mg weekly (oral or parenteral) for patients with non-organ-threatening and normal renal function [38,39,40,41,42,43,44]. A randomized controlled trial compared methotrexate with cyclophosphamide in patients without critical organ manifestations, termed the NORAM study [45]. At 6 months, methotrexate was as effective as cyclophosphamide in disease control; 90% of patients in the MTX group achieved remission, and 94% in the CYC group achieved remission [45]. However, long-term (median 6 years) follow-up demonstrated that MTX-treated patients were more likely to relapse and needed to be treated with glucocorticoids and further immunosuppression for a longer time compared with CYC-treated patients [46]. Furthermore, MTX was shown to be less effective in induction remission in patients with more extensive disease (P = 0.04) or pulmonary disease (P = 0.03) as remission was delayed among these patients [45]. Therefore, MTX should be considered as an alternative to cyclophosphamide for non-organ-threatening disease according to the EULAR/ERA-EDTA recommendations [28]. The recommendations also listed the types of patients for whom MTX could be considered: nasal and paranasal disease without bony involvement (erosion), cartilage collapse, olfactory dysfunction or deafness; skin involvement without ulceration; myositis (skeletal muscle only); non-cavitating pulmonary nodules/infiltrate without haemoptysis or when cyclophosphamide or rituximab were not available, contraindicated or undesirable for the patient [28]. Folic acid supplement is suggested during MTX treatment.
Plasma exchange
The main indications for plasma exchange (PLEX) as an adjunctive therapy with standard remission induction are AAV with anti-glomerular basement membrane (GBM) antibody, diffuse alveolar haemorrhage and severe renal impairment. The randomized MEPEX study (Methylprednisolone versus Plasma Exchange) [47] enrolled 137 new diagnoses of severe renal vasculitis (serum creatinine level > 500 µmol/L or dependent on dialysis). In addition to oral prednisolone and cyclophosphamide, patients were randomly treated with seven sessions of plasma exchange (PLEX, n = 70) or three infusions of 1 g of pulse methylprednisolone daily (MP, n = 67). PLEX was superior in terms of patient survival and renal recovery, with 69 and 43% of those treated with PLEX who were alive and dialysis independent at 6 and 12 months, respectively, compared to 49 and 19% of those treated with MP, respectively [47]. However, there were no significant differences in end-stage renal disease (ESRD) and mobility in the subsequent observation (median 3.95 years), indicating that the long-term prognosis and overall benefit of plasma exchange for severe AAV remain uncertain [48].
CCX 168
As AAV pathogenesis therapies continue to evolve, complement activation through alternative pathways has been demonstrated to be associated with the progression of AAV [49, 50]. The common pathway component C5a is demonstrated to play a significant role in ANCA-mediated neutrophil activation [51, 52]. Several trials were designed to evaluate the efficacy and safety of CCX168, a C5a-receptor inhibitor since 2011. Recently, the result from CLEAR study (C5aR inhibitor on Leukocytes Exploratory ANCA-associated Renal Vasculitis) showed that C5a receptor inhibition with avacopan was effective in replacing high-dose glucocorticoids in treating vasculitis [53]. In this study, 67 newly diagnosed or relapsing vasculitis were randomly assigned to receive avacopan (30 mg, twice daily) plus reduced-dose prednisone (20 mg daily, n = 22), avacopan (30 mg, twice daily, n = 22) without prednisone, or placebo plus prednisone starting at 60 mg daily (control group, n = 23). All patients received cyclophosphamide or rituximab. At 12 weeks, 86, 81, and 70% of the avacopan with reduced prednisone, avacopan with no prednisone groups, and high-dose glucocorticoid group achieved clinical response, respectively, while the incidence of adverse events in three groups was similar (86, 96, and 91%, respectively) [53]. This study showed that both avacopan groups were non-inferior to high-dose glucocorticoid for remission induction.
Maintenance of remission
Despite the significant benefit of corticosteroid plus cyclophosphamide for AAV patients, relapse and drug toxicity are related to this approach. Relapse remains a major challenge, although most patients achieved remission under proper treatment [19, 45]. The relapse rate is higher after discontinuation of immunosuppression or glucocorticoids [19, 54]. Therefore, long-term maintenance of remission is necessary to reduce relapse, and the optimal duration is 24 months according to the 2016 recommendations [28]. Considering the adverse effects caused by long-term exposure to cyclophosphamide, the most commonly used medication in maintenance therapy, there are less toxic immunosuppressive agents, such as azathioprine, methotrexate, rituximab, leflunomide, and mycophenolate mofetil, that can be combined with or without low-dose glucocorticoids.
Azathioprine
Azathioprine (AZA) is the first-line medication for remission maintenance. In the CYCAZAREM study [19] (Cyclophosphamide versus Azathioprine for Early Remission Phase of Vasculitis), following induction of remission with prednisolone plus oral CYC, 144 newly diagnosed AAV patients were randomized to receive either AZA (2 mg/kg/day) or CYC (1.5 mg/kg/day). At 18 months, no significant difference was observed in the relapse rates in both arms (15.5% in the AZA-treated group and 13.7% in the CYC-treated group), demonstrating that AZA and CYC appear to be comparably efficacious in preventing relapse in maintenance therapy. This study has demonstrated that converting from CYC to AZA once patients achieve remission is an effective way to maintain treatment efficacy while reducing adverse events from the cumulative dose of long-term CYC use. White blood cells in peripheral blood should be monitored during AZA treatment.
Methotrexate
Methotrexate has been effectively used for maintenance therapy after inducing remission [55, 56]. A prospective, open-label trial, termed WEGENT, by the French Vasculitis Group was conducted in 126 patients and randomly treated them with AZA (2 mg/kg/day) or MTX (starting at 0.3 mg/kg/week and reaching 25 mg/kg/week) for 12 months [57]. Adverse events occurred in 29 AZA recipients and 35 MTX recipients (P = 0.29), and seven patients (11%) in the AZA arm reached the primary end point (adverse events requiring therapy discontinuation), as compared to 12 patients (19%) in the MTX arm (P = 0.21). Furthermore, relapse rates in the follow-up observation (29 ± 13 months) were similar in both groups (36 and 33%, respectively) [57]. This study showed that AZA and MTX were comparable in both safety and efficacy for maintenance therapy. However, the use of MTX is limited in AAV remission due to its renal clearance. MTX should not be used in patients with renal insufficiency (Scr < 177 µmol/L), and folic acid supplementation is helpful during MTX treatment.
Leflunomide
Metzler et al. reported a randomized controlled trial evaluating the efficacy and safety in a comparison of leflunomide (30 mg/day) and MTX (starting at 7.5 mg/week and reaching 20 mg/week after 8 weeks) for 2 years in 54 patients who achieved remission with CYC and prednisolone [58]. Leflunomide was more effective than MTX in remission maintenance, but it caused a high rate of adverse events, including hypertension and infection. Therefore, leflunomide is not routinely used as the first-line maintenance therapy and would be an option in the event of intolerance to AZA or MTX.
Mycophenolate mofetil (MMF)
MMF was indicated to represent an alternative to CYC for induction and maintenance of remission in MPO–ANCA-associated MPA with mild-to-moderate renal disease in some uncontrolled studies [59,60,61,62]. However, a randomized controlled trial, IMPROVE, that recruited 154 newly diagnosed AAV patients (after CYC induction) from 42 centers in 11 European countries demonstrated that at a median follow-up of 36 months, MMF (starting at 2 g/day) was less effective than AZA (starting at 2 mg/kg/day) without significant difference in adverse events [63]. Therefore, MMF may be considered for use in patients with intolerance to other agents.
Rituximab
As mentioned above, no maintenance treatment was conducted in either the RAVE or RITUXVAS trial. The MAINRITSAN study (Maintenance of Remission using Rituximab in Systemic ANCA-associated vasculitis) is the first randomized controlled trial to compare RTX (low dose at 500 mg every 6 months) to AZA (dosed at 2 mg/kg/day and tapered until 22 months) for maintenance therapy. It was conducted in 115 AAV patients following standard remission with CYC plus glucocorticoids [64]. At 28 months, 5% (3/57) of patients in the RTX arm suffered major relapse compared to 29% (17/58) of patients in the AZA arm, and there was not a significant difference in the adverse event rates [64]. Although RTX seemed to be more effective than AZA for maintenance in this trial, the result is limited, because the dose of AZA was tapered from 12 months (starting at 2 mg/kg/day for 12 months, and then 1.5 mg/kg/day for 6 months, and 1 mg/kg/day for 4 months) [64]. Further evaluation of the efficacy and optimal dose of RTX in maintenance remission is still needed.
Treatment of relapsing and refractory disease
Relapse was defined as re-occurrence, new onset of disease attributable to active vasculitis, or worsened disease activity. “Major relapse” is defined as “recurrence or new onset of potentially organ- or life-threatening disease attributable to active vasculitis”. “Minor relapse” is defined as “recurrence or new onset of disease attributable to active vasculitis that is neither potentially organ- nor life-threatening” [65]. Relapse in AAV is a well-recognized, independent risk factor for subsequent progression to end-stage renal disease (ESRD) [66]. Major relapse of organ-threatening or life-threatening disease is suggested to be treated as a new disease, with the combination of glucocorticoids and either cyclophosphamide or rituximab to induce remission [28]. Rituximab is preferred for use in relapse of organ-threatening or life-threatening disease considering the clinical data in the relapse subgroup of the RAVE study and the cumulative toxicity caused by long-term cyclophosphamide use. To treat non-severe relapse in AAV (so-called “minor relapse”), the intensification of immunosuppressive agents, such as an increased dose of glucocorticoids or AZA, is suggested. Modification of the immunosuppressive remission maintenance regimen was recommended by the EULAR/ERA-EDTA recommendation in 2016 [28] based on a study that demonstrated a higher severe relapse rate caused by increasing the glucocorticoid dose in patients with minor relapse [67]. It remains important to avoid excessive exposure to cyclophosphamide, because most malignancies occur at a cumulative dose of cyclophosphamide over 36 g [68].
Patients with refractory disease include those who have unchanged or increased activity after 4 weeks of treatment or an inadequate response (less than 50% reduction in the disease activity score in 6 weeks) and those who have chronic persistent disease (at least one major or three minor items on the disease activity score after 12 weeks of treatment) [65]. A switch from cyclophosphamide to rituximab or from rituximab to cyclophosphamide is suggested for such patients. For patients who inadequately respond to pulsed cyclophosphamide or rituximab or when rituximab is unavailable, a switch to oral cyclophosphamide can be considered [69]. Intravenous immunoglobulins (0.4 g/kg for 5 days, 2 g per cycle) may also benefit patients who fail to achieve remission and have persistent low activity [70,71,72].
Outcomes of AAV
As mentioned above, AAV is usually fatal before the introduction of standard immunosuppressive treatment, and it has a 1-year mortality rate of approximately 80% [73]. Patients with generalized AAV have a very poor prognosis if the disease is not properly diagnosed and treated [74]. The use of glucocorticoids and cyclophosphamide has dramatically improved the outcomes of AAV patients and could increase the 5-year survival rate to 69–91% in patients with GPA and 45–76% in patients with MPA [75]. Advanced age, secondary infection, especially pulmonary involvement, and initial renal function are independent predictors of mortality [73]. Despite the effective control of disease activity, treatment-related adverse effects, such as infections, urothelial malignancy, and infertility, have received closer attention [76, 77]. Of note, infection has become the leading cause of patient death. In a 5-year follow-up cohort study by EUVAS (n = 535, median age 61 years, 53% of GPA), infections contributed to 48% of deaths in the first year, which was followed by active vasculitis (19%), while mortality after the first year was mainly related to cardiovascular disease, malignancy, and infection (26, 22, and 20%, respectively) [18]. Most infections are associated with Staphylococcus aureus and P. jirovecii, and prophylaxis and effective treatment against any infections play a significant role during remission. Furthermore, it was found that patients with older age, renal dysfunction, a lower lymphocyte count, or pulmonary involvement are more likely to suffer from infection [78]. A recent study revealed that the CD4 lymphocyte count had a higher predictive value than the total lymphocyte count for overall infections [79]. Therefore, strict monitoring of the lymphocyte count in the peripheral blood (no less than 600/mm3), especially the CD4+ lymphocyte count (no less than 200/mm3), is useful for decreasing treatment-related adverse effects and improving prognosis.
Relapse and treatment resistance are also crucial issues for AAV outcomes. Although most patients could achieve remission after induction therapy, relapse remains common for patients with AAV, even while receiving maintenance treatment. For example, in the CYCAZAREM study, the relapse rates were 13.7% in the CYC group and 15.5% in the AZA group at 18 months [19], and in the NORAM study, relapse rates were 46.5% in MTX arm and 69.5% in CYC arm at 18 months, respectively [45]. Severe relapse, such as pulmonary haemorrhage, may be life-threatening. In the long-term follow-up of patients in four EUVAS trials, i.e., MEPEX, CYCAZAREM, NORM, and CYCLOPS studies, a total of 201 patients (38%) experienced at least one relapse between 44 and 62 months, and renal insufficiency at enrolment (creatinine level > 200 μmol/L) was strongly associated with a reduced risk of relapse [80]. The relapse rate increases with time [81] and patients with GPA are more likely to relapse than those with MPA (18 versus 4%, respectively) [19]. PR3–ANCA and cardiovascular involvement at presentation were independent factors associated with a higher risk of relapse [80] [80], whereas PR3–ANCA and pulmonary and/or ENT involvement were observed in two cohort studies from USA and France [82]. Cyclophosphamide-sparing strategies with pulsed intravenous cyclophosphamide or methotrexate, instead of oral cyclophosphamide, seem to increase the risk of relapse [26, 83, 84]. Some patients insufficiently respond to glucocorticoids and cyclophosphamide therapy. Treatment resistance occurs in 23% of 334 treated patients, particularly females, black patients, and those with severe kidney disease [66]. Another study documented that older age and MPO–ANCA may be predictors of treatment resistance [82].
As one of the most commonly involved organs in AAV, renal involvement is another key issue in AAV patients. Renal survival was documented to be 57% at 30 months and 82% at 57 months [85, 86]. In addition, 38% of MPO–ANCA patients and 15% of PR3–ANCA patients progressed to end-stage renal disease (ESRD) [87]. Patients with poor renal function have worse renal survival outcomes. The higher the serum creatinine at the time of diagnosis is, the poorer the renal survival [88]. In the follow-up of patients in the above-mentioned MEPEX trial (n = 137, median 3.95 years), 51% (n = 70) of patients died, and 41% (n = 56) developed ESRD [47]. A study from The Netherlands described similar results, where 23% patients on dialysis at the time of diagnosis died within 6 months of follow-up and another 29% continued to depend on dialysis [89]. Relapse was reported to be a factor that increased the risk of progression to ESRD by 4.7 times [66]; in addition, it is an independent predictor of ESRD. The age, percentage of normal glomeruli, tubular atrophy, and intraepithelial infiltrates in the renal biopsy were found to be predictors of renal function in patients with severe renal dysfunction caused by ANCA glomerulonephritis [19]. In 2010, Berden et al. proposed a histopathological classification of ANCA-associated glomerulonephritis, i.e., focal, crescentic, mixed and sclerotic categories, and renal survival rates in their cohort for these four categories were 93, 76, 61, and 50%, respectively [90]. Another study from Europe had similar findings [91] and revealed that this classification system could predict renal outcomes. However, a study by Chang et al. investigated Chinese patients with AAV and found that the probability of progressing to ESRD was increased with ascending categories of focal, mixed, crescentic, and sclerotic glomerulonephritis [92]. Such difference between the Chinese and European cohorts might be attributed to the following reasons. First, in the Chinese cohort, most patients have MPO–ANCA and thus had more chronic lesions. Second, plasma exchange was not sufficiently employed in the Chinese cohort [92].
Summary
Ethnicity, geographical factors, age, and distinct phenotypes of AAV may contribute to the different occurrence rates of AAV. Considerable strides have been made in the treatment of AAV in past decades. To induce remission, glucocorticoids in combination with cyclophosphamide remain the standard therapy for generalized AAV, while methotrexate can be used as an alternative to cyclophosphamide in patients with non-organ-threatening disease. Rituximab is non-inferior to cyclophosphamide for AAV remission induction and is more effective in patients with relapsing disease. For maintenance therapy, azathioprine, rituximab, and methotrexate are well-accepted options. The C5a receptor may be an important treatment target. Infection has become the leading cause of death instead of active AAV per se. The intensity of immune suppression should be properly adjusted to control disease manifestations and prevent relapse, as well as to avoid adverse events related to the standard immunosuppressive treatment.
References
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC et al (2013) 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheumatol 65:1–11
Watts RA, Lane SE, Scott DG et al (2001) Epidemiology of vasculitis in Europe. Ann Rheum Dis 60:1156–1157
Watts RA, Mahr A, Mohammad AJ et al (2015) Classification, epidemiology and clinical sub-grouping of antineutrophil cytoplasmic anti- body (ANCA)-associated vasculitis. Nephrol Dial Transplant 30(suppl 1):i14–i22
Andrews M, Edmunds M, Campbell A et al (1990) Systemic vasculitis in the 1980s—is there an increasing incidence of Wegener’s granulomatosis and microscopic polyarteritis? J R Coll Phys Lond 24:284–288
Knight A, Ekbom A, Brandt L et al (2006) Increasing incidence of Wegener’s granulomatosis in Sweden, 1975–2001. J Rheumatol 33:2060–2063
Fujimoto S, Ra Watts, Kobayashi S et al (2011) Comparison of the epidemiology of anti-neutrophil cytoplasmic antibody-associated vasculitis between Japan and the U.K. Rheumatology (Oxford) 50:1916–1920
Zeft AS, Schlesinger M, Keenan H et al (2010) Wegener’s granulomatosis and environmental factors in Western Montana. Rheumatol Rep 1:e8
Watts RA, Lane SE, Bentham G et al (2000) Epidemiology of systemic vasculitis: a ten-year study in the United Kingdom. Arthritis Rheum 43:414–419
Ormerod AS, Cook MC (2008) Epidemiology of primary systemic vasculitis in the Australian Capital Territory and south-eastern New South Wales. Intern Med J 38:816–823
Koldingsnes W, Nossent H (2000) Epidemiology of Wegener’s granulomatosis in northern Norway. Arthritis Rheumatol 43:2481–2487
Gibson A, Stamp LK, Chapman PT et al (2006) The epidemiology of Wegener’s granulomatosis and microscopic polyangiitis in a Southern Hemisphere region. Rheumatology (Oxford) 45:624–628
Watts Richard A, Mooney Janice, Skinner Jane et al (2012) The contrasting epidemiology of granulomatosis with polyangiitis (Wegener’s) and microscopic polyangiitis. Rheumatology 51:926–931
Chen M, Yu F, Zhang Y, Zhao MH (2005) Clinical and pathological characteristics of Chinese patients with antineutrophil cytoplasmic autoantibody associated systemic vasculitis: a study of 426 patients from a single center. Postgrad Med J 81:723–727
Chien-Sheng Wu, Hsieh Chi-Jeng, Peng Yu-Sen, Chang Ting-Hui, Zong-Ying Wu (2015) Antineutrophil cytoplasmic antibody-associated vasculitis in Taiwan: A hospital-based study with reference to the population-based National Health Insurance database. J Microbiol Immunol Infect 48:477–482
Liu LJ, Chen M, Yu F, Zhao MH, Wang HY (2008) Evaluation of a new algorithm in classification of systemic vasculitis. Rheumatology (Oxford) 47:708e12
Watts RA, Scott DG (2013) L32. ANCA vasculitis over the world. What do we learn from country differences? Presse Med 42:591–593
Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W et al (2009) EULAR recommendations for the management of primary small and medium vessel vasculitis for the European Vasculitis Study Group. Ann Rheum Dis 68:310–317
Flossmann O, Berden A, de Groot K et al (2011) Long-term patient survival in ANCA- associated vasculitis. Ann Rheum Dis 70:488–494
Jayne D, Rasmussen N, Andrassy K et al (2003) A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 349(1):36–44
EUVAS. CYCLOPS. http://www.vasculitis.org/protocols/CYCLOPS.pdf. Accessed 10 Mar 2008
Haubitz M, Bohnenstengel F, Brunkhorst R, Schwab M, Hofmann U, Busse D (2002) Cyclophosphamide pharmacokinetics and dose requirements in patients with renal insufficiency. Kidney Int 61:1495–1501
Adu D, Pall A, Luqmani RA et al (1997) Controlled trial of pulse versus continuous prednisolone and cyclophosphamide in the treatment of systemic vasculitis. QJM 90:401–409
Haubitz M, Frei U, Rother U et al (1991) Cyclophosphamide pulse therapy in Wegener’s granulomatosis. Nephrol Dial Transplant 6:531–534
Guillevin L, Cordier JF, Lhote F et al (1997) A prospective, multicenter, randomized trial comparing steroids and pulse cyclophosphamide versus steroids and oral cyclophosphamide in the treatment of generalized Wegener’s granulomatosis. Arthritis Rheumatol 40:2187–2198
de Groot K, Harper L, Jayne DR et al (2009) Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 150(10):670–680
Harper L, Morgan MD, Walsh M, Hoglund P, Westman K, Flossmann O et al (2012) Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long- term follow-up. Ann Rheum Dis 71:955–960
de Groot K, Adu D, Savage CO, European Vasculitis Study Group (EUVAS) (2001) The value of pulse cyclophosphamide in ANCA-associated vasculitis: meta-analysis and critical review. Nephrol Dial Transplant 16(10):2018–2027
Yates M, Watts RA, Bajema IM, Cid MC et al (2016) EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 75:1583–1594
Specks U, Fervenza FC, McDonald TJ, Hogan MC (2001) Response of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheumatol 44(12):2836–2840
Keogh KA, Wylam ME, Stone JH, Specks U (2005) Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 52(1):262–268
Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U (2006) Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial. Am J Respir Crit Care Med 173(2):180–187
Jones RB, Ferraro AJ, Chaudhry AN et al (2009) A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 60(7):2156–2168
Stasi R, Stipa E, DelPoeta G, Amadori S, Newland AC, Provan D (2006) Long-term observationofpatientswithanti-neutrophilcytoplasmicantibody-associated vasculitis treated with rituximab. Rheumatology (Oxford) 45(11):1432–1436
Eriksson P (2005) Nine patients with anti-neutrophil cytoplasmic antibody-positive vasculitis successfully treated with rituximab. J Intern Med 257(6):540–548
Smith KG, Jones RB, Burns SM, Jayne DR (2006) Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: remission, relapse, and re-treatment. Arthritis Rheumatol 54(9):2970–2982
Jones RB et al (2010) Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 363:211–220
Stone JH et al (2010) Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363:221–232
Hoffman GS, Leavitt RY, Kerr GS et al (1992) The treatment of Wegener’s granulomatosis with glucocorticoids and methotrexate. Arthritis Rheumatol 35:1322–1329
Sneller MC, Hoffman GS, Talar-Williams C et al (1995) An analysis of forty-two Wegener’s granulomatosis patients treated with methotrexate and prednisone. Arthritis Rheumatol 38:608–613
Stone JH, Tun W, Hellman DB (1999) Treatment of non-life threatening Wegener’s granulomatosis with methotrexate and daily prednisone as the initial therapy of choice. J Rheumatol 26:1134–1139
Langford CA, Talar-Williams C, Sneller MC (2000) Use of methotrexate and glucocorticoids in the treatment of Wegener’s granulomatosis. Long-term renal outcome in patients with glomerulonephritis. Arthritis Rheumatol 43:1836–1840
Stone JH (2005) Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med 352:351–361
De Groot K, Mühler M, Reinhold-Keller E et al (1998) Induction of remission in Wegener’s granulomatosis with low dose methotrexate. J Rheumatol 25:492–495
Metzler C, Hellmich B, Gause A et al (2004) Churg Strauss syndrome—successful induction of remission with methotrexate and unexpected high cardiac and pulmonary relapse ratio during maintenance treatment. Clin Exp Rheumatol 22(6 SUPPL.):S-52–S-61
De Groot K, Rasmussen N, Bacon PA et al (2005) Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 52(8):2461–2469
Faurschou M, Westman K, Rasmussen N et al (2012) Brief report: long-term outcome of a randomized clinical trial comparing methotrexate to cyclophosphamide for remission induction in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 34(10):3472–3477
Jayne DR, Gaskin G, Rasmussen N et al (2007) Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 18(7):2180–2188
Walsh M, Casian A, Flossmann O et al (2013) Long-term follow-up of patients with severe ANCA-associated vasculitis comparing plasma exchange to intravenous methylprednisolone treatment is unclear. Kidney Int 84(2):397–402
Xiao H et al (2002) Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Investig 110:955–963
Gou SJ, Yuan J, Chen M, Yu F, Zhao MH (2013) Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int 83:129–137
Schreiber A et al (2009) C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol 20:289–298
Xiao H, Dairaghi DJ, Powers JP, Ertl LS, Baumgart T, Wang Y et al (2014) C5a receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol 25:225–231
Jayne DR, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, Burst V, Grundmann F, Jadoul M, Szombati I, Tesař V, Segelmark M, Potarca A, Schall TJ, Bekker P, CLEAR Study Group (2017) Randomized trial of C5a receptor inhibitor Avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. doi:10.1681/ASN.2016111179
Walsh M, Merkel PA, Mahr A, Jayne D (2010) Effects of duration of glucocorticoid therapy on relapse rate in antineutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis. Arthritis Care Res 62:1166–1173
Langford CA, Talar-Williams C, Barron KS et al (2003) Use of a cyclophosphamide-induction methotrexate-maintenance regimen for the treatment of Wegener’s granulomatosis: extended follow-up and rate of relapse. Am J Med 114:463–469
Reinhold-Keller E, Fink COE, Herlyn K et al (2002) High rate of renal relapse in 71 patients with Wegener’s granulomatosis under maintenance of remission with low-dose methotrexate. Arthritis Rheum 47:326–332
Pagnoux C et al (2008) Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med 359:2790–2803
Metzler C et al (2007) Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis. Rheumatology (Oxford) 46:1087–1091
Silva F et al (2010) Mycophenolate mofetil for induction and maintenance of remission in microscopic polyangiitis with mild to moderate renal involvement—a prospective, open-label pilot trial. Clin J Am Soc Nephrol 5:445–453
Koukoulaki M, Jayne DR (2006) Mycophenolate mofetil in anti-neutrophil cytoplasm antibodies-associated systemic vasculitis. Nephron Clin Pract 102:c100–c107
Langford CA, Talar-Williams C, Sneller MC (2004) Mycophenolate mofetil for remission maintenance in the treatment of Wegener’s granulomatosis. Arthritis Rheum 51:278–283
Nowack R, Gobel U, Klooker P, Hergesell O, Andrassy K, van der Woude FJ (1999) Mycophenolate mofetil for maintenance therapy of Wegener’s granulomatosis and microscopic polyangiitis: a pilot study in 11 patients with renal involvement. J Am Soc Nephrol 10:1965–1971
Hiemstra TF, Walsh M, Mahr A, Savage CO, de Groot K, Harper L et al (2010) Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA 304:2381–2388
Guillevin L, Pagnoux C, Karras A et al (2014) Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 371(19):1771–1780
Hellmich B, Flossmann O, Gross WL et al (2007) EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti-neutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis 66:605–617
Hogan SL et al (2005) Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med 143:621–631
Miloslavsky EM, Specks U, Merkel PA et al (2015) Outcomes of non-severe relapses in antineutrophil cytoplasmic antibody-associated vasculitis treated with glucocorticoids. Arthritis Rheumatol 67:1629–1636
Faurschou M, Sorensen IJ, Mellemkjaer L, Loft AG, Thomsen BS, Tvede N et al (2008) Malignancies in Wegener’s granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol 35:100–105
Seror R, Pagnoux C, Ruivard M et al (2010) Treatment strategies and outcome of induction-refractory Wegener’s granulomatosis or microscopic polyangiitis: analysis of 32 patients with first-line induction-refractory disease in the WEGENT trial. Ann Rheum Dis 69:2125–2130
Muso E, Ito-Ihara T, Ono T et al (2004) Intravenous immunoglobulin (IVIg) therapy in MPO-ANCA related polyangiitis with rapidly progressive glomerulonephritis in Japan. Jpn J Infect Dis 57:S17–S18
Jayne DRW, Chapel H, Adu D et al (2000) Intravenous immunoglobulin for ANCA-associated systemic vasculitis with persistent disease activity. QJM 93:433–439
Fortin PM, Tejani AM, Bassett K et al (2013) Intravenous immunoglobulin as adjuvant therapy for Wegener’s granulomatosis. Cochrane Database Syst Rev 1:CD007057
Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, Plaisance M, Pusey CD, Jayne DR (2003) Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 41:776–784
Fauci AS, Wolff SM (1973) Wegener’s granulomatosis: studies in eighteen patients and a review of the literature. Medicine 52:535–561
Mukhtyar C, Flossmann O, Hellmich B, Bacon P, Cid M, Cohen-Tervaert JW, Gross WL, Guillevin L, Jayne D, Mahr A, Merkel PA, Raspe H, Scott D, Wittel J, Yazici H, Luqmani RA, European Vasculitis Study Group (EUVAS) (2008) Outcomes from studies of anti- neutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League against Rheumatism systemic vasculitis task force. Ann Rheum Dis 67:1004–1010
Talar-Williams C, Hijazi YM, Walther MM, Linehan WM, Hallahan CW, Lubensky I, Kerr GS, Homan GS, Fauci AS, Sneller MC (1996) Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med 124:477–484
Homan GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, Rottem M, Fauci AS (1992) Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 116:488–498
Lai QY, Ma TT, Li ZY, Chang DY, Zhao MH, Chen M (2014) Predictors for mortality in patients with antineutrophil cytoplasmic autoantibody-associated vasculitis: a study of 398 Chinese patients. J Rheumatol 41:1849–1855
Shi YY, Li ZY, Zhao MH, Chen M (2015) The CD4 lymphocyte count is a better predictor of overall infection than the total lymphocyte count in ANCA-associated vasculitis under a corticosteroid and cyclophosphamide regimen: a retrospective cohort. Medicine (Baltimore) 94:e843
Walsh M, Flossmann O, Berden A et al (2012) Risk factors for relapse of antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Rheumatol 64:542–548
Mukhtyar C, Luqmani R (2007) Disease-specific quality indicators, guidelines, and outcome measures in vasculitis. Clin Exp Rheumatol 25(6 Suppl 47):120–129
Pagnoux C, Hogan SL, Chin H, Jennette JC, Falk RJ, Guillevin L et al (2008) Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small vessel vasculitis: comparison of two independent cohorts. Arthritis Rheumatol 58:2908–2918
Faurschou M, Westman K, Rasmussen N, de Groot K, Flossmann O, Hoglund P et al (2012) Brief report: long-term outcome of a randomized clinical trial comparing methotrexate to cyclophosphamide for remission induction in early systemic antineutrophil cytoplasmic anti- body-associated vasculitis. Arthritis Rheumatol 64:3472–3477
Walsh M, Faurschou M, Berden A, Floss-Mann O, Bajema I, Hoglund P et al (2014) Long-term follow-up of cyclophosphamide compared with azathioprine for initial maintenance therapy in ANCA-associated vasculitis. Clin J Am Soc Nephrol 9:1571–1576
Hogan SL, Nachman PH, Wilkman AS et al (1996) Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 7:23–32
Koldingsnes W, Nossent H (2002) Predictors of survival and organ damage in Wegener’s granulomatosis. Rheumatology 41:572–581
Mohammad AJ, Segelmark M (2014) A population- based study showing better renal prognosis for proteinase 3 antineutrophil cytoplasmic antibody (ANCA)-associated nephritis versus myeloperoxidase ANCA-associated nephritis. J Rheumatol 41:1366–1373
Westman KW, Selga D, Isberg PE et al (2003) High proteinase 3-anti-neutrophil cytoplasmic antibody (ANCA) level measured by the capture enzyme- linked immunosorbent assay method is associated with decreased patient survival in ANCA-associated vasculitis with renal involvement. J Am Soc Nephrol 14:2926–2933
de Joode AA, Sanders JS, Stegeman CA (2013) Renal survival in proteinase 3 and myeloperoxidase ANCA-associated systemic vasculitis. Clin J Am Soc Nephrol 8:1709–1717
Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K et al (2010) Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21:1628–1636
Hilhorst M, Wilde B, van Breda VP, van Paassen P, Cohen-Tervaert JW (2013) Estimating renal survival using the ANCA-associated GN classification. J Am Soc Nephrol 24:1371–1375
Chang DY, Wu LH, Liu G, Chen M, Kallenberg CG, Zhao MH (2012) Re-evaluation of the histopathologic classification of ANCA-associated glomerulonephritis: a study of 121 patients in a single center. Nephrol Dial Transplant 27:2343–2349
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Lei Shi declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Shi, L. Anti-neutrophil cytoplasmic antibody-associated vasculitis: prevalence, treatment, and outcomes. Rheumatol Int 37, 1779–1788 (2017). https://doi.org/10.1007/s00296-017-3818-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3818-y