Abstract
The TNF inhibitors etanercept (ETA) and adalimumab (ADA) are approved for treating patients older than 2 years with polyarticular juvenile idiopathic arthritis (JIA). Because long-term experience of treating children younger than 4 years is limited, we evaluated the efficacy and safety of ETA or ADA in patients aged 2–4 years. This prospective, long-term, observational registry study documented baseline demographics, clinical characteristics, disease activity parameters, and safety of patients treated with ETA or ADA. Efficacy was determined using the JADAS-10, the JADAS criteria for minimal disease activity (MDA) and remission, and the PedACR response criteria after 3, 6, 12, 18, and 24 months. Between January 2001 and March 2015, 85 patients with polyarticular JIA aged 2–4 years started anti-TNF-α treatment. Seventy-four (54 girls) patients were treated with ETA and 11 (7 girls) with ADA. After 6/12/24 months of treatment, JADAS-MDA was reached by 55/58/58 % of ETA patients and 50/71/66 % of ADA patients. Furthermore, JADAS-Remission was achieved by 35/44/50 % of ETA patients and 16/28/66 % of ADA patients. PedACR 50/70/90 response was achieved by 64/54/41 % of ETA patients and 56/33/22 % of ADA patients at the last treatment observation. Discontinuation because of remission or inefficacy was recorded in 24 (29 %) and 28 (33 %) patients, respectively. Seventy-nine adverse events and four serious adverse events were reported. Administration of ETA and ADA in JIA patients younger than 4 years was efficacious, well tolerated, and safe. Patients younger than 4 years may show marked improvement following anti-TNF-alpha therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2011, etanercept (ETA) and in 2013, adalimumab (ADA) were approved for the treatment of patients older than 2 years with juvenile idiopathic arthritis (JIA). The use of tumor necrosis factor (TNF)-α inhibitors (TNFi) has increased significantly in children younger than 4 years. However, clinical experience and published data on TNFi treatment in this patient population remain limited. Four studies have investigated ETA treatment in JIA patients younger than 4 years, including two reports from the German Biologika in der Kinderrheumatologie (BIKER) registry, but with very limited patient numbers. Bracaglia et al. [1] and one previous BIKER study [2] each included 25 patients younger than 4 years of age treated with ETA. Gimenez-Roca et al. [3] investigated 27 patients younger than 4 years treated with TNFi (23 ETA, 4 ADA) and the BIKER study of Windschall et al. [4] included 13 patients younger than 2 years of age. Efficacy and safety were generally comparable with studies in patients older than 4 years [1–4]. In one international, multicenter, open-label, phase 3b study in 32 polyarticular JIA patients aged 2–4 years, the efficacy and safety of ADA was analyzed, leading to the approval of ADA [5]. Recently, high tolerability and efficacy was observed following ADA treatment as a first and second biological agent in patients with different categories of JIA [6].
The BIKER registry has been collecting data on ETA treatment in children with JIA since 2001 and ADA treatment in children with JIA since 2008. Patients in all JIA categories who are treated with ETA and ADA are followed by the registry.
This study analyzed BIKER registry data from young patients who started TNFi treatment (ETA or ADA) aged 2–4 years. We focused on evaluating safety and efficacy of ETA and ADA and compared our results with published data on TNFi treatment in JIA patients to better understand if there are potential differences between very young and older JIA patients that might influence treatment decisions in methotrexate (MTX) refractory disease. Given the observational design of the registry, no efficacy comparison between the two TNFi cohorts was intended.
Patients and methods
Patients
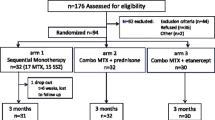
All polyarticular JIA patients aged 2–4 years in the German BIKER registry that newly started treatment with ETA or ADA between January 2001 and March 2015 were included in the analysis. Efficacy was analyzed in patients if they had assessments at baseline and at least one follow-up visit.
Patient demographics and clinical data
Treatment decisions and classification of patient JIA categories were made by the responsible pediatric rheumatologist and were not influenced by the registry.
Patient characteristics included sex, age, diagnosis, disease duration, previous treatments, and initial concomitant treatment and comorbidities. Clinical data included antinuclear antibody (ANA)-positivity; HLA-B27; morning stiffness; the number of tender, swollen, and active joints; the number of joints with limited motion; the physician’s assessment of global disease activity (visual analog scale [VAS]); patient’s/parent’s assessment of global disease activity and pain (both using VAS); erythrocyte sedimentation rate (ESR); C-reactive protein; and functional assessment using the Childhood Health Assessment Questionnaire (CHAQ) disability index.
Measurement of efficacy
Efficacy was measured using standardized disease activity parameters including functional ability, total number of active joints, number of joints with limited motion, patient’s and physician’s global assessment (VAS), ESR, C-reactive protein, and CHAQ. The JADAS-10 activity score, including the four components of the number of active joints, patient’s and physician’s global assessment (VAS), and ESR, was analyzed at baseline and after 3, 6, 12, 18, and 24 months [7]. JADAS-10 criteria for minimal disease activity (MDA, JADAS-10 ≤3.8) and remission (JADAS-10 ≤1) were used [8]. Improvement was also determined using the PedACR response criteria as well as the proposed criteria for inactive disease and remission between start and last observation on drug [9]. Reasons for discontinuation were recorded.
Analysis of safety
For adverse events (AEs), the investigator assessed and recorded the AE in detail on the AE form, including the date and time of onset, description, severity, time course, duration and outcome, relationship of the AE to the study drug, and alternative etiology for events not considered “probably related” to medication.
Serious adverse events (SAEs) were defined as events that were fatal or life threatening and resulted in a persistent or major disability or incapacity, required prolonged inpatient hospitalization, or led to a congenital anomaly or birth defect. The number and rates of AEs and SAEs were counted and compared between ETA and ADA as well as with published rates for JIA patients [2, 5].
Statistical analysis
Demographic and baseline characteristics were summarized using descriptive statistics. Categorical data were expressed as mean, median, standard deviations, and interquartile ranges as well as maximum and minimum values, and nominal and ordinal data were expressed as absolute values and percentages. Baseline characteristics were compared between ETA and ADA patients using a t test and the chi-square test, and rates per exposure years were compared using a Wald test (z test). P values less than 0.05 were considered statistically significant. Statistical analysis was performed using SPSS version 19 software (SPSS Inc., Chicago, Illinois, USA).
Compliance with ethical standards
The BIKER registry was approved by the local ethics committee of the Aerztekammer Duesseldorf and the University of Halle-Wittenberg. Written parental consent was obtained for all study participants. The study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Results
Between January 2001 and March 2015, a total of 85 patients with polyarticular JIA aged 2–4 years and treated with ETA or ADA were identified in the BIKER registry database. Seventy-four patients were treated with ETA as a first biologic agent and 11 received ADA. Demographic data and patient characteristics are shown in Table 1. The age (mean ± SD) at disease onset was 1.8 ± 0.7 years (ETA) and 1.6 ± 0.7 years (ADA). The age at start of therapy was 3.1 ± 0.5 years (ETA) and 3.5 ± 0.5 years (ADA). The mean disease duration until the start of treatment was 1.3 ± 0.8 years (ETA) and 1.8 ± 0.7 years (ADA). Thus, the onset age was similar but the age at baseline was numerically but not statistically significantly higher in the ADA cohort, as was the disease duration.
Prior to biological treatment, 78 (92 %) patients were exposed to MTX. Pretreatment involved nonsteroidal anti-inflammatory drugs (NSAIDs) in 58 (68 %) patients and systemic corticosteroids in 38 (45 %) patients. Four patients received cyclosporine A and one patient received azathioprine. Uveitis, as a concomitant disease, was reported in two (3 %) ETA patients and five (45 %) ADA patients. Thus, patients with prior uveitis were significantly more likely to receive ADA (odds ratio, 30.0; 95 % confidence interval [CI], 4.8–188; p < 0.001).
Initial combination treatment with NSAIDs was used in a total of 47 (55 %) patients (42 ETA, 5 ADA), oral corticosteroids in 29 (34 %) patients (23 ETA, 6 ADA), MTX in 64 (75 %) patients (54 ETA, 10 ADA), cyclosporine A in 4 (4.7 %) patients (only ETA), leflunomide in 2 ETA patients, and azathioprine in 1 ETA patient. A total of 13 (15 %) ETA patients and only 1 (9 %) ADA patient received TNFi monotherapy.
At baseline, several disease activity indicators were numerically higher in the ETA than in the ADA cohort without being statistically different (Table 1). For example, the number of active joints was higher in patients treated with ETA (7.7 ± 10.4) than in patients treated with ADA (2.5 ± 3.1) (Table 1). At baseline, mean ESR was 32.5 ± 26.1 in ETA patients (80 % with elevated ESR, defined as being >20 mm/h) and 19.4 ± 13.3 in ADA patients (55 % with elevated ESR).
The physician’s assessment of global disease activity (VAS) was higher in patients in the ETA cohort (58.3 ± 28.9) than in patients treated with ADA (41.9 ± 31.4).
Functional capacity was evaluated using the CHAQ-disability index (DI). The CHAQ-DI was statistically significantly higher in patients treated with ETA (mean 0.9 ± 0.8) compared with patients treated with ADA (0.4 ± 0.5; p < 0.05). In summary, there was a trend towards a higher disease activity with a more pronounced joint involvement in the ETA cohort, as well as a higher functional impairment.
Efficacy
Marked improvement from baseline to the last observation was observed in the majority of patients. Both patient cohorts demonstrated a relevant clinical benefit by a decrease (% change from baseline) in active joints (ETA, −49 %; ADA, −36.7 %), the parent’s/patient’s global assessment of overall disease activity on the VAS (ETA, −60 %; ADA, −24 %), the physician’s global assessment of overall disease activity on the VAS (ETA, −44 %; ADA, −61 %), and the CHAQ-DI (ETA, −61.2 %; ADA, −100 %).
A clear improvement in JADAS-10 score was also demonstrated in the majority of ETA and ADA patients already after 3 and 6 months of therapy (Fig. 1). The improvement within the first 12 months of therapy was very similar in both treatment cohorts. Between 12 and 24 months of therapy, the only a modest change in the median JADAS-10 was observed. After 24 months of therapy, however, the median JADAS-10 score showed a marked decrease with ETA or ADA treatment in comparison with baseline. Given the observational design of the registry and the smaller number of patients treated with ADA, no efficacy comparison between the two TNFi cohorts was performed. After 6/12/24 months of treatment, JADAS-MDA was achieved by 55/58/58 % (ETA) and 50/71/66 % (ADA) and JADAS-Remission was achieved by 35/44/50 % (ETA) and 16/28/66 % (ADA) of patients (Fig. 2). A PedACR 50/70/90 response of 64/54/41 % was achieved in ETA patients and of 56/33/22 % in ADA patients at last observation on drug.
JADAS-10 scores (Juvenile Arthritis Disease Activity Score, ref. 7) shown as median values over 24 months of observation (ETA etanercept cohort, n = 31; ADA adalimumab cohort, n = 7)
Discontinuation because of remission was recorded in 24 (32 %) patients in the ETA cohort. Only one (1.2 %) patient was reported to have successfully stopped TNFi treatment within a 12-month period. A total of 12 (50 %) patients who stopped TNFi treatment because of remission experienced a flare (reported to the registry to date). The ETA/ADA-free interval until a flare was extremely variable and ranged from 4 months to 6 years (mean 8.4 months). No patient discontinued ADA because of disease remission. Discontinuation because of inefficacy was reported in 31 (36 %) patients, 28/74 of whom were in the ETA cohort.
Safety
Tolerability was unremarkable in most of the patients (Table 2). Seventy-nine AEs and four SAEs (two infections, one new onset of type 1 diabetes mellitus, and one arthritis exacerbation) were reported.
Sixty-four AEs occurred in patients treated with ETA (rate 0.9/patient; 39.8/100 patient years; 95 % CI 31.1–50.8). Twenty-two infections occurred in the ETA group (rate 13.6/100 patient years; 95 % CI 9.0–20.7). There were three SAEs, two of which were serious infections. In the ADA cohort, 15 AEs were recorded (rate 1.4/patient; 71.4/100 patient years; 95 % CI 43.1–118). There were seven infections (rate 33/100 patient years; 95 % CI 16–70) and one SAE (one new onset of type 1 diabetes mellitus) in this cohort.
While the total exposure time for ETA was 161 years and for ADA was 21 years, the rate of AEs as well as the rate of infections was significantly higher in the ADA cohort (relative risk [RR], 1.8 [95 % CI 1.0–3.2]; p < 0.001; and RR 2.48 [95 % CI 1.0–5.7]; p = 0.001), respectively. However, these data must be considered preliminary as the total exposure time to ADA was limited.
Eleven cases of uveitis, including four flares of pre-existing uveitis, were observed following treatment with ETA (rate 0.15/patient; 6.8/100 patient years). One case of new onset uveitis was reported with ADA. No malignancy or death and, except for a single case of autoimmune diabetes mellitus in the ADA cohort, no further autoimmune disorder was observed. Despite the diabetes mellitus case, all events resolved without permanent damage. There were no cases of tuberculosis or any other opportunistic infection reported.
Discontinuation because of intolerance was reported in one patient in the ADA cohort (allergic reaction) and in two patients in the ETA group (uveitis and an injection site reaction).
Discussion
Our study aimed to analyze and report data on safety and efficacy of ETA and ADA treatment in young patients aged 2–4 years, a patient cohort in which limited TNFi treatment experience has been reported. The results of our registry study, which included the largest number of JIA patients younger than 4 years treated by TNFi reported to date, indicate that ADA and ETA are generally well tolerated in children aged 2–4 years. The infection rate was higher in the ADA cohort than in the ETA cohort, but no serious infections were associated with ADA treatment in our study. This led to significantly higher exposure-adjusted rates of AEs in patients treated with ADA. No tuberculosis or any other opportunistic infection was reported, and no malignancy or death occurred in our patient cohort. However, experience with using ETA and ADA to treat patients younger than 4 years must be considered in the context of the number of patients, which remains limited.
Only one new autoimmune disorder (type 1 diabetes mellitus) occurred following treatment (ADA), and was probably unrelated to TNFi therapy.
Twelve cases of uveitis were observed following TNFi treatment (11 with ETA).
ADA confers advantages in the treatment of uveitis. ETA has no obvious efficacy with respect to the occurrence or course of uveitis [10–12]. However, the uveitis flare rate in our ETA patients was similar to the published rate of uveitis following therapy with ETA [11]. The presence of only one case of new onset uveitis in our ADA cohort is therefore difficult to interpret.
In our ETA patient cohort, the rate of AEs was higher compared with published data from patients aged less than 4 years or from older children [2, 13–18]. Supporting our results, a higher rate of infection was reported in JIA patients aged 2–4 years who were treated with ETA (73.3 % compared with subjects aged 5–11 years [52.2 %] and 12–17 years [36.4 %]) in the CLIPPER trial. However, serious infections were still very infrequent in patients in those age groups [19]. Safety results from the ADA cohort are in agreement with published data from patients aged 2–4 years who were treated with ADA [5].
Published data on efficacy in very young children treated with anti-TNFi are increasing, but most studies (as mentioned previously) have included limited numbers of patients [1–4]. Previous studies in younger cohorts have included higher numbers of systemic JIA (SJIA) patients [2]. However, improved and alternative options to treat SJIA are available at present [20–22]. Therefore, only a few patients with SJIA were recorded in the TNFi cohort of the BIKER registry, particularly in recent years, and were not included in our young patient cohort.
In our study, disease activity parameters at baseline were higher in ETA patients than in ADA patients. The preference for ADA in the context of concomitant uveitis may be responsible for differences between the two cohorts in the articular disease activity.
A high TNFi response rate was observed, with 58 % (ETA) and 66 % (ADA) of patients achieving minimal disease activity (JADAS-10) after 24 months of treatment. Previously, nearly all patients were refractory to MTX. PedACR 50/70/90 response rates achieved with ETA at last observation on drug appear lower than those previously reported in patients aged younger than 4 years, where the PedACR 70 response rate was 64 % at the last observation [2]. However, in our ETA patients who were observed for 24 months (n = 31), the JADAS-10 improvement was excellent, as shown by a marked decrease of the median JADAS-10 score and a high rate of patients in JADAS-Remission (50 %) after 24 months of treatment. These response rates were comparable with those from previous studies in older children treated with ETA [13, 15, 23].
Our ADA patients achieved a lower PedACR 50/70/90 response rate at last observation but it must be considered that the disease activity parameters at baseline were not as high as those in ETA patients prior to biological treatment. Our results from clinical practice appeared inferior to those of an ADA study by Kingsbury et al. [5], where the PedACR 70 response rate after 24 weeks was 61 %. However, JADAS-10 improvement, with minimal disease activity achieved in 58 % of ADA patients after 24 months of therapy, indicated a strong treatment response, in agreement with the results by Kingsbury et al. In summary, our young patient cohort improved greatly following TNFi treatment with either agents.
The reported flare rate of 50 % among our patients who discontinued TNFi medication because of remission corresponds well with data from JIA and rheumatoid arthritis patients in the literature [24–26] and confirms that flare rates in JIA are high, and the discontinuation of medications is challenging.
In conclusion, treatment with ETA and ADA leads to marked improvement in young patients with JIA with achievement of minimal disease activity and even remission in a high rate of patients. Patients treated with ETA showed good efficacy and safety outcomes. The small cohort of patients treated with ADA also showed good efficacy, albeit with a slightly higher infection rate. In summary, our findings indicate that there is no reason for concern in treating very young patients with TNFi, but must be considered as preliminary because of the small number of patients studies. However, our experience may facilitate treatment decision-making in young JIA patients with MTX refractory disease.
References
Bracaglia C, Buonuomo PS, Tozzi AE et al (2012) Safety and efficacy of etanercept in a cohort of patients with juvenile idiopathic arthritis under 4 years of age. J Rheumatol 39(6):1287–1290
Tzaribachev N, Kuemmerle-Deschner J, Eichner M, Horneff G (2008) Safety and efficacy of etanercept in children with juvenile idiopathic arthritis below the age of 4 years. Rheumatol Int 28:1031–1034
Gimenez-Roca C, Iglesis E, Torrente-Segarra V et al (2015) Efficacy and safety of TNF-alpha antagonists in children with juvenile idiopathic arthritis who started treatment under 4 years of age. Rheumatol Int 35:323–326
Windschall D, Müller T, Becker I, Horneff G (2015) Safety and efficacy of etanercept in children with juvenile idiopathic arthritis below the age of 2 years. Rheumatol Int 35:613–618
Kingsbury DJ, Bader-Meunier B, Patel G, Arora V, Kalabic J, Kupper H (2014) Safety, effectiveness, and pharmacokinetics of adalimumab in children with polyarticular juvenile idiopathic arthritis aged 2 to 4 years. Clin Rheumatol 33:1433–1441
Schmeling H, Minden K, Foeldvari I, Ganser G, Hospach T, Horneff G (2014) Efficacy and safety of adalimumab as first and second used biologic agent in juvenile idiopathic arthritis—the German biologics JIA registry (BiKeR). Arthritis Rheum 66:2580–2589
Consolaro A, Ruperto N, Bazso A et al. (2009) Pediatric Rheumatology International Trials Organization. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 61:685–666
Consolaro A, Bracciolini G, Ruperto N et al (2012) Remission, minimal disease activity, and acceptable symptom state in juvenile idiopathic arthritis: defining criteria based on the juvenile arthritis disease activity score. Arthritis Rheum 64:2366–2374
Wallace C, Giannini E, Huang B et al (2011) American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res 63:929–936
Saurenmann RK, Levin AV, Feldman BM, Laxer RM, Schneider R, Silverman ED (2006) Risk of new-onset uveitis in patients with juvenile arthritis treated with anti-TNFalpha agents. J Pediatr 149:833–836
Schmeling H, Horneff G (2005) Etanercept and uveitis in patients with juvenile idiopathic arthritis. Rheumatol 44:1008–1011
Foeldvari I, Becker I, Horneff G (2015) Uveitis events during adalimumab, etanercept, and methotrexate therapy in juvenile idiopathic arthritis: data from the biologics in pediatric rheumatology registry. Arthritis Care Res 67:1529–1535
Horneff G, Schmeling H, Biedermann T et al (2004) The German etanercept registry for treatment of juvenile idiopathic arthritis. Ann Rheum Dis 63:1638–1644
Horneff G, De Bock F, Foeldvari I et al (2009) Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA registry. Ann Rheum Dis 68:519–525
Giannini EH, Ilowite NT, Lovell DJ et al (2009) Long-term safety and effectiveness of etanercept in children with selected categories of juvenile idiopathic arthritis. Arthritis Rheum 60:2794–2804
Lovell DJ, Giannini EH, Reiff A et al (2000) Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med 342:763–769
Lovell DJ, Giannini EH, Reiff A et al (2008) Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum 58:1496–1504
Prince FH, Twilt M, ten Cate R et al (2009) Long-term follow-up on effectiveness and safety of etanercept in juvenile idiopathic arthritis: the Dutch national register. Ann Rheum Dis 68:635–641
Horneff G, Burgos-Vargas R, Constantin T et al (2014) Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann Rheum Dis 73:1114–1122
Quartier P, Taupin P, Bourdeaut F et al (2003) Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthritis Rheum 48:1093–1101
De Benedetti F, Brunner H, Ruperto N et al (2012) Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med 367:2385–2395
Ruperto N, Brunner H, Quartier P et al (2012) Two randomized trials of Canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med 367:2396–2406
Windschall D, Müller T, Becker I, Horneff G (2015) Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis-related arthritis and psoriasis arthritis. Clin Rheumatol 34:61–69
Postepski J, Kobusinska K, Olesinska E, Osinska V, Opoka-Winiarska V (2013) Clinical remission in juvenile idiopathic arthritis after termination of etanercept. Rheumatol Int 33:2657–2660
Chang C, Meyer R, Reiff A (2015) Impact of medication withdrawal method on flare-free survival in patients with juvenile idiopathic arthritis on combination therapy. Arthritis Care Res 67:658–666
Ghiti Moghadam M, Vonkeman HE, Ten Klooster PM et al (2016) Stopping tumor necrosis factor inhibitor treatment in patients with established rheumatoid arthritis in remission or with stable low disease activity: a pragmatic multicenter, open-label randomized controlled trial. Arthritis Rheumatol 68:1810–1817
Acknowledgments
This study would not have been possible without the collaboration of numerous German and Austrian pediatric rheumatologists, patients, and their parents.
Collaborators
Michael Borte, Frank Dressler, Maria Fasshauer, Dirk Föll, Ivan Foeldvari, Toni Hospach, Christian Hümer, Markus Hufnagel, Jasmin Kümmerle-Deschner, Bernd-Ulrich Keck, Thomas Keller, Rolf Küster, Kirsten Minden, Thomas Müller, Martina Prelog, Betina Rogalski, Otto Schofer, Michaela Sailer-Höck, Frank Weller-Heinemann, and Olaf Zimmermann.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D Windschall has received research grants from Roche and Pfizer and speaker’s fees from AbbVie, Novartis, and Pfizer. G Horneff has received research grants from AbbVie, Pfizer, and Roche, and speaker’s fees from AbbVie, Novartis, Pfizer, and Roche.
Funding
The registry is supported by an unrestricted grant from Pfizer, Germany; AbbVie, Germany; and Roche, Germany. The companies had no chance to influence the analysis of data or drafting of the manuscript.
Rights and permissions
About this article
Cite this article
Windschall, D., Horneff, G. Safety and efficacy of etanercept and adalimumab in children aged 2 to 4 years with juvenile idiopathic arthritis. Clin Rheumatol 35, 2925–2931 (2016). https://doi.org/10.1007/s10067-016-3439-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3439-y