Abstract
Aim of this study was to evaluate the efficacy and tolerability of intra-articular (IA) clodronate, compared to saline solution, in patients with symptomatic knee osteoarthritis (KOA). In this double-blind phase 3 randomized clinical trial, patients were randomized to receive once weekly IA injection of 2 mg clodronate or placebo for 4 weeks with 12 weeks of follow-up. The primary objective was the sum of spontaneous, on passive movement, and at digital pressing pain relief assessed by visual analogue score (VAS) of 0–100 at 5 weeks after the final injection. Improving in Western Ontario MacMaster (WOMAC) scale, Lequesne index, consumption of acetaminophen, and physician or patient overall judgment were secondary objectives. Study population included 80 patients, 67 women and 13 men aged 66 ± 6 (SD) years. A significant improvement for all efficacy parameters was observed at all-time points in both groups. A significant difference in favor to clodronate in VAS for pain was observed 5 weeks after the last injection (−114.6 vs. −87.2 for clodronate and placebo group, respectively; p < 0.05). The improvements in Lequesne index, global KOA evaluation from both patients and investigators, and the WOMAC pain subscale were significantly greater in the clodronate group. The proportion of patients that did not require acetaminophen was significantly greater in the clodronate group (about 10 vs. 30 % for clodronate and placebo group, respectively; p < 0.05). IA 2 mg clodronate is associated with small and transient symptomatic and functional benefits and it is safe in KOA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a common chronic disorder characterized by joint cartilage wearing as the central feature, associated with concomitant change in the synovial and subchondral bone metabolism [1]. Knee osteoarthritis (KOA) is a major cause of disability affecting about 10 % of people over the age of 55 years [2]. Current treatments aim to alleviate both pain and functional disability by a combination of non-pharmacological and pharmacological approaches, while the efficacy of disease modifying drugs is controversial [3, 4].
The pathogenesis of OA remains a subject of controversy [5]. OA initially presents mostly with destruction of the articular cartilage [6], which is followed by osteophyte formation, cystic degeneration and then sclerosis of the subchondral bone [7]. This “cartilage first” hypothesis has been challenged, and it was hypothesized that also changes in subchondral bone may play an important pathophysiological role [8]. These changes include the subchondral cortical plate and the underlying cancellous bone. The subchondral bone in OA has been shown to have a decreased number and thinning of cancellous trabeculae and localized osteoporosis [9, 10], but also increased bone mineral density (BMD) as assessed by dual X-ray absorptiometry [11, 12], and increased bone turnover, micro-fractures, and bone marrow lesions on magnetic resonance imaging (MRI) [13–15]. A recent systematic review on MRI abnormalities in KOA concluded that knee pain is associated with bone marrow lesions and effusion [16].
Therefore, subchondral bone might be a key target for OA treatment [17]. In particular, subchondral bone turnover and osteoclast functions are likely to be important elements in the pathogenesis of OA [17]. In patients with KOA, fewer MRI subchondral lesions after treatment with drugs that suppress bone turnover, including bisphosphonates, have been observed [18]. Bisphosphonate therapy was found to decrease the incidence and progression of osteophytes in animal models [19–22]. Moreover, a direct chondro-protective effect of bisphosphonates has been postulated [23]. However, bisphosphonate use has been explored in a number of studies on patients with spondylosis and/or KOA with inconclusive results. An improvement in subjective pain was reported in one uncontrolled study of etidronate [24], and the treatment with alendronate was associated with less spinal osteophytes and disk-space narrowing progression than placebo in a post hoc analysis of fracture intervention trial [25]. A treatment with risedronate was associated with a trend of pain relief, improvements in patient global assessment of disease scores and progress in delaying radiographic progression in patients with KOA in a pilot study [26], but these results were not confirmed in a properly powered study [27]. Zoledronic acid has been reported to reduce subchondral bone marrow lesions and knee pain [28]. Recently, data from NIH osteoarthritis initiative strengthens the concept that treatment with bisphosphonates may be associated with beneficial symptomatic effects and perhaps structural benefits in people with OA [29].
A major limitation in the studies assessing the efficacy of bisphosphonates in OA is the need of high doses with potential safety concerns [30], which are largely taken up by bone tissue and therefore, poorly concentrated on subchondral bone. An alternative approach is represented by the administration of intra-articular (IA) bisphosphonate.
In a prior multicenter study, IA clodronate was apparently as efficacious as hyaluronic acid, with a significant progressive improvement in pain and functional outcomes with both drugs extending for at least 2–4 weeks after the last injection [31]. IA hyaluronic acid is recommended by prestigious scientific societies [3, 4, 32], but long-term efficacy is still highly controversial. A recent systematic review and meta-analysis of 89 trials on IA visco-supplementation of the knee concluded that the benefit on pain and function is minimal or absent with a high risk of flares up [33]. These conclusions may represent a relevant draw back for our original study with IA clodronate, persisting the possibility that both IA clodronate and hyaluronic acid had exclusively a relevant “placebo” effect.
The “placebo” effect of saline injection has been described in particular in the first weeks after IA injection [34–37]. For this reason with this study, we wanted to compare in a double-blind design the tolerability and efficacy of IA clodronate versus saline solution after 5-week period after the last injection.
Materials and methods
This is a randomized, single-center, double-blind phase 3 study aimed to evaluate efficacy and tolerability of IA clodronate 2 mg versus placebo in KOA.
Men and non-pregnant women aged between 50 and 75 years, suffering from KOA defined according to American College of Rheumatology criteria [38], radiographically confirmed with a Kellgren–Lawrence scale [39], with X-rays performed in the last 6 months, symptomatic for at least 3 months, were eligible. The Kellgren and Lawrence system is a method of classifying the severity of knee osteoarthritis on a five grade 0–4 scale : grade 0—no radiographic features of OA are present; grade 1—doubtful joint space narrowing (JSN) and possible osteophytic lipping; grade 2—the presence of definite osteophytes and possible JSN on anteroposterior weight-bearing radiograph; grade 3—multiple osteophytes, definite JSN, sclerosis, possible bony deformity; grade 4–large osteophytes, marked JSN, severe sclerosis, and definitely bony deformity. Additional inclusion criteria were baseline spontaneous pain not <40 mm on visual analogue score (VAS) scale of 100 mm and reduced joint mobility greater than grade 2 on a 4-point scale (arbitrarily assessed by the investigator using a scale from 1 complete to 4 totally compromised).
Patients were excluded if Kellgren–Lawrence score was 1, if they had osteoarthritis secondary to other joint diseases, if they were receiving anticoagulant therapy or if they had received systemic steroidal drugs, chondro-protective agents, or intra-articular drugs in the 30 days preceding the study. Patients were also excluded if they had been on bisphosphonates in the previous year or if they had undergone knee surgery in the past (excluding arthroscopy) or washing, diagnostic or surgical arthroscopy in the previous 180 days. Patients with known hypersensitivity to study drugs or similar drugs, including acetaminophen or suffering from other diseases that require use of anticoagulants, were excluded. Individuals coming from outside our province and then unlikely to be able to attend all the visits and those with a history or suspected alcoholism or drug addiction, patients with poor compliance or unable to provide informed consent were also excluded.
The primary objective was the sum of spontaneous, on passive movement, and at digital pressure pain (4 kg/m2) [40] relief of the index knee, all evaluated by VAS of 0–100 mm reported by patient at clinical evaluation, at week 8 (5 weeks after the last injection) in order to minimize the expected placebo effect of the injections. We use the sum of different aspects of pain to assure a more global assessment of joint pain.
Improving in Western Ontario MacMaster (WOMAC) scale, Lequesne index, consumption of acetaminophen, and physician or patient overall judgment were secondary objectives.
The WOMAC Index [41] is a three-dimensional, self-administered measure specific to OA, looking at pain, stiffness and physical function in OA of hip or knee. There are 24 questions, five on pain, two on stiffness and 17 on physical function.
The Lequesne-algo-functional index for knee [42], looking at pain, maximum distance walked and activities of daily living. Questions are asked and points allocated according to the patient’s reply on a 3-point Likert scale (0–2).
The consumption of acetaminophen tablets was reported at each visit in total number/week (counting the returned pills that were provided weekly by the investigators); for simplification, we later divided patients in requiring or not the rescue drug.
Outpatients who attended our clinic complaining of knee pain due to KOA, and meeting the inclusion criteria and without exclusion ones, were proposed to take part to the study. At baseline, eligible patients without any stratification were randomized at a 1:1 ratio for blocks of 4, using a randomization list generated with computer software to receive either once weekly IA injection of 2 mg clodronate or placebo for 4 weeks with further 6 fortnightly follow-up visits. The injected volume was 1 ml. The vials containing clodronate or placebo were prepared at the Abiogen Pharma (Pisa SpA, Italy) laboratories, where stability and the final concentration of clodronate were periodically tested. The treatment codes were kept by Abiogen Pharma, and they were disclosed after the registration of the locked database at the competent office of Ministry of Health. The placebo and active vials were totally indistinguishable. If both knees met the inclusion criteria, only the worst received the treatment to avoid the bias due to the certain influence of a worse knee taking the better as study target. Four days before the first injection patients were asked to discontinue any nonsteroidal anti-inflammatory drugs, and physical therapy and rehabilitation procedures were not permitted over the entire follow-up. During the study (i.e., the 4 days preceding the first injection and afterward), patients were allowed pain relief with acetaminophen as necessary.
The enrolled subject could discontinue the study at any time on his own initiative, without prejudice to the possibility to make use of other therapies. The date and reasons for discontinuation were reported in the appropriate case report form, indicating one of the following reasons: (1) adverse event; (2) serious adverse event; (3) therapeutic effect is not satisfactory; (4) the appearance of the exclusion criteria; (5) uncooperative patient; (6) withdrawal of consent; and (7) administrative problems.
The safety population comprised all patients receiving at least one injection of the study medication. Adverse events observed by the investigators or reported by the patients spontaneously or following a non-leading question were coded using the medical dictionary for regulatory activities. Particular attention was paid to local painful reactions at the injection site, postinjection reactions (e.g., effusions), and acute pseudoseptic arthritis.
The clinical study was conducted in accordance with the ethics principles of the Declaration of Helsinki and was approved by the local ethics committees. Each enrolled patient signed the informed consent to participate in the study.
The trial is registered in the National Observatory on clinical investigation of medicinal: EUDRACT 2009-012956-26.
Sample size and statistical analysis
The sample size of 40 patients per treatment group, assuming a retention rate of 85 %, has 90 % power, with a 0.05 two-sided significance level, to detect a difference between treatments of 20 mm in the cumulative VAS value, assuming that the common standard deviation is 24 mm according with our previous experience [31]. Demographic and main clinical characteristics of the treatment and placebo groups were summarized by descriptive statistics. Treatment groups have been compared at each visit carrying out a mixed model for repeated measures, using the change from baseline as dependent variable, treatment, visit, and the treatment by visit interaction as fixed factors, baseline as covariate, and patient as random effect [43]. Differences between treatments have been reported as least-square mean estimates. Intention-to-treat (ITT) analysis, conducted using data from all randomized patients who received at least one dose of study drug and the last value carried forward procedure, was included. Statistical significance was determined using a p value ≤0.05 (two tailed).
All analyses were performed with SPSS, version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
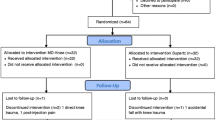
Eighty patients, 67 women and 13 men aged 66 ± 6 (SD) years, were recruited from the outpatients clinic with the service for intra-articular injections. Nine screened patients were excluded because not fulfilling the inclusion or exclusion criteria. This service is fully reimbursed by the regional health service, and this might explain why all patients offered to participate in the trial gave their consent. Two patients drop out: one on clodronate after the second injection for family problems and the second (on placebo) during 12-week follow-up for an adverse event unrelated to treatment (Fig. 1). There were no additional missing values. The main clinical characteristics of the study population are listed in Table 1. Only 12 patients had been on bisphosphonates and all of them discontinued the treatment at least 18 months before recruitment.
A significant improvement for all efficacy parameters was observed at all-time points in both groups. The difference between the two groups in favor to clodronate for the primary end point of the sum of the three VAS pain scores at 8-week follow-up (5 weeks after the last injection) was 27.4 mm thus fulfilling the assumption made for the esteem of the statistical power. The difference between the two groups completely disappeared at 12-week follow-up, 9 weeks after the last injection (Table 2). The mean changes for the three individual VAS pain scores are also listed in Table 2.
The improvements in global KOA evaluation from both patient and investigator (Fig. 2), Lequesne index and WOMAC pain subscale, were significantly greater in clodronate treated patients at 8-week follow-up (5 weeks after the final injection) (Fig. 3), but the changes in global WOMAC score were not significantly different.
Approximately half of the patients required acetaminophen treatment during the 4 days preceding the first injection. This proportion, together with the mean number of tablets (data not shown), remained stable in the placebo group while they progressively declined in the active group, particularly during the posttreatment follow-up (Fig. 4).
The results of these analyses and the related statistical significances did not change when modified ITT analyses conducted using data from all randomized patients who received at least one dose of study drug and the last value carried forward procedure were adopted.
The mean reported pain at the site of IA injection was significantly higher in clodronate group (Fig. 5), but local pain never led to treatment discontinuation. No other side effects related to the treatment were reported by all patients, included the 2 who abandoned the trial in advance. Side effects, considered unrelated to treatment, were reported by 15 patients in the control group (flu-like syndromes, diarrhea, abdominal pain at the time of the infusions and flu-like syndromes, abdominal pain, low back pain, and headache during follow-up) and by 10 in the clodronate group (flu-like syndromes, abdominal pain at the time of infusions and low back pain, diarrhea, neck stiffness and in one case severe cough that led to hospitalization for suspected lung cancer during follow-up).
Discussion
In this study, we have shown that the knee IA injection of 2 mg clodronate is associated with significantly greater benefits than the infusion of saline solution in a double-blind randomized clinical trial. A striking finding of our study is the impressive 35–45 % beneficial effect observed in both groups for the primary efficacy parameters throughout the study, with the two groups diverging only after the end of the fourth injection. A similar effect of saline injection has been reported by others [34–37], and it was attributed to the injection per se, aspiration of synovial fluid, “washing” effect of the saline solution, education of the patients, or placebo effect.
In our previous dose-finding study [31] with IA clodronate, we found a trend of dose–response relationship up to 2 mg clodronate and no significant differences between clodronate and hyaluronic acid. In that study, a placebo group was not included and the interpretation of the results could not be conclusive regarding the pharmacological efficacy of clodronate since an equivalent placebo effect could be attributed to both treatments. The efficacy of hyaluronic acid in KOA is still debated. The analysis from meta-analyses yielded conflicting results: Some suggest small to strong effects [44–47], but others failed to show an effect superior to placebo [48, 49] attributing the results of other analysis to the presence of publication bias that may have been responsible of an overestimation of the observed small effects [50]. The recent systematic review and meta-analysis [33] concluded that the benefit of visco-supplementation on pain and function is minimal or nonexistent.
The results of the present trial do indicate an additional small benefit of clodronate over saline solution. Most indices are slightly but significantly better and tend to affect both pain and functionality as expressed by WOMAC score and Lequesne index. The differences are limited to the first month after the last injection but tend to disappear thereafter.
Bisphosphonates may work through a variety of mechanisms, such as the suppression of local bone turnover or via inhibition of local pro-inflammatory cytokines or nociceptors. Defective subchondral bone metabolism in osteoarthritis could modify chondrocytes in cartilage via local factors released during subchondral bone remodeling. A direct stimulation of collagen and proteoglycan synthesis [19] and MMP inhibition and downregulation [23] have been also reported. TGF-β1 activated during osteoclastic bone resorption in subchondral bone seem to initiate the pathological changes of osteoarthritis [51] and inhibition of this process by bisphosphonates could be a potential therapeutic approach to treating this disease. Bisphosphonates can exert a mild immune-modulating activity via inhibition of pro-inflammatory cytokines [52] or white blood cells subtypes [53, 54]. Clodronate containing liposomes have been tested in a model of rheumatoid arthritis with evidence of synovial macrophage depletion [55]. Moreover, a recent study using IA zoledronic acid in a rat OA model reported a suppressive effect on synovial inflammation [56]. Increased subchondral bone turnover may contribute to pain in OA and targeting of osteoclasts may reduce pain associated with OA [57]. Bisphosphonates also have effects on cells other than osteoclasts, such as macrophages and microglia, including those that produce the pro-nociceptive β-NGF, which may explain their effect in algo-dystrophic syndromes [58]. Bisphosphonates modulate the release of protons from activated osteoclasts, and this may reduce the acidic sensitization of bone nociceptors [59], and a direct effect on the synovial glial cells has also been reported [60].
From secondary analysis of patients treated with bisphosphonates or strontium ranelate for osteoporosis, a trend of reduction of joint space loss has been reported [25, 26, 29, 61], but this was not confirmed in a larger ad hoc study with risedronate [27]. Some have speculated that this clinical trial was underpowered, but it is unlikely that new properly designed studies will be undertaken in the future, taking into consideration the newly arisen safety issues of long-term bisphosphonate treatment, attributed to the long skeletal retention and over-suppression of bone turnover.
Even though a direct evidence that the bisphosphonate may diffuse from cartilage to bone is missing, it is likely that the approach that we adopted with the IA administration of clodronate generates local high concentration of the drug on joint tissues and subchondral bone, thus circumventing the problem of systemic administration of bisphosphonates being largely taken up by the entire skeleton [62]. A direct effect of clodronate on cartilage cannot be ruled out since clodronate was shown to bind fibroblast growth factor 2 and polysulphated or polyphosphated derivatives [63] and to modify cartilage oligomeric matrix protein [64].
Whatever the mechanism of action, the bisphosphonate injected IA and then likely bound to sub-chondral bone is expected to exert its action for quite long time. The reason for the persistent effect of bisphosphonates is still very controversial with some attributing this to the bisphosphonates absorbed on the resting surfaces and others to re-circulation of the bisphosphonates buried in the bone tissue. In both cases, the persistence of the effect is related with the affinity for the mineralized tissue. Clodronate is by far the bisphosphonate with the lowest affinity [65] and its activity on bone turnover even after very high doses is expected to disappear within few days after discontinuation. In our previous study [31], even though insignificant, the best results were obtained with Clodronate 1 mg twice a week for 2 weeks rather than 2 mg once a week.
The main limitation of our present study is the limited benefit achieved with clodronate versus placebo and that makes our results still inconclusive regarding the possibility to develop a new therapeutic strategy for KOA. Other limitations are the lack of useful informations on structural modification effects, such as radiological, laboratory, or synovial histological data, but the study was aimed to clinical outcomes. The significant difference between the two treatments in the global pain scores persisted for a month after the last injection. The difference observed versus saline injection was somewhat lower than that assumed in our power calculation and this may raise concerns regarding the statistical power of our study. The substantial effects observed after each weekly injection of saline solution might have obscured the long-term superior efficacy of clodronate. In future studies, the possibility to test monthly injections of both saline solution and clodronate together with greater doses of clodronate are enviable, taking into consideration the good local tolerability of 2 mg. The real value of this study is to provide some additional support to the hypothesis that subchondral bone turnover plays a role in the development and maintenance of KOA.
In conclusion, we have seen that IA 2 mg clodronate is safe and is associated with small symptomatic and functional benefits in KOA patients in addition to those seen with saline solution, with benefits appreciable for a few weeks after the treatment course.
References
Herrero-Beaumont G, Roman-Blas JA, Castañeda S, Jimenez SA (2009) Primary osteoarthritis no longer primary: three subsets with distinct etiological, clinical, and therapeutic characteristics. Semin Arthritis Rheum 39:71–80
Peat G, McCarney R, Croft P (2001) Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 60:91–97
Hochberg MC, Altman RD, April KT et al (2012) American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 64:465–474
Zhang W, Nuki G, Moskowitz RW et al (2010) OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 18:476–499
Herrero-Beaumont G, Roman-Blas JA, Largo R, Berenbaum F, Castaneda S (2011) Bone mineral density and joint cartilage: four clinical settings of a complex relationship in osteoarthritis. Ann Rheum Dis 70:1523–1525
Bush PG, Hall AC (2003) The volume and morphology of chondrocytes within non-degenerate and degenerate human articular cartilage. Osteoarthritis Cartilage 11:242–251
Goldring MB, Goldring SR (2010) Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann NY Acad Sci 1192:230–237
Zhang ZM, Li ZC, Jiang LS, Jiang SD, Dai LY (2010) Micro-CT and mechanical evaluation of subchondral trabecular bone structure between postmenopausal women with osteoarthritis and osteoporosis. Osteoporos Int 21:1383–1390
Karvonen RL, Miller PR, Nelson DA, Granda JL, Fernandez-Madrid F (1998) Periarticular osteoporosis in osteoarthritis of the knee. J Rheumatol 25:2187–2194
Messent EA, Ward RJ, Tonkin CJ, Buckland-Wright C (2005) Cancellous bone differences between knees with early, definite and advanced joint space loss: a comparative quantitative macroradiographic study. Osteoarthritis Cartilage 13:39–47
Davies-Tuck ML, Wluka AE, Wang Y et al (2009) The natural history of bone marrow lesions in community-based adults with no clinical knee osteoarthritis. Ann Rheum Dis 68:904–908
Dore D, Quinn S, Ding C et al (2010) Subchondral bone and cartilage damage: a prospective study in older adults. Arthritis Rheum 62:1967–1973
Bergman AG, Willen HK, Lindstrand AL, Pettersson HAT (1994) Osteoarthritis of the knee: correlation of subchondral MR signal abnormalities with histopathologic and radiographic features. Skeletal Radiol 23:445–448
Zanetti M, Bruder E, Romero J, Hodler J (2000) Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology 215:835–840
Hernandez-Molina G, Guermazi A, Niu J et al (2008) Central bone marrow lesions in symptomatic knee osteoarthritis and their relationship to anterior cruciate ligament tears and cartilage loss. Arthritis Rheum 58:130–136
Yusuf E, Kortekaas MC, Watt I, Huizinga TW, Kloppenburg M (2011) Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis 70:60–67
Karsdal MA, BayJensen AC, Lories RJ et al (2014) The coupling of bone and cartilage turnover in osteoarthritis: opportunities for bone antiresorptives and anabolics as potential treatments ? Ann Rheum Dis 73:336–348
Carbone LD, Nevitt MC, Wildy K et al (2004) The relationship of antiresorptive drug use to structural findings and symptoms of knee osteoarthritis. Arthritis Rheum 50:3516–3525
Muehleman C, Green J, Williams JM et al (2002) The effect of bone remodeling inhibition by zoledronic acid in an animal model of cartilage matrix damage. Osteoarthritis Cartilage 10:226–233
Hayami T, Pickarski M, Wesolowski GA et al (2004) The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum 50:1193–1206
Funck-Brentano T, Lin H, Hay E et al (2012) Targeting bone alleviates osteoarthritis in osteopenic mice and modulates cartilage catabolism. PLoS One 7:e33543
Panahifar A, Maksymowych WP, Doschak MR (2012) Potential mechanism of alendronate inhibition of osteophyte formation in the rat model of post-traumatic osteoarthritis: evaluation of elemental strontium as a molecular tracer of bone formation. Osteoarthritis Cartilage 20:694–702
Teronen O, Heikkila P, Konttinen YT et al (1999) MMP inhibition and downregulation by bisphosphonates. Ann NY Acad Sci 878:453–465
Fujita T, Fujii Y, Okada SF, Miyauchi A, Takagi Y (2001) Analgesic effect of etidronate on degenerative joint disease. J Bone Miner Metab 19:251–256
Neogi T, Nevitt MC, Ensrud KE, Bauer D, Felson DT (2008) The effect of alendronate on progression of spinal osteophytes and disc space narrowing. Ann Rheum Dis 67:1427–1430
Spector TD, Conaghan PG, Buckland-Wright JC et al (2005) Effect of risedronate on joint structure and symptoms of knee osteoarthritis: results of the BRISK randomized, controlled trial. Arthritis Res Ther 7:R625–R633
Bingham CO, Buckland-Wright JC, Garnero P et al (2006) Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year Multinational Knee Osteoarthritis Structural Arthritis Study. Arthritis Rheum 54:3494–3507
Laslett LL, Dore DA, Quinn SJ et al (2012) Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann Rheum Dis 71:1322–1328
Laslett LL, Kingsbury SR, Hensor EM, Bowes MA, Conaghan PG (2014) Effect of bisphosphonate use in patients with symptomatic and radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. Ann Rheum Dis 73:824–830
Abrahamsen B (2010) Adverse effects of bisphosphonates. Calcif Tissue Int 86:421–435
Rossini M, Viapiana O, Ramonda R et al (2009) Intra-articular clodronate for the treatment of knee osteoarthritis: dose ranging study vs hyaluronic acid. Rheumatology 48:773–778
Jordan KM, Arden NK, Doherty M et al (2003) EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 62:1145–1155
Rutijes AWS, Juni P, da Costa BR et al (2012) Viscosupplementation for Ostearthritis of the knee: a systematic review and meta-analysis. Ann Int Med 157:180–191
Bellamy N, Campbell J, Robinson V et al (2006) Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2:CD005328
Jørgensen A, Stengaard-Pedersen K, Simonsen O et al (2010) Intra-articular hyaluronan is without clinical effect in knee osteoarthritis: a multicentre, randomised, placebo-controlled, double-blind study of 337 patients followed for 1 year. Ann Rheum Dis 69:1097–1102
Lundsgaard C, Dufour N, Fallentin E, Winkel P, Gluud C (2008) Intra-articular sodium hyaluronate 2 mL versus physiological saline 20 mL versus physiological saline 2 mL for painful knee osteoarthritis: a randomized clinical trial. Scand J Rheumatol 37:142–150
Zhang W, Robertson J, Jones AC, Dieppe PA, Doherty M (2008) The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis 67:1716–1723
Altman R, Asch E, Bloch D et al (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29:1039–1049
Kellgren MJ (1963) The epidemiology of chronic rheumatism: atlas of standard radiographs, 2nd edn. Blackwell Scientific, Oxford, UK
Wolfe F, Smythe HA, Yunus MB et al (1990) The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33:160–172
Bellamy N, Buchanan WW, Goldsmith CH et al (1998) Validation Study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Lequesne M, Méry C, Samson M et al (1987) Indexes of severity for osteoarthritis of the hip and knee. Scand J Rheumatol 65(Suppl):85–89
Ranstam J, Turkiewicz A, Boonen S et al (2012) Alternative analyses for handling incomplete follow-up in the intention-to-treat analysis: the randomized controlled trial of balloon kyphoplasty versus non-surgical care for vertebral compression fracture (FREE). BMC Med Res Methodol 24(12):35
Wang CT, Lin J, Chang CJ, Lin YT, Hou SM (2004) Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. A meta-analysis of randomized controlled trials. J Bone Joint Surg Am 86-A:538–545
Bellamy N, Campbell J, Robinson V et al (2006) Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2:CD005321
Reichenbach S, Blank S, Rutjes AW et al (2007) Hylan versus hyaluronic acid for osteoarthritis of the knee: a systematic review and meta-analysis. Arthritis Rheum 57:1410–1418
Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE (2011) Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis—meta-analysis. Osteoarthritis Cartilage 19:611–619
Arrich J, Piribauer F, Mad P et al (2005) Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ 172:1039–1043
Medina JM, Thomas A, Denegar CR (2006) Knee osteoarthritis: should your patient opt for hyaluronic acid injection? J Fam Pract 55:669–675
Lo GH, LaValley M, McAlindon T, Felson DT (2003) Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA 290:3115–3121
Zhen G, Wen C, Jia X et al (2013) Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nature Med 19(6):704–712. doi:10.1038/nm.3143
Frith JC, Mönkkönen J, Auriola S, Mönkkönen H, Rogers MJ (2001) The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum 44:2201–2210
Rossini M, Adami S, Viapiana O et al (2012) Long-term effects of amino-bisphosphonates on circulating γδ T cells. Calcif Tissue Int 91:395–399
Rossini M, Adami S, Viapiana O et al (2013) Acute phase response after zoledronic acid is associated with long-term effects on white blood cells. Calcif Tissue Int 93:249–252
Barrera P, Blom A, Van Lent PL et al (2000) TI: synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum 43:1951–1959
Cinar BM, Ozkoc G, Bolat F et al (2014) Intra articular zoledronic acid in rat osteoarthritis model: significant reduced synovitis may indicate chondroprotective effect. Knee Surg Sports Traumatol Arthrosc [Epub ahead of print]
Sagar DR, Ashraf S, Xu L et al (2013) Osteoprotegerin reduces the development of pain behaviour and joint pathology in a model of osteoarthritis. Ann Rheum Dis [Epub ahead of print]
Varenna M, Adami S, Rossini M et al (2013) Treatment of complex regional pain syndrome type I with neridronate: a randomized, double-blind, placebo-controlled study. Rheumatology 52:534–542
Yanow J, Pappagallo M, Pillai L (2008) Complex regional pain syndrome (CRPS/RSD) and neuropathic pain: role of intravenous bisphosphonates as analgesics. ScientificWorldJournal 8:229–236
Dehghani F, Conrad A, Kohl A, Korf HW, Hailer NP (2004) Clodronate inhibits the secretion of proinflammatory cytokines and NO by isolated microglial cells and reduces the number of proliferating glial cells in excitotoxically injured organotypic hippocampa slice cultures. Exp Neurol 189:241–251
Reginster JY, Badurski J, Bellamy N et al (2013) Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: results of a double-blind, randomised placebo-controlled trial. Ann Rheum Dis 72:179–186
Fleisch H (2007) Introduction to bisphosphonates, History and functional mechanisms. Orthopade 36:103–109
Rose K, Finger IE, Ferenz KB (2011) Interaction of clodronate with fibroblast growth factor 2 reduces FGF2-activity in endothelial cells. Biomed Pharmacother 65:46–51
Gomez-Barrena E, Lindroos L, Ceponis A et al (2006) Cartilage oligomeric matrix protein (COMP) is modified by intra-articular liposomal clodronate in an experimental model of arthritis. Clin Exp Rheumatol 24:622–628
Nancollas GH, Tang R, Phipps RJ et al (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38:617–627
Acknowledgments
Abiogen Pharma provided, free of charge, all medications. The study was independently designed by three of the investigators (SA-MR-DG) and Abiogen Pharma provided administrative assistance in order to have the protocol written in accordance with the current national and international requirements.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration number: EUDRACT 2009-012956-26.
Rights and permissions
About this article
Cite this article
Rossini, M., Adami, S., Fracassi, E. et al. Effects of intra-articular clodronate in the treatment of knee osteoarthritis: results of a double-blind, randomized placebo-controlled trial. Rheumatol Int 35, 255–263 (2015). https://doi.org/10.1007/s00296-014-3100-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-014-3100-5