Abstract
To investigate the burden of ankylosing spondylitis in the Czech Republic as a baseline for future health economic evaluations. Data were obtained from two cross-sectional studies Beda I (2005) and Beda II (2008), performed in 1,008 and 509 patients, respectively. Methodology used was Cost-of-Illness prevalence-based analysis bottom-up approach. Analysis was performed from payer (health insurance companies) and societal perspective (including productivity costs using friction cost approach). Mean age of sample in Beda I and Beda II was 50.2 and 52.5 years, male were present by 61.0 and 62.7 %; average disease duration was 23.0 and 26.4 years, respectively. Mean total annual costs per patient in the sample were €4,782 in Beda I and €5806 in Beda II. Average direct costs per patient in the sample per year are estimated at €1,812 (Beda I) and €2,588 (Beda II) with the average productivity costs €2,970 (Beda I) and €3,218 (Beda II). We observed a small decrement in percentage (6.7 %) of productivity costs for Beda II as an influence of higher consumption of biologic drugs, hence higher direct costs and possible productivity preservation. The largest direct cost burdens were spa procedures (45.3 %, Beda I) and biological drugs (52.8 %, Beda II). Unique analysis of the burden of the AS in the Central-Eastern Europe presents health care resource and cost consumption by comparing two cross-sectional prevalence-based studies. Further analysis should be carried to obtain data connecting health status with costs consumption in order to analyse the AS from this perspective.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Disease and the epidemiology
Ankylosing spondylitis (AS) is a chronic progressive autoimmune inflammatory condition that primarily affects the spine and sacroiliac joints causing pain and stiffness in and around the spine. Involvement of other joints, enthuses and extra-articular structures is also possible [1]. AS leads to irreversible structural changes and consequently to impaired spinal mobility and reduced quality of life (QoL).
Because the onset of AS can occur at a relatively young age, usually during early adulthood, typically presenting in young men, patients have to adjust to their disease for most of their lives [2, 3]. Similarly, to other chronic diseases, AS can affect quality of life, morbidity, mortality, participation in paid and unpaid work and health care costs [3]. Previous studies have shown that the economic impacts of AS on the society or patients were substantial, and the costs are driven by the costs of losses of work capacity [4, 5].
The AS affects about 0.1–1.4 % of the European population, depending on the geographical region studied [3]. According to a recent epidemiology study, the prevalence in the Czech Republic was explored to be much lower (0.1 %) which could be caused by generally later diagnosis of the disease in the Czech settings [6]. Early diagnosis is critical as patients are to delay the occurrence of irreversible damage. The delay is 9 years in the Czech Republic [7].
Treatment for AS in the Czech Republic generally follows the ASAS/EULAR (Assessment of SpondyloArthritis International Society/European League against Rheumatism) recommendations [8]. Constant physiotherapy, nonsteroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), intra-articular injection of corticosteroids are the main medication for AS as well as regular spa procedures [9]. The current guidelines define the failure of the standard treatment as a start of the antitumour necrosis factor (anti-TNF) treatment according to the international recommendation [10].
From the socioeconomic point of view, it is important to use the treatments that control the disease activity and can prevent or slow disease progression to avoid or delay the high health care costs, productivity losses and additional payments via welfare benefit combined with low quality of life (QoL) associated with severe diseases [11].
The purpose of this analysis was to estimate the costs of patients with AS in the Czech Republic as a basis of the future health economic evaluations of various preventive and treatment strategies and for policy and decision-makers better understanding of burden of this disease.
Methods
Data
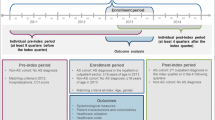
The data were obtained from a cross-sectional studies Beda I (2005) [12] in which patients’ data were collected with the recall time of 12 months in the form of questionnaires [12]. The same patients’ sample was accessed in the follow-up study Beda II (2008). All patients were members of ankylosing spondylitis patients’ organisation, Bechterev Club. The validated Czech language versions of standardised questionnaires were used [13].
The questionnaires contained questions regarding sociodemographic characteristics of patients, medical history and courses of disease, type of follow-up and therapy (separately evaluating the biological treatment), rehabilitation, medical devices, therapy of side effects, quality of life, current health status and work capacity (including questions of days absent to work due to AS––absenteeism and work status––either working status, partially or fully disabled).
Patient reported outcomes, HAQ-DI (Health Assessment Questionnaire-Disability Index) [14] and BASDAI (Bath Ankylosing Spondylitis Disease Activity Index) [15] were also included in the questionnaire. The evaluation of quality of life (QoL) and health state was extended to EQ-5D (EuroQoL), SF-36 (the Short Form-36 Health Status Questionnaire) and ASQoL in Beda II in 2008. The study has been described in details previously [12].
Analysis
According to the data provided from the questionnaires, the methodology used for analysing the burden of the AS was limited to the Cost-of-Illness analysis (COI).
The analysis was performed from the societal perspective, including perspective of health care payer (direct medical costs) with production loss (productivity costs). According to the latest health economics guidelines, we should have rather called this perspective in this reference case analysis as a limited societal perspective [16]. Individual resource quantities were multiplied with specific unit costs obtained from publicly available sources. Direct costs (including expenses incurred obtaining medical services or the treatment for the disease, e.g. outpatient and inpatient treatment, treatment and assistive devices, medicines, etc.) were obtained from the database, which includes the price list published by Health Insurance Companies in the Czech Republic [17]. Prescription drug costs were calculated based on the average daily dose obtained from information system AISLP (Information system of human, homoeopathic and veterinary registered drugs for the Czech and Slovak Republic).
For calculation of productivity costs, we used friction costs approach where the costs of lost production are valued with the cost of labour for defined period, referred as friction period. After this period, productivity of particular workplace is returned, and the productivity costs to society are zero. In contrast to human capital approach, these methods represent rather macroeconomic perspective and measure actual production losses [18, 19]. As a denominator, we used average gross income in year 2008. As the friction period, we used period of 130 workdays (6 months). We calculated productivity costs incurred by long and short-term absence from paid work, including days on sick leave, reductions in working time because of AS and early retirement. The absence from paid work due to sick leave (in this paper referred as a absenteeism) expressed as amount of working days absent from paid work comprised the number of days patients spent with inpatient or outpatient health care (outpatient visits, inpatient care, spa, rehabilitation, admission of biological treatment and intra-articular injections). Productivity costs incurred by absenteeism were calculated by multiplying number of days absent to paid work due to AS (maximum of 130 days––friction period) and average daily gross income in year 2008—€42.9.
We also included productivity costs due to early retirement, either to be partially or fully disabled. Conditions of the disability are defined by the law about pension (No. 155/1955 Coll.). Full disability and partial disability are according that law described as at least 66 and 33 % decrease at the work productivity caused by worsening of the health condition of the individual for more than 6 months. Practically, we counted productivity costs attributed to partial and full disability by multiplying average gross income for 6 months (friction period) in year 2008 with coefficients of 0.83 and 0.495 for full and partial disability. The coefficients are mean values of 66 and 100, 33 and 66 % for full and partial disability, respectively.
For calculation of productivity costs, we included all patients being partially or fully disabled at the time of analysis, no matter how long they are disabled. In the discussion part, we will focus on how much would be productivity costs changed if we would include just patients being newly disabled.
The non-health care costs, such as private household help, transportation and exercise, were not available in the cohort study [12]; therefore, they could not be included in the analysis.
The results are presented as the mean annual cost per patient, and time horizon of the data provided was one year. Price year of the analysis was 2008. All costs were converted to Euro (2008) [20].
Nevertheless, as costs are highly correlated with both physical function and disease activity, the mean costs are dependent on the sample included in the study. Unfortunately, due to the limitation of the reported data on the cohort study, it was not possible to present the costs related to the disease severity (HAQ and BASDAI).
Results
Patient demographics
The data were obtained from a cross-sectional studies Beda I (2005) and its follow-up study Beda II (2008) [12]. In total, 1,008 questionnaires were received in 2005 compared with 509 replies in 2008. Basic characteristics of the samples are recorded in Table 1. In Beda II, the mean time of duration of the disease was 26.4 years, which refers to the 3 years offset from the Beda I survey where the reported median of the disease duration was 23 years. A total of 59 (Beda I) and 56.8 % (Beda II) of patients had a BASDAI score ≥4.0. Full and partial disability is provided to patients by the state government and is based on the degree of disability (Table 1).
Resource consumption
Details on resource consumption are presented in Table 2.
The hospital admission was reported for 30 patients (3 %) in Beda I and 38 patients (7.5 %) in Beda II with a mean length of stay to be 16 days (based on a survey from the rheumatology centres in Prague in the Czech Republic), establishing the mean sample to 0.4 (Beda I) and 1.2 days (Beda II) per year.
The results of Beda I compared with Beda II show no difference in the total of number of outpatient visits to specialists, the most often to the rheumatologist (78.0, 76.4 %), next most visited were general practitioners (16.0, 14.9 %), respectively. On an annual basis, the mean number of outpatient visit in Beda I and Beda II was almost similar (4.5 and 4.4 visits per patient, respectively).
Around 80.0 % of patients in both samples underwent the laboratory tests and diagnostics (bloods, urinalyses, etc.). The use of medical devices increased from 53 % (Beda I) up to 60.7 % (Beda II).
The physical therapy has historically played an important role in the management of AS in the Czech Republic and is therefore well developed and organised [12]. The consumption of the physiotherapy increased from 38 to 45.2 % patients between Beda I and Beda II as well as the mean number of days of physiotherapy is 3.3 (Beda I) and 4.8 days (Beda II) per patient per year.
The amount of spa procedures decreased from 79 (Beda I) to 73.1 % (Beda II) although the average length of stay of underwent spa treatment remained almost the same, 21.6 days (Beda I) compared with 20.1 days (Beda II) per patient per year.
A total of 88.0 % (Beda I) and 85.5 % (Beda II) of patients used chronic medication, most frequently nonsteroidal anti-inflammatory drugs (NSAIDs, predominantly nimesulide, ibuprofen, meloxicam, diclofenac), disease-modifying antirheumatic drugs (DMARDs, predominantly sulfasalazine and methotrexate) and gastroprotectants (mostly proton pump inhibitors). The intra-articular injections were used by 14.2 % of patients with a mean number 0.5 of injections per patient per year (Beda I) compared with 9.2 % of patients and 0.4 injections per patient per year (Beda II).
In Beda I, the biological drugs (infliximab, etanercept, adalimumab) were used by 2.9 % of the sample (29 patients) compared with 6.3 % of the sample (32 patients) reported in Beda II.
In the samples of biologic drug consumers, infliximab was used by 17 patients (58.6 %) in Beda I and by 20 patients (61.8 %) in Beda II. Infliximab was applied as an infusion given 5 mg per kg on 0., 2., 6. and then on every 6 weeks. Etanercept used by 11 patients (36.7 %) in Beda I and by 7 patients (23.5 %) in Beda II. It was used as dosage of 25 mg twice a week. Adalimumab was used by only 1 patient (3.3 %) in Beda I compared with 5 patients (14.7 %) in Beda II., 40 mg s. c. was applied in every 2 weeks.
Costs
The direct and productivity costs of both samples are described in Tables 3, 4. The distribution/percentage of direct and productivity costs was slightly decreased in Beda II for productivity costs, with productivity costs representing 60.7 % (Beda I) and 55.4 % (Beda II) (Table 3). This phenomenon can be probably interpreted as an influence of increasing consumption of biologic treatment in both ways; higher direct costs and possible productivity preservation.
The mean total annual costs per patient in the sample were €4,782 in Beda I and €5,806 in Beda II, which shows 17.6 % increase between two samples.
In total, the medication used contributed to the direct costs by 39.4 % in Beda I and by 56.5 % in Beda II per patient per year.
Discussion
It is often difficult to compare direct costs between countries, mainly due to price differences for health care resources and due to the fact that different countries have different organisation of health care and medical tradition. Further studies vary in design, type of patients included and data collection. Even with these limitations, it is clear that there is substantial societal cost related to the medical treatment for AS [4].
Kobelt et al [11] reported the only health care costs €2,700 in Spain (price year 2005) compared with only health care costs in the United Kingdom (€2,600) (price year 2002) and in Canada (€1,800) (price year 2003), which is comparable with the calculated health care costs in the Czech Republic €1,812 (Beda I) and €2,588 (Beda II) (Table 3).
In the Czech Republic, prescribed medication associated with AS accounted for 18 (Beda I) and 27 % (Beda II) of the total costs, which are comparable with other findings. Ara et al. report that the prescribed medication accounted for 20 % of the total costs, and Boonen et al. report that pharmaceutical treatments contribute 13, 25 and 30 % of total costs in AS patients in the Netherlands, Belgium and France, respectively [5, 21].
Mean number of outpatient consultation in Beda I and Beda II (4.5 and 4.4 visits per patient, respectively) is higher then what have been found in the United Kingdom (2.2 visits per patient) and in Canada (3.7 visits per patient) and much less then in Spain (7.5 visits per patient), indicating strong differences in patients’ management [11, 22, 23].
In our two cohorts, only 2.9 % (Beda I) and 6.3 % patients (Beda II) were treated with biologic drugs, significantly less compared to average proportion of AS patients on biologics in the EU and USA [11, 12]. This discrepancy is further highlighted by the fact that, unlike in RA, the role of traditional DMARDs in the treatment for AS is rather limited.
In Beda I, biological drugs represented 34.2 % of direct costs compared with the results of Beda II where biological drugs represented 52.8 % of direct costs (Table 4).
The high contribution of biological drugs to the total costs is caused by high cost of the treatment, €21,534 per one patient with biological treatment in Beda I and €17,046 per one patient with biological treatment in Beda II. Current efforts to increase the number of patients treated with biologic agents in the Czech Republic are restrained mainly by the financial limits set by third party payers; therefore, physicians must select appropriate treatment candidates among patients with very high disease activity only [12].
As more effective and also more expensive treatments are introduced in the health care systems, the pharmacoeconomic studies become more important tool in the decision making process. Our study has found out that the ratio between direct and productivity costs reveals changes over the time, with an increase (relative and absolute) in direct costs in contrast to relative decrease and simultaneously small absolute increase in productivity costs in Beda I and Beda II, respectively. This is mainly effect of higher consumption of biologic drug therapy. Biologics, on one hand contribute to higher investment in direct medical costs, however, preserve productivity and revealed productivity costs savings and provide higher QoL. In Beda II patients on biologics reported lower BASDAI (mean 4.06, median 3.86 in contrast patients not treated with biologics mean 4.52, median 4.40), they reported lower rate of invalidity as well––65.6 % in contrast to 67.1 % not treated with biologics. Patients on biologics revealed lower consumption of spa procedures (one of the biggest costs driver)––62.5 % compare to 73.8 % for patients not being treated with biologics.
According to the methodology for calculation of productivity costs used, friction costs approach with inclusion of all patients with disability, no matter how long they disable are, we calculated productivity costs of 62 and 55 % of total costs in Beda I and Beda II, respectively. In case of inclusion of patients just newly disabled, 29 and 25, 20 and 16 % of all partly and fully disabled patients in Beda I and Beda II, respectively, we would get much lower productivity costs. Specifically, €1,327 and €1,176 give 42 and 31 % of total costs in Beda I and Beda II, respectively. Regarding the methodology used, we have to be very careful in results comparison, particularly within productivity costs estimation. Not just use of human capital or friction costs approach plays important role; however, inclusion of exiting or just newly disabled patients to productivity costs calculation determine the results. As a cost under estimation could be considered the fact that the costs of informal care by family were not available, thus they could not be included in the calculations, and costs of unpaid work and leisure time were not included either. Another cost underestimation is caused by poor evidence about the complications associated with the AS as orthopaedic surgery of pelvis and backbone, complications associated with osteoporosis (fractures, neurological complications) and side effects of the medication treatment as gastrointestinal problems, and nevertheless, early death caused by amyloidosis.
Taking into account the latest epidemiologic data about prevalence of AS in the Czech Republic (94.2/100,000) [24], the total burden of AS in the Czech Republic in 2005 and 2008 would had been €47 and €57 mill., respectively. Taking into account just medical costs, the burden for health care system would have been €18 and €25 mill. in year 2005 and 2008, respectively, which represented 0.23 and 0.29 % of total health care budget in 2005 and 2008, respectively. For interpretation of these figures, we are a bit careful, since patients in our study were from patients’ organisation, where we can expect older and more disabled subgroup of patients, which could affect the cost estimation, particularly in productivity costs. However, according to the higher penetration of biologics in recent years, we can expect higher percentage of total health care budget of AS in coming years. The increasing tendency is observable within our study.
This is the first Czech calculation that presents the burden of the AS, the health care resource and cost consumption comparing two cross-sectional studies that offer us the possibility to compare the evolution of health care consumption and productivity and productivity costs trend within three-year period. To our knowledge, a similar detailed pharmacoeconomic evaluation was not performed in any of the Eastern Bloc countries. These studies should be considered as a base for further more detailed analysis.
References
Keat A, Barkham N, Bhalla A, Gaffney K, Marzo-Ortega H, Paul S et al (2005) BSR guidelines for prescribing TNF-alpha blockers in adults with ankylosing spondylitis. report of a working party of the British Society for rheumatology. Rheumatology 44:939–947
McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y et al (2007) Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation. Health Technol Assess 11:1–158
Boonen A, van der Linden SM (2006) The burden of ankylosing spondylitis. J Rheumatol 78(Suppl):4–11
Boonen A, van der Heijde D, Landewe R, Guillemin F, Rutten-van Molken M, Dougados M (2003) Direct costs of ankylosing spondylitis and its determinants: an analysis among three European countries. Ann Rheum Dis 62:732–740
Boonen A, van der Heijde D, Landewe R, Spoorenberg A, Shouten H, Rutten-van Molken M (2002) Work status and productivity costs due to ankylosing spondylitis: comparison of three European countries. Ann Rheum Dis 61:429–437
Hanova P, Pavelka K, Dostal C, Holcatova I, Pikhart H (2006) Epidemiology of rheumatoid arthritis, juvenile idiopathic arthritis and gout in two regions of the Czech Republic in a descriptive population-based survey in 2002–2003. Clin Exp Rheum 24(5):499–507
Pavelka K (2006) Early diagnosis of ankylosing spondylitis. Vnitr Lek 52(7–8):726–729 Czech
Zochling J, van der Heijde D, Burgos-Vargas R, Collantes E, Davis JC Jr, Dijkmans B et al (2006) ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 65(4):442–452
Pavelka K, Stolfa J, Vencovsky J (2004) Supplement of standard protocol for treatment ofankylosing spondylitis. Ces Revmatol 1:30–035 Czech
Braun J, Pham T, Sieper J, Davis J, van der Linden S, Dougados M et al (2003) International ASAS consensus statement for the use of the anti-tumour necrosis factor agents in patients with ankylosing spondylitis. Ann Rheum Dis 62(9):817–824
Kobelt G, Sobocki P, Mulero J, Gratacos J, Pocovi A, Collantes-Estevez E (2006) The burden of ankylosing spondylitis in Spain. Value Health 11(3):408–415
Forejtova S, Mann H, Stolfa J, Vedral K, Fenclova I, Nemethova D et al (2008) Factors influencing health status and disability of patients with ankylosing spondylitis in the Czech Republic. Clin Rheumatol 27:1005–1013
Sleglova O, Dusek J, Olejarova M, Hornatova H, Draska L, Vencovsky J (2004) Evaluation of status and quality of life in patients with ankylosing spondylitis––validation of Czech versions of bath questionnaires––BAS-G, BADAI and BASFI. Ces Revmatol 2:43–54 Czech
Fries JF, Spitz P, Kraines RG, Holman HR (1980) Measurement of patient outcome in arthritis. Arthr Rheum 23(2):137–145
Garrett S, Jetkinson T, Kennedy LG, Whitelock H, Gaisford P, Callin A (1994) A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing spondylitis disease activity Index. J Rheumatol 21(12):2286–2291
Garrison LP Jr, Mansley EC, Abbott TA, Bresnahan BW, Hay JW, Smeeding J (2010) Good research practices for measuring drug costs In cost-effectiveness analyses: a societal perspective: the Ispor drug cost task force report: part II. Value Health 13(1):8–13
[Price list of VZP, version 650. Health Insurance Companies]. http://www.vzp.cz/poskytovatele/ciselniky/hromadne-vyrabene-lecive-pripravky-a-potraviny-pro-zvlastni-lekarske-ucely#archiv (accessed 19 September 2011) [Czech]
Larg A, Moss JR (2011) Cost-of-Illness Studies. A Guide to Critical Evaluation. Pharmacoeconomics 29(8):653–671
van Asselt AD, Dirksen CD, Arntz A, Severens JL (2008) Difficulties in calculating productivity costs: work disability associated with borderline personality disorder. Value Health 11(4):637–644
[Central bank exchange rate fixing –averages]. http://www.cnb.cz/en/financial_markets/foreign_exchange_market/exchange_rate_fixing/currency_average.jsp?code=EUR (accessed 5 June 2011) [Czech]
Ara RM, Packham JC, Haywood KL (2008) The direct costs associated with ankylosing spondylitis patients attending a UK secondary care rheumatology unit. Rheumatology 47:68–71
Kobelt G, Andlin-Sobocki P, Brophy S, Jonsson L, Calin A, Braun J (2004) The burden of ankylosing spondylitis and the cost-effectiveness of treatment with infliximab. Rheumatology 43:1158–1166
Kobelt G, Andlin-Sobocki P, Maksymowych W (2006) Cost and quality of life of patients with ankylosing spondylitis in Canada. J Rheumatol 33:289–295
Hanova P, Pavelka K, Holcatova I, Pikhart H (2010) Incidence and prevalence of psoriatic arthritis, ankylosing spondylitis, and reactive arthritis in the first descriptive population-based study in the Czech Republic. Scand J Rheumatol 39:310–317
Acknowledgement
Authors would like to acknowledge Mr. Vedral from patient´s organisation for the cooperation and consultation during the manuscript writing. This work was supported by grant number 000 000 23728 – Ministry of Health of Czech Republic, by grant number SVV 265 005 - Charles University in Prague.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petříková, A., Doležal, T., Klimeš, J. et al. The economic burden of the ankylosing spondylitis in the Czech Republic: comparison between 2005 and 2008. Rheumatol Int 33, 1813–1819 (2013). https://doi.org/10.1007/s00296-012-2542-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-012-2542-x