Abstract
Scleroderma renal crisis (SRC) has been associated with the use of corticosteroids (CS) in retrospective studies. Using an evidence-based approach, we undertook a systematic review of the literature to identify prospective studies in which scleroderma patients were administered CS to ascertain the risk of SRC in those patients. A comprehensive search was conducted using Medline, EMBASE, the Cochrane Library, and Web of Science. All original prospective clinical studies were eligible if they enrolled SSc patients newly treated with CS. Selected studies were reviewed, and data extraction was systematically performed for the dose and duration of the CS intervention as well as the occurrence of SRC. Twenty-six studies with a total of 500 SSc patients commencing new CS therapy were included in the systematic review. Ten definite cases of SRC, equivalent to a rate of 2%, were identified. In the subset of early diffuse patients, the rate of SRC was 4%. All 10 definite cases of SRC occurred in patients who received medium- to high-dose CS therapy. Seven cases occurred in the setting of stem cell transplant. CS are associated with SRC, although this may be due to confounding by disease severity and/or co-intervention. Great caution must continue to be exerted when initiating such therapy, especially in high doses and in the early diffuse subset of SSc patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) is a connective tissue disease of uncertain aetiology characterized by the pathophysiologic triad of vascular damage, fibrosis of the skin and internal organs, and autoimmunity and inflammation [1]. Scleroderma renal crisis (SRC) is one of its most feared complications. It is characterized by acute renal failure usually accompanied by malignant hypertension [2]. Although the advent of ACE inhibitors has dramatically improved the outcome from SRC [3], mortality remains high, climbing up to 50% within a year in one study of early diffuse systemic sclerosis (dcSSc) patients [4]. A significant number of patients who survive require short- and/or long-term dialysis, and some eventually require renal transplant [2].
Dating back to 1951, there has been concern about corticosteroids (CS) and their potential role in precipitating SRC in SSc [5]. Multiple case reports [6–11] and retrospective studies subsequently supported a possible association [12–14]. In a case–control study of 110 SSc patients from the Pittsburg cohort who developed SRC between 1981 and 1993, new use of prednisone in dosages ≥15 mg/day was associated with a fourfold increase in the onset of SRC (odds ratio 4.37, 95% confidence interval 2.03–9.43) [12]. On the other hand, in a recent retrospective analysis of the 134 patients who participated in the D-penicillamine trial [15], CS were not an independent risk factor for SRC in early dcSSc [4]. However, in subgroup analysis, low dosages of prednisone (mean 7.4 mg/day) were associated with the onset of SRC only in patients with severe skin involvement with large joint contractures.
The data thus remain conflicting and suboptimal, and studies based on prospective data are needed to clarify the role of CS in precipitating SRC [12, 16]. Our objective was therefore to perform a systematic review of the literature to identify prospective studies of SSc patients newly treated with CS to determine whether these patients were at an increased risk of SRC.
Methods
Search strategy and study selection criteria
In August 2009, a comprehensive search was performed on four databases including Medline, EMBASE, the Cochrane Library, and Web of Science (see “Appendix” for a template of the complete search) by one author (GT) assisted by a professional librarian. Using the Ovid search engine, Medline and EMBASE were searched using the following terms:
-
1.
systemic sclerosis, scleroderma
-
2.
prospective studies, clinical trial
-
3.
steroid, corticosteroid, glucocorticoid, prednisone, prednisolone, methylprednisolone, dexamethasone
-
4.
stem cell transplantation, antithymocyte globulin (these terms were added because we were aware that transplant protocols were including CS to prevent serum sickness from antithymocyte globulin therapy.)
The Cochrane Library and Web of Science database were searched employing the terms from 1 to 4 above and adding the following limits:
-
5.
NOT “multiple sclerosis”, “tuberous sclerosis”, “nodular sclerosis”, “nuclear sclerosis”, “biliary sclerosis”, “localized scleroderma”
There was no language exclusion. The search was limited to published studies.
The abstracts of each reference identified by the search were reviewed to determine which studies would be selected for full-length review. In addition, relevant references from selected papers were also hand-searched by one author (GT) for potential inclusion in the review.
Studies were selected for inclusion in the systematic review according to the following criteria:
-
1.
the study presented original data;
-
2.
the study was prospective;
-
3.
the study included SSc patients; overlap syndromes were acceptable as long as patients met criteria for SSc as well;
-
4.
the study included patients newly treated with CS as part of the study protocol; route of administration was either oral or intravenous;
-
5.
in the case of duplication with multiple articles publishing data on the same cohort, complementary articles and/or data sets were collapsed;
-
6.
studies with a mixed patient population were included if, from the full text, a subset of patients with SSc could be separately characterized, and their outcome independently assessed.
Description of studies
Data from each selected study were extracted by one investigator (GT) and verified by a second investigator (MH) using a structured data extraction form. Differences were resolved by consensus. The following information was systematically extracted:
-
1.
Author, journal, year of publication, country where study was done;
-
2.
Study design (e.g., pilot, prospective, randomized trial);
-
3.
Characteristics of the study population: sample size, age, percentage of female patients, disease duration, and percentage of patients with diffuse skin involvement;
-
4.
The dose and duration of CS intervention as well as the main co-intervention(s). CS dose was defined as high, medium, and low dose as follows:
-
(a)
High dose: Initial administration in IV pulses, regardless of frequency, or in doses greater than 30 mg/day of prednisone equivalent;
-
(b)
Medium dose: Initial dosage between 16 and 30 mg/day, regardless of the tapering regimen; and
-
(c)
Low-dose: Dosage never exceeded 15 mg/day, regardless of duration;
-
(a)
-
5.
Follow-up period; and
-
6.
SRC outcome: definite occurrence, definite non-occurrence, or no specific mention of SRC in the paper.
Results
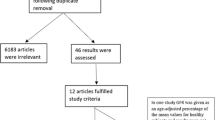
The search process identified 50 results from Medline, 230 from EMBASE, 125 from Web of Science, and 10 from the Cochrane Library (Fig. 1). After excluding duplicates, there was a total of 344 results. Title and abstract review led to the exclusion of 316 articles: 197 were reviews, seminars, or comments; 19 did not involve SSc patients, (e.g., graft-versus-host disease, localized scleroderma, other rheumatic diseases); 11 were case reports or case series; 29 were retrospective studies; 23 were cross-sectional studies; and 37 were prospective trials but without CS intervention. Thus, 28 articles were selected for full-text review [2, 17–44]. Of these, 6 were excluded: 3 did not have a CS intervention despite employing high-dose immunosuppression therapy; [39–41] one did not have an adequately characterized CS subgroup [42]; and two did not have CS as part of their study protocol, in addition to having a poorly characterized CS subgroup [43, 44]. In addition, 2 articles [37, 38] had complementary data sets and were collapsed. On the other hand, hand searching of the references of the 28 articles selected for full-text review yielded an additional 5 studies [45–49]. Thus, we included a total of 26 data sets in the systematic review [19–40, 47–51].
The characteristics of the studies included in the systematic review are presented in Table 1. The 26 data sets included a total of 500 patients. Overall, 81% of the patients were women, which is consistent with the gender distribution usually seen in SSc. Eighty percent (80%) of the patients had dcSSc. This is likely due to the fact that 11 of the 26 studies included only early dcSSc patients. The mean disease duration was 30 months (or 2.5 years), and the mean follow-up period was 17 months.
Ten definite SRC cases were reported among the 500 patients, equivalent to a rate of 2%. One other study also reported two patients who died because of progressive cardiopulmonary and renal disease, but these patients were not explicitly labeled as having had SRC. Of the 10 definite cases, 8 had received pulse CS therapy, 2 medium doses of CS, and none low-dose CS. Seven definite cases occurred in the context of autologous hematopoietic stem cell transplantation protocols. The two possible cases had both received antithymocyte globulin. Considering only the 11 studies limited to early dcSSc patients [18, 21, 22, 25, 27, 28, 30, 31, 33, 38, 49], we identified 9 definite SRC cases in 226 patients, which is equivalent to a rate of 4%.
Discussion
In this systematic review, we found that 2% of all SSc patients and 4% of early diffuse patients treated with CS developed SRC. In the literature, SRC has been reported to occur in 5–18% of SSc patients [13, 14, 50, 51]. Of these, 75–86% have been reported to occur within the first 4 years of disease [13, 50]. Taking the most conservative numbers, we thus estimate that the incidence of SRC within the first 4 years of disease is approximately 3.75% (5 × 75%). This is equivalent to an annual incidence rate of 0.94%. Using similar estimates for diffuse patients only, the annual incidence of SRC in the first 4 years would be approximately 2.34% [14]. Thus, the rates found in this systematic review are approximately twice those expected, both for SSc in general (2 vs. 0.94%) and in the early diffuse subset (4 vs. 2.34%). However, there is tremendous uncertainty present, on the one hand from the heterogeneity of the studies reviewed and on the other around the expected estimates calculated from the literature. Thus, it is difficult to conclude whether the doubling of rates found in this study is real or whether it would fall within the confidence intervals of the expected rates, if those were known. Also, the patients included in this review consisted largely of early, diffuse SSc patients with severe internal organ involvement, poor prognostic features and who were also treated with other potentially nephrotoxic therapies, such as total body irradiation, antithymocyte globulin, and cyclophosphamide. Thus, we cannot exclude the fact that our findings may also reflect, in part, some underlying confounding, with early SSc patients with severe organ involvement being most likely to receive CS and other co-interventions but also at greatest risk of developing SRC. On the other hand, patients included in this systematic review were possibly at lower risk of SRC, with several study protocols excluding patients with a history of prior SRC or renal abnormalities and others using “prophylactic” ACE inhibitors. Moreover, follow-up of some patients was as short as 20 days [48]. Finally, since there is no universally agreed definition, it is possible that mild or normotensive episodes of SRC may have been overlooked. These considerations would tend to have resulted in selecting low-risk patients and under-reporting of cases and, in turn, an under-estimation of the true rate of SRC in patients treated with CS. Thus, our results can be viewed as conservative estimates of the association between CS and SRC.
A limitation of this study is underscored by our relatively small sample size and number of outcomes. CS are infrequently used in SSc, and SRC is indeed rare. Moreover, the studies included in this review are highly heterogeneous, in terms of country of origin, spectrum of disease (including patients with limited and diffuse disease and different disease duration and severity), steroid regimen, and co-interventions. Thus, the association between CS and SRC remains one that is particularly difficult to evaluate [16].
On the other hand, the strength of our data is based on an exhaustive review of the literature and on data collected prospectively. EUSTAR recently published 14 recommendations for the treatment of systemic sclerosis. One of the recommendations suggested that steroids were associated with a higher risk of SRC, and thus, patients on steroids should be carefully monitored for blood pressure and renal function. However, given that this recommendation was based on retrospective studies (level 2 and 3), the strength of the recommendation was only C. Our systematic review of prospective studies now provides level 1 evidence on the subject. Thus, for now, our estimates remain the best, albeit conservative, estimates of the risk of SRC associated with CS in SSc.
Our study provides additional support for the association between CS and SRC previously reported in retrospective studies but does not eliminate the possibility that the association may be due to confounding by disease severity or by co-intervention. Thus, great caution must continue when initiating CS therapy in SSc, especially at higher doses and for the early diffuse subset of patients.
References
Varga J (2008) Systemic sclerosis: an update. Bull NYU Hosp Jt Dis 66(3):198–202
Steen V (2003) Scleroderma renal crisis. Rheum Dis Clin North Am 29:315–333
Steen V, Costantino J, Shapiro A, Medsger TJ (1990) Outcome of renal crisis in systemic sclerosis: relation to availability of angiotensin-converting-enzyme (ACE) inhibitors. Ann Intern Med 114(3):249–250
DeMarco P, Weisman M, Seibold J, Furst D, Wong W, Hurwitz E (2002) Predictors and outcomes of scleroderma renal crisis: the high-dose versus low-dose D-Penicillamine in early diffuse systemic sclerosis trial. Arthritis Rheum 46(11):2983–2989
Lunseth JH, Baker LA, Shifrin A (1951) Chronic scleroderma with acute exacerbation during corticotropin therapy; report of a case with autopsy observations. AMA Arch Intern Med 88(6):783–792
Sharnoff J, Carideo H, Stein I (1951) Cortisone-treated scleroderma. JAMA 145(16):1230–1232
Helfrich DJ, Banner B, Steen VD, Medsger TA Jr (1989) Normotensive renal failure in systemic sclerosis. Arthritis Rheum 32(9):1128–1134
Yamanishi Y, Yamana S, Ishioka S, Yamakido M (1996) Development of ischemic colitis and scleroderma renal crisis following methylprednisolone pulse therapy for progressive systemic sclerosis. Intern Med 35(7):583–586
Kohno K, Katayama T, Majima K et al (2000) A case of normotensive scleroderma renal crisis after high-dose methylprednisolone treatment. Clin Nephrol 53(6):479–482
Lee AT, Burnet SP (2002) Corticosteroid-induced scleroderma renal crisis. Med J Aust 177(8):459
Naniwa T, BAnno S, Takahashi N, Maeda S, Hayami Y, Ueda R (2005) Normotensive scleroderma renal crisis with diffuse alveolar damage after corticosteroid therapy. Mod Rheumatol 15(2):134–138
Steen VD Jr (1998) Case-control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum 41(9):1613–1619
Teixeira L, Mouthon L, Mahr A et al (2008) Mortality and risk factors of scleroderma renal crisis: a French retrospective study of 50 patients. Ann Rheum Dis 67(1):110–116
Penn H, Howie A, Kingdon E et al (2007) Scleroderma renal crisis: patient characteristics and long-term outcomes. QJM 100(8):485–494
Clements P, Furst D, Wong W, Mayes M, White B (1999) High-dose versus low-dose D-Penicillamine in early diffuse systemic sclerosis: analysis of a two-year, double-blind, randomized, controlled clinical trial. Arthritis Rheum 42(6):1194–1203
Denton C (2008) Renal manifestations of systemic sclerosis- clinical features and outcome assessment. Rheumatology 47(suppl 5):v54–v56
Tarkowski A, Andersson-Gare B, Aurell M (1993) Use of anti-thymocyte globulin in the management of refractory systemic autoimmune diseases. Scand J Rheumatol 22(6):261–266
Sharada B, Kumar A, Kakker R et al (1994) Intravenous dexamethasone pulse therapy in diffuse systemic sclerosis. A randomized placebo-controlled study. Rheumatol Int 14(3):91–94
Pai BS, Srinivas CR, Sabitha L, Shenoi SD, Balachandran CN, Acharya S (1995) Efficacy of dexamethasone pulse therapy in progressive systemic sclerosis. Int J Dermatol 34(10):726–728
Matteson EL, Shbeeb MI, McCarthy TG, Calamia KT, Mertz LE, Goronzy JJ (1996) Pilot study of antithymocyte globulin in systemic sclerosis. Arthritis Rheum 39(7):1132–1137
Davas EM, Peppas C, Maragou M, Alvanou E, Hondros D, Dantis PC (1999) Intravenous cyclophosphamide pulse therapy for the treatment of lung disease associated with scleroderma. Clin Rheumatol 18(6):455–461
Stratton RJ, Wilson H, Black CM (2001) Pilot study of anti-thymocyte globulin plus mycophenolate mofetil in recent-onset diffuse scleroderma. Rheumatology 40(1):84–88
Pakas I, Ioannidis JPA, Malagari K, Skopouli FN, Moutsopoulos HM, Vlachoyiannopoulos PG (2002) Cyclophosphamide with low or high dose prednisolone for systemic sclerosis lung disease. J Rheumatol 29(2):298–304
Giacomelli R, Valentini G, Salsano F et al (2002) Cyclophosphamide pulse regimen in the treatment of alveolitis in systemic sclerosis. J Rheumatol 29(4):731–736
Calguneri M, Apras S, Ozbalkan Z et al (2003) The efficacy of oral cyclophosphamide plus prednisolone in early diffuse systemic sclerosis. Clin Rheumatol 22(4–5):289–294
Griffiths B, Miles S, Morgan A et al (1999) Pulse intravenous methylprednisolone and cyclophosphamide is effective in treating interstitial lung disease in patients with systemic sclerosis. Arthritis Rheum 42(9):717
Apras S, Ertenli I, Ozbalkan Z et al (2003) Effects of oral cyclophosphamide and prednisolone therapy on the endothelial functions and clinical findings in patients with early diffuse systemic sclerosis. Arthritis Rheum 48(8):2256–2261
Nadashkevich O, Davis P, Fritzler M, Kovalenko W (2006) A randomized unblinded trial of cyclophosphamide versus azathioprine in the treatment of systemic sclerosis. Clin Rheumatol 25(2):205–212
Hoyles RK, Ellis RW, Wellsbury J et al (2006) A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum 3962–3970
Valentini G, Paone C, La Montagna G et al (2006) Low-dose intravenous cyclophosphamide in systemic sclerosis: an open prospective efficacy study in patients with early diffuse disease. Scand J Rheumatol 35(1):35–38
Liossis SNC, Bounas A, Andonopoulos AP (2006) Mycophenolate mofetil as first-line treatment improves clinically evident early scleroderma lung disease. Rheumatology 45(8):1005–1008
Airo P, Danieli E, Rossi M et al (2007) Intravenous cyclophosphamide for interstitial lung disease associated to systemic sclerosis: results with an 18-month long protocol including a maintenance phase. Clin Exp Rheumatol 25(2):293–296
Oyama Y, Barr WG, Statkute L et al (2007) Autologous non-myeloablative hematopoietic stem cell transplantation in patients with systemic sclerosis. Bone Marrow Transplant 40(6):549–555
Yiannopoulos G, Pastromas V, Antonopoulos I et al (2007) Combination of intravenous pulses of cyclophosphamide and methylprednizolone in patients with systemic sclerosis and interstitial lung disease. Rheumatol Int 27(4):357–361
Beretta L, Caronni M, Raimondi M et al (2007) Oral cyclophosphamide improves pulmonary function in scleroderma patients with fibrosing alveolitis: experience in one centre. Clin Rheumatol 26(2):168–172
Vanthuyne M, Blockmans D, Westhovens R et al (2007) A pilot study of mycophenolate mofetil combined to intravenous methylprednisolone pulses and oral low-dose glucocorticoids in severe early systemic sclerosis. Clin Exp Rheumatol 25(2):287–292
McSweeney PA, Nash RA, Sullivan KM, Storek J, Crofford LJ, Dansey R (2002) High-dose immunosuppressive therapy for severe systemic sclerosis: initial outcomes. Blood 100(5):1602–1610
Nash RA, McSweeney PA, Crofford LJ, Abidi M, Chen C-S (2007) High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis: long-term follow-up of the US multicenter pilot study. Blood 110(4):1388–1396
Farge D, Marolleau JP, Zohar S et al (2002) Autologous bone marrow transplantation in the treatment of refractory systemic sclerosis: early results from a French multicentre phase I-II study. Br J Haematol 119(3):726–739
Farge D, Passweg J, Van Laar JM et al (2004) Autologous stem cell transplantation in the treatment of systemic sclerosis: report from the EBMT/EULAR registry. Ann Rheum Dis 63(8):974–981
Tsukamoto H, Nagafuji K, Horiuchi T et al (2006) A phase I-II trial of autologous peripheral blood stem cell transplantation in the treatment of refractory autoimmune disease. Ann Rheum Dis 65(4):508–514
Asboe-Hansen G (1975) Treatment of generalized scleroderma with inhibitors of connective tissue formation. Acta Derm Venereol 55(6):461–465
Silver RM, Miller KS, Kinsella MB, Smith EA, Schabel SI (1990) Evaluation and management of scleroderma lung disease using bronchoalveolar lavage. Am J Med 88(5):470–476
Silver RM, Warrick JH, Kinsella MB, Staudt LS, Baumann MH, Strange C (1993) Cyclophosphamide and low-dose prednisone therapy in patients with systemic sclerosis (scleroderma) with interstitial lung disease. J Rheumatol 20(5):838–844
Becker H, Loers E, Helmke K, Federlin K (1986) Therapy of rheumatic diseases with inosiplex. Immun Infekt 14(3):93–99
Akesson A, Scheja A, Lundin A, Wollheim FA (1994) Improved pulmonary function in systemic sclerosis after treatment with cyclophosphamide. Arthritis Rheum 37(5):729–735
Behr J, Vogelmeier C, Beinert T et al (1996) Bronchoalveolar lavage for evaluation and management of scleroderma disease of the lung. Am J Respir Crit Care Med 154(2 Pt 1):400–406
Antoniades L, Sfikakis PP, Mavrikakis M (2001) Glucocorticoid effects on myocardial performance in patients with systemic sclerosis. Clin Exp Rheumatol 19(4):431–437
Takehara K (2004) Treatment of early diffuse cutaneous systemic sclerosis patients in Japan by low-dose corticosteroids for skin involvement. Clin Exp Rheumatol 22(3 Suppl 33):S87–S89
Steen V, Medsger T (2000) Long-term outcomes of scleroderma renal crisis. Ann Intern Med 133(8):600–603
Denton C, Lapadula G, Mouthon L, Muller-Ladner U (2009) Renal complications and scleroderma renal crisis. Rheumatology 48:iii32–iii35
Acknowledgments
This study was funded by an Établissement de Jeunes Chercheurs award from the Fonds de la Recherche en Santé du Québec. Dr Hudson is supported by a New Investigator Award from the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding author
Appendix: search strategy
Appendix: search strategy
Medline
Database: Ovid MEDLINE(R) <1950 to August Week 1 2009>
Search Strategy:
-
1.
Systemic sclerosis.mp. or Scleroderma, Systemic/(14470)
-
2.
Clinical Trial/(456645)
-
3.
Prospective Studies/(266598)
-
4.
(steroid* or corticosteroid* or glucocorticoid* or prednisone or prednisolone or methylprednisolone or dexamethasone).mp. [mp = title, original title, abstract, name of substance word, subject heading word] (382704)
-
5.
stem cell transplantation/or antithymocyte globulin/(20222)
-
6.
3 or 2 (662329)
-
7.
4 or 5 (400729)
-
8.
6 and 1 and 7 (50)
EMBASE
Database: EMBASE <1980 to 2009 Week 32>
Search Strategy:
-
1.
Systemic sclerosis.mp. or Scleroderma, Systemic/(7829)
-
2.
Clinical Trial/(551097)
-
3.
Prospective Studies/(84348)
-
4.
(steroid* or corticosteroid* or glucocorticoid* or prednisone or prednisolone or methylprednisolone or dexamethasone).mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (457911)
-
5.
stem cell transplantation/or antithymocyte globulin/(24097)
-
6.
3 or 2 (609254)
-
7.
4 or 5 (471731)
-
8.
6 and 1 and 7 (230)
Web of science (August 2009—125 results)
-
Topic = (scleroderma OR systemic sclerosis).
-
AND Topic = (prospective OR trial)
-
AND Topic = (steroid* OR corticosteroid* OR glucocorticoid* OR prednisone OR prednisolone OR methylprednisolone OR dexamethasone OR stem cell transplantation OR antithymocyte globulin)
-
NOT Topic = (“multiple sclerosis” OR “tuberous sclerosis” OR “nodular sclerosis” OR “nuclear sclerosis” OR “biliary sclerosis” OR “localized scleroderma”)
-
Timespan = All Years. Databases = SCI-EXPANDED, SSCI, A&HCI
The Cochrane Library (August 2009—10 results)
-
(Advanced Search)
-
“scleroderma” OR systemic sclerosis in Title, Abstract or Keywords
-
and prospective OR trial in Title, Abstract or Keywords
-
and steroid* OR corticosteroid* OR glucocorticoid* OR prednisone OR prednisolone OR methylprednisolone OR dexamethasone OR stem cell transplantation OR antithymocyte globulin in Title, Abstract or Keywords
-
not “multiple sclerosis” OR “tuberous sclerosis” OR “nodular sclerosis” OR “nuclear sclerosis” OR “biliary sclerosis” OR “localized scleroderma” in Title, Abstract or Keywords
Rights and permissions
About this article
Cite this article
Trang, G., Steele, R., Baron, M. et al. Corticosteroids and the risk of scleroderma renal crisis: a systematic review. Rheumatol Int 32, 645–653 (2012). https://doi.org/10.1007/s00296-010-1697-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-010-1697-6