Abstract
To explore the possibility of step-down method and low dose of etanercept for long-term stable remission of patients with juvenile idiopathic arthritis (JIA). Patients with JIA were enrolled into this study between February 2008 and March 2010 and then followed up for 2 years. The inclusion criteria were clinical remission and use of etanercept for therapy. On the first year of the study, the dose of etanercept was kept at 0.4 mg/kg per week, the half dose of what those patients had been used. On the second year, the dose of etanercept was further lowered to 0.4 mg/kg per month. DMARDs were allowed in this study. MR images were performed to observe joint changes. The primary end point was disease flare defined according to clinical and/or radiological data. The flare rate curve was analyzed by Kaplan–Meier, and logistic regression model was used to find factors associating with disease flare. MRI was performed to prove no active changes or progressions of bone erosions on joints. Thirty-one patients were enrolled in this study. There were 4 patients experiencing disease flare during the first 12th month. During the second year, disease flare was not occurred. Thus, the cumulative flare rate was 12.9 % on 12th month and then unchanged on the second year. Logistic regression model indicates there are no differences in sex, age of disease initiation, disease duration, subtypes, DMARDs, HLA-B27, months of etanercept duration and scores on MRI between patients with remission and those experiencing flares. At the end of the study, MRI found no progressions of joints to the patients keeping stable remission. Step-down method can be used for etanercept tapering. Long-term remission and low flare rate can be got by this method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease in children. It is a diagnosis of exclusion and defined as inflammatory arthritis with unknown etiology occurring in those under 16 years of age, present for greater than 6 weeks. JIA was grouped on the basis of clinical and laboratory features into 7 subsets by International League Against Rheumatism (ILAR) [1]. JIA often persist into adulthood and can lead to severe long-term disability and is associated with life-threatening complications [2, 3].
Recent major advances in treatment, especially the advent of TNF-alpha antagonists, have been greatly improved outcomes for children with JIA [4–14]. Inactive disease and remission are now regarded as the objective of therapy, and biologics have been playing an important role to reach this objective. Etanercept (recombinant human tumor necrosis factor-α receptor II: IgG Fc fusion protein for injection, rhTNFR:Fc) is one kind of TNF-alpha antagonists which is subcutaneously injected twice a week at a dose of 0.4 mg/kg. Sufficient advice exists for starting etanercept therapy once the diagnosis is established. Although etanercept can induce disease remission to many children with JIA, it still has to be used for years, even though patients may respond to initial therapies [5, 6, 14–17].
Physicians have to balance the risk of doing too little (i.e., withdrawing medication and provoking flares) versus the risk of doing too much (i.e., continuing medication despite a stable remission and thereby accepting the risk of adverse effects). Actually, there was occasional information about patients with rheumatoid arthritis (RA) or JIA who stopped using biologics after remission [17–19]. Inactive disease was one of the four main reasons for etanercept withdrawal [17]. However, in a letter to Ann Rheum Dis., the flare rate after etanercept withdrawal was described to be as high as 47 % [20], and another article reported a flare rate of 100 % (11/11) in JIA patients after etanercept withdrawal [21]. Those results were enough to discourage any attempting to stop etanercept. But those two reports both had small samples and stopped etanercept once for all. Thus, we wanted to explore whether step-down method and long-term use of low dose of etanercept could keep stable remission of majority JIA patients.
Methods
Patients
Patients with enthesitis-related subtype (ERA), polyarthritis subtype (pJIA) or extended oligoarthritis subtype of JIA (extended oJIA) were enrolled into this study between February 2008 and March 2010. The inclusion criteria were clinical remission and continuous use of etanercept. Clinical remission was defined as clinically documented ID for at least 6 months [22]. ID is defined as no joints with active arthritis; no fever, rash, serositis, splenomegaly or generalized lymphadenopathy attributable to arthritis; no active uveitis; no elevation in erythrocyte sedimentation rate (ESR), C-reactive protein level or both attributable to arthritis; and disease activity by physician’s global assessment on a visual analog scale ranging from 0 to 10 cm [22]. Stable remission was defined as the absence of any flare, that is, continuous remission after dose tapering.
Patients were excluded if they had received glucocorticoids, biologics other than etanercept prior to inclusion. DMARDs in this study were methotrexate (MTX 10–15 mg/m2 per week), sulfasalazine (SSZ 30–40 mg/kg/d) and leflunomide (LEF 10–15 mg/d).
Independent ethics committees at RenJi hospital, Jiaotong University, Shanghai, approved the study protocol.
Study design
The study was designed as a prospective, open-label, observational medication-withdrawal clinical trial. On the first year, the dose of etanercept was 0.4 mg/kg per week, the half dose of what those patients had been used at. On the second year, the dose of etanercept was further lowered to 0.4 mg/kg per month.
Patients were evaluated clinically in follow-up examinations every 3 months for 2 years. Disease flare was defined as occurrence of any sign of active arthritis and/or active systemic symptoms, that is, when any of the criteria for ID was no longer met. Progressions on MRI, that is, increasing in bone erosions, bone edema, and synovial effusion, were also viewed as evidence for poor therapy effects. The number of patients who had disease flare and/or progressions on MRI was recorded.
Magnetic resonance imaging
MR images were performed at inclusion, then once per year or when disease flared. MR images of sacroiliac joints, hip joints and knees were scanned in a 1.0T Gyroscan NT whole-body MRI system (Philips Medical Systems). The sequences obtained were coronal and transverse T1-weighted TSE, T2-weighted TSE, T2-TSE SPAIR.
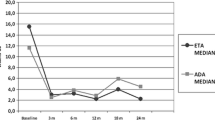
The numbers of patients with bone erosions, bone edema and synovial effusion on MRI were recorded. Synovial effusion and bone edema were evaluated separately, and one point was given for one abnormality. On bone erosion, the subsequent images were compared to the first one and the severity was scored 0–2; 0 meant no bone erosion, and if more than 50 % of articular surface was affected, it was scored as 2. The scoring system was partially based on previous literature [23, 24]. Two experienced radiologist readers were assigned to evaluate every image independently.
Data collection
Those patients were followed up every 3 months for 2 years, and data were collected for each patient from each visit. Data collected at the first clinical visit included sex, age, disease onset and duration, subtype, imaging results, laboratory test results and medication use for induction therapy. At each subsequent clinical visit, data were collected for each of the ID criterion as published by Wallace et al. [21]. An abnormal ESR was defined as 20 mm/h. Imaging data were obtained from imaging reports and original images.
Statistical method
The primary outcome of the study was the flare rate. Demographic and baseline characteristics were summarized using descriptive statistics. Data are given as mean or median as well as range. Flare rate was analyzed by Kaplan–Meier curve. Factors with a P value <0.05 between patients with flares and without flares were considered to be associated with disease flare by way of logistic regression model. Software of SAS 6.12 was used to analysis in this study.
Results
Thirty-one patients with JIA in remission were enrolled in this study between February 2008 and March 2010. These patients were then followed up for 2 years after enrollment. On the first year, these patients were given etanercept at 0.4 mg/kg per week and there were 4 patients experiencing disease flare and quitting. On the second year, 21 patients among the rest 27 patients were administrated etanercept at the dose of 0.4 mg/kg per month and the other 6 patients were still given the same dose as the first year due to their parents’ willingness. Disease flare was not occurred on the second year. Thus, the cumulative flare rate was 12.9 % on 12th month and then unchanged until the end of the study. The study flow can be found in Fig. 1.
Among three subtypes, ERA was in the majority of patients. Among subtypes, there are no differences in age, disease duration, etanercept duration, erythrocyte sedimentation rate and C-reactive protein at inclusion. HLA-B27 positivity is only seen in ERA with a rate of 68.2 %. Due to inactivity status of disease, MRI showed no signs of bone edema and synovial effusion at inclusion, while bone erosions definitely occurred to 8 patients. The characteristics of those patients are shown in Table 1.
The data of 4 patients with disease flare were summarized in Table 2. A cumulative flare rate was 12.9 % (4/31) at 12th month and then unchanged until 24th month (Fig. 2). Factors associating with disease flare were analyzed by way of logistic regression analysis. However, the result indicated there were no differences in sex, age of disease initiation, disease duration, subtypes, DMARDs, HLA-B27, bone erosions on MRI and months of etanercept use between patients keeping stable remission and those experiencing flares.
At inclusion, bone edema and synovial effusion were not found on MRI and bone erosions occurred to 8 patients on MRI. At the end of the first year, bone edemas were present to 4 patients who experienced disease flare. At the end of the second year, there are no progressions of bone erosions on MRI to the patients keeping stable remission (Table 3).
Discussion
Many chronic inflammatory diseases regularly take a relapsing course. Treatment over years is often necessary, even though patients may respond to initial therapies. Sufficient advice exists for starting therapies once the diagnosis is established or for escalating treatment if the therapy is not sufficient to induce remission. However, in clinical practice, physicians are frequently faced with the question of what to do with patients who are clinically well after induction of remission. Physicians have to decide whether continuation of drug therapy is meaningful. There really exited phenomenon that some patients stopped using etanercept due to inactive disease. But the flare rate or disease course after etanercept stopping in JIA had been rarely reported before. A brief letter to Ann Rheum Dis. described a flare rate of 47 % (9/19) in JIA patients after stopping etanercept [20]. Another article reported a flare rate of 100 % (11/11) in JIA patients after etanercept withdrawal [21].
But the common point of those two reports was that etanercept was stopped without process of tapering. Evidences from the use of glucocorticoids in arthritis emphasize step-down method for glucocorticoids tapering. So, we brought this idea into etanercept therapy, and the whole process of tapering was divided into two steps of dose, that is, 0.4 mg/kg per week and 0.4 mg/kg per month. Besides clinical assessment of disease conditions, MR images were also performed to prove no disease activity or progression during the study.
From our study, a cumulative flare rate of 12.9 % (4/31) was observed among first 12th month. Logistic regression analysis was used to find out factors associating with disease flare. But none of factors analyzed had significant effect on disease flare. On the second year, major patients (21/27) were given etanercept once a month which is very close to full withdrawal of etanercept. And the rest 6 patients were given the same dose as the first year due to parents’ willingness. During the second year, no patient was found to have disease flare. Our result was superior to the previous report [20, 21]; especially on the second year, the dose of etanercept was very low which was close to full withdrawal of etanercept.
The achievement of low flare rate may be due to strictly sticking to the ID criteria. Only those patients who had fully fitted in with ID criteria and kept that status for more than half year were allowed to taper etanercept both in the two steps. MR images provided another safe belt to prove no inflammation on joints.
Foell and his coworkers conducted a clinical trial of methotrexate withdrawal which found out a flare rate of 16.8 % when MTX was used as only drug for maintenance therapy on remitted JIA patients [25]. Although direct comparison cannot be made, our flare rate seems similar to Foell’s work, which may suggest that low dose of etanercept could be used for long-term maintenance therapy of JIA.
Our study firstly provided detailed information about process of etanercept tapering which lasted for 2 years. Our results of low flare rate proved that step-down method was effective in etanercept tapering and long-term stable remission to major patients could be got with the help of DMARDs. Our results also suggested possibility of etanercept withdrawal after JIA patients had achieved clinical remission.
References
Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J, Prieur AM, Suarez-Almazor ME (2004) International league of associations for rheumatology. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31(2):390–392
Prakken B, Albani S, Martini A (2011) Juvenile idiopathic arthritis. Lancet 377(9783):2138–2149
Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, Ilowite NT, Kimura Y, Laxer RM, Lovell DJ, Martini A, Rabinovich CE, Ruperto N (2011) 2011 American college of rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthr Care Res (Hoboken) 63(4):465–482
Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, Stein LD, Gedalia A, Ilowite NT, Wallace CA, Whitmore J, Finck BK (2000) Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric rheumatology collaborative study group. N Engl J Med 342(11):763–769
Lovell DJ, Reiff A, Jones OY, Schneider R, Nocton J, Stein LD, Gedalia A, Ilowite NT, Wallace CA, Whitmore JB, White B (2006) Pediatric rheumatology collaborative study group. Long-term safety and efficacy of etanercept in children with polyarticular-course juvenile rheumatoid arthritis. Arthr Rheum 54(6):1987–1994
Lovell DJ, Reiff A, Ilowite NT, Wallace CA, Chon Y, Lin SL, Baumgartner SW (2008) Pediatric rheumatology collaborative study group. Safety and efficacy of up to 8 years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthr Rheum 58(5):1496–1504
Horneff G, Schmeling H, Biedermann T, Foeldvari I, Ganser G, Girschick HJ, Hospach T, Huppertz HI, Keitzer R, Küster RM, Michels H, Moebius D, Rogalski B (2004) Paediatric rheumatology collaborative group. The German etanercept registry for treatment of juvenile idiopathic arthritis. Ann Rheum Dis 63(12):1638–1644
Kietz DA, Pepmueller PH, Moore TL (2001) Clinical response to etanercept in polyarticular course juvenile rheumatoid arthritis. J Rheumatol 28(2):360–362
Kietz DA, Pepmueller PH, Moore TL (2002) Therapeutic use of etanercept in polyarticular course juvenile idiopathic arthritis over a two year period. Ann Rheum Dis 61(2):171–173
Horneff G, De Bock F, Foeldvari I, Girschick HJ, Michels H, Moebius D (2009) German and Austrian paediatric rheumatology collaborative study Group. Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA Registry. Ann Rheum Dis 68(4):519–525
Lahdenne P, Vahasalo P, Honkanen V (2003) Infliximab or etanercept in the treatment of children with refractory juvenile idiopathic arthritis: an open label study. Ann Rheum Dis 62(3):245–247
Quartier P, Taupin P, Bourdeaut F, Lemelle I, Pillet P, Bost M, Sibilia J, Koné-Paut I, Gandon-Laloum S, LeBideau M, Bader-Meunier B, Mouy R, Debré M, Landais P, Prieur AM (2003) Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthr Rheum 48(4):1093–1101
Robinson RF, Nahata MC, Hayes JR, Rennebohm R, Higgins G (2003) Quality-of-life measurements in juvenile rheumatoid arthritis patients treated with etanercept. Clin Drug Investig 23(8):511–518
Prince FH, Twilt M, ten Cate R, van Rossum MA, Armbrust W, Hoppenreijs EP, van Santen-Hoeufft M, Koopman-Keemink Y, Wulffraat NM, van Suijlekom-Smit LW (2009) Long-term follow-up on effectiveness and safety of etanercept in juvenile idiopathic arthritis: the Dutch national register. Ann Rheum Dis 68(5):635–641
Giannini EH, Ilowite NT, Lovell DJ, Wallace CA, Rabinovich CE, Reiff A, Higgins G, Gottlieb B, Singer NG, Chon Y, Lin SL (2009) Pediatric rheumatology collaborative study group. Long-term safety and effectiveness of etanercept in children with selected categories of juvenile idiopathic arthritis. Arthr Rheum 60(9):2794–2804
Papsdorf V, Horneff G (2011) Complete control of disease activity and remission induced by treatment with etanercept in juvenile idiopathic arthritis. Rheumatology (Oxford) 50(1):214–221
Tynjala P, Vahasalo P, Honkanen V, Lahdenne P (2009) Drug survival of the first and second course of anti-tumour necrosis factor agents in juvenile idiopathic arthritis. Ann Rheum Dis 68(4):552–557
Bejarano V, Conaghan PG, Quinn MA, Saleem B, Emery P (2010) Benefits 8 years after a remission induction regime with an infliximab and methotrexate combination in early rheumatoid arthritis. Rheumatology (Oxford) 49(10):1971–1974
van der Kooij SM, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Güler-Yüksel M, Zwinderman AH, Kerstens PJ, van der Lubbe PA, de Beus WM, Grillet BA, Ronday HK, Huizinga TW, Breedveld FC, Dijkmans BA, Allaart CF (2009) Drug-free remission, functioning and radiographic damage after 4 years of response-driven treatment in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis 68(6):914–921
Prince FH, Twilt M, Simon SC, van Rossum MA, Armbrust W, Hoppenreijs EP, Kamphuis S, van Santen-Hoeufft M, Koopman-Keemink Y, Wulffraat NM, ten Cate R, van Suijlekom-Smit LW (2009) When and how to stop etanercept after successful treatment of patients with juvenile idiopathic arthritis. Ann Rheum Dis 68(7):1228–1229
Pratsidou-Gertsi P, Trachana M, Pardalos G, Kanakoudi-Tsakalidou F (2010) A follow-up study of patients with juvenile idiopathic arthritis who discontinued etanercept due to disease remission. Clin Exp Rheumatol 28(6):919–922
Wallace CA, Ruperto N, Giannini E (2004) Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol 31(11):2290–2294
Murray JG, Ridley NT, Mitchell N, Rooney M (1996) Juvenile chronic arthritis of the hip: value of contrast-enhanced MR imaging. Clin Radiol 51(2):99–102
Nistala K, Babar J, Johnson K, Campbell-Stokes P, Foster K, Ryder C, McDonagh JE (2007) Clinical assessment and core outcome variables are poor predictors of hip arthritis diagnosed by MRI in juvenile idiopathic arthritis. Rheumatology (Oxford) 46(4):699–702
Foell D, Wulffraat N, Wedderburn LR, Wittkowski H, Frosch M, Gerss J, Stanevicha V, Mihaylova D, Ferriani V, Tsakalidou FK, Foeldvari I, Cuttica R, Gonzalez B, Ravelli A, Khubchandani R, Oliveira S, Armbrust W, Garay S, Vojinovic J, Norambuena X, Gamir ML, García-Consuegra J, Lepore L, Susic G, Corona F, Dolezalova P, Pistorio A, Martini A, Ruperto N (2010) Paediatric Rheumatology International Trials Organization (PRINTO). Methotrexate withdrawal at 6 versus 12 months in juvenile idiopathic arthritis in remission: a randomized clinical trial. JAMA 303(13):1266–1273
Conflict of interest
We have no conflict of interests to others.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cai, Y., Liu, X., Zhang, W. et al. Clinical trial of etanercept tapering in juvenile idiopathic arthritis during remission. Rheumatol Int 33, 2277–2282 (2013). https://doi.org/10.1007/s00296-012-2642-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-012-2642-7