Abstract
Biologic agents are increasingly being used to treat adult patients with systemic lupus erythematosus (SLE). However, the available data on biologic agents' use in childhood-onset SLE (cSLE) remains limited. To collate available evidence related to the efficacy and safety of using biologic agents in cSLE. The study followed the PRISMA checklist for reporting the data and conducted a thorough search using PubMed, Cochrane Library, and Scopus from January 2005 to August 2023. Only articles meeting specific criteria were included, focusing on cSLE, the use of biologic agents, and having outcome measures at six- and 12-month follow-ups for safety and efficacy. Case reports were excluded, and four independent reviewers screened the articles for accuracy, with a fifth reviewer resolving any discrepancies that arose to achieve a consensus. The final selection included 18 studies with a total of 593 patients treated with biologic agents for severe and/ or refractory cSLE. The most common indication for using biologic agents was lupus nephritis. Rituximab was used in 12 studies, while belimumab was used in six studies. The studies evaluated the efficacy of biologic agents based on SLE disease activity scores, laboratory parameter improvements, and reduced corticosteroid dosage. Positive outcomes were reported, with improvements in renal, hematologic, and immunologic parameters along with mild adverse effects, mostly related to mild infections and infusion reactions. Belimumab and rituximab have shown promise as potential treatments for severe and refractory cSLE cases, leading to decreased disease activity and complete or partial remission in many patients with an acceptable safety profile. However, further research is needed to better understand their benefits and potential risks in these patients.
Key Points |
• This review emphasizes the lack of sufficient randomized controlled trials exploring the use of biologics in childhood systemic lupus erythematosus (cSLE). • Treatment plans for cSLE are being derived from those used for adult systemic lupus erythematosus. • According to current evidence, belimumab and rituximab can be potential treatment options for refractory and severe cases of cSLE. • Additional studies are required to reach more definitive conclusions. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic Lupus erythematosus (SLE) is a complex autoimmune disease affecting multiple systems, characterized by a wide range of clinical and laboratory findings and an unpredictable course [1, 2]. Childhood-onset SLE (cSLE) accounts for 20% of all lupus cases and differs from adult-onset SLE (aSLE) in terms of disease severity and long-term outcomes. cSLE often presents with more severe manifestations, including lupus nephritis and hematological issues [3,4,5,6,7]. Despite advances in the management of cSLE, yet most of the available treatment is not evidence-based, it is either anecdotal reports or an expert’s opinion [8]. It is not unusual for treating physicians to consider off-label medication as therapeutic options for refractory cases of cSLE and monogenic lupus [9, 10].
Current treatments for SLE are not definitive cures but have improved patients' life expectancy and long-term outcomes. Corticosteroids and conventional disease-modifying antirheumatic drugs (cDMARDs) are commonly used immunosuppressants and immunomodulators for SLE treatment. However, they come with significant side effects, including increased infection risk and potential long-term complications like growth delay and osteoporosis.
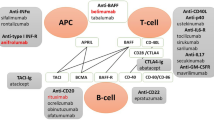
Biologic disease-modifying antirheumatic drugs (bDMARDs) offer a more targeted approach, acting on specific immunological targets such as B lymphocytes or certain cytokines [11,12,13]. The use of bDMARDs in the treatment of SLE, especially aSLE, has been increasing [14,15,16]. However, data on their efficacy and safety in cSLE are limited, which creates a gap in treating children with SLE and might further affect overall treatment outcomes. Hence, this systematic review aims to summarize available data on the use of bDMARDs in treating cSLE, providing insights into their potential benefits and risks as an alternative therapeutic option.
Methods
Search sources and strategies
With the subject terms "childhood SLE", "childhood lupus", "juvenile SLE", "early onset SLE", "monogenic lupus", "biologic agents", "biologic treatment", "rituximab", "belimumab", "tocilizumab", "anifrolumab", "eculizumab", "anti-TNF agents", "abatacept", and "JAK inhibitor", a comprehensive literature review was conducted using PubMed, Cochrane Library and Scopus from January 2005 to August 2023. Eligible studies focused on the use of bDMARDs for cSLE and reported outcome measures after at least six- and/or 12-month follow-ups. Our inclusion criteria included clinical trials, cohort studies, case–control studies, pilot studies, and case series with more than five patients. Exclusion criteria included non-English articles; metanalyses, systematic reviews, literature reviews, case reports or case series with fewer than five patients; inaccessible full texts; or insufficient outcome measures.
Reference lists of retrieved articles were checked, and citations from the electronic search were downloaded. Additionally, articles from other sources were entered manually through reference lists and hand searching. Duplicate articles were removed, and each included article was given a unique identifier (author name and year of publication) in an Excel sheet for review.
All the selected articles were subsequently analyzed using the PICO principle [17]: Patients (patients diagnosed with cSLE), Intervention (biologic agents), Comparison (Placebo or conventional treatment), Outcome (Disease response and side effects).
Screening criteria
Four reviewers independently screened the publications based on titles and then the abstracts using predefined inclusion criteria. Full-text articles were evaluated if they met the relevant criteria. The review followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 2020 checklist to minimize bias and ensure a systematic approach (Fig. 1).
Data extraction
Data from each selected study were extracted using a Google Sheet, with three sections: general information (author, publication year, country, journal), specific information (study design, sample, biologic agents used), and outcome analysis (disease activity, quality of life, symptom control, drug side effects). The Excel sheet and data extraction form were pilot tested with a small number of articles, and based on the findings, they were revised before use in the main study. Data extraction was done twice, and any discrepancies were discussed and resolved by the reviewers.
Generating results
Initially, 56 studies were identified and screened for relevance. After removing duplicates, reviews, and applying specific criteria, 18 studies were found to meet all the inclusion criteria.
Quality of evidence
The quality of evidence in the included studies was evaluated using the Jadad five-point scale for the clinical trials and the Newcastle–Ottawa scale (NOS) for non-randomized studies. The scores were reported in Table 1, and the assessment was done independently by one author.
Results
A total of 2655 publications were retrieved initially, and after screening titles and abstracts, 56 studies met the inclusion criteria, but 14 of them could not be accessed. Further eligibility checks led to the exclusion of 24 studies due to various reasons, resulting in 18 studies being included in the review. The selected studies comprised 13 retrospective, three prospective, one clinical trial, and one pilot study. Table 2 shows the characteristics of the selected publications.
All the accessible data on belimumab and rituximab consisted of retrospective cohorts and case series. Notably, only a single controlled randomized clinical study was identified, demonstrating that belimumab is well-tolerated and offers clinical benefits. It is worth noting that these studies received favorable quality scores when assessed using the NOS.
The total number of patients with cSLE in these studies was 593, with most of them being female (87%), and an average age of 14.7 years at the initiation of bDMARDs treatment either for severe and/or active disease refractory to corticosteroids and other conventional treatments or due to toxicity from conventional treatment regimens. The most common organ manifestations in the studies were renal manifestations, as shown in Fig. 2.
Rituximab was evaluated in 12 publications, and belimumab was evaluated in six, with variations in dosing regimens for rituximab, ranging from 375 mg/m2 given weekly to 750 mg/m2 given two weeks apart [17,18,19,20,21,22,23,24,25]. However, in one study, a dose of 1000 mg/1.73m2 was used, and in the other study the dose ranged from 350–450 mg/m2 [26, 27]. Belimumab was typically administered at the standard dose of 10 mg/kg every four weeks [28,29,30].
Efficacy of rituximab and belimumab
Table 3 shows the summary of results assessing the efficacy of biologic agents in cSLE patients.
Disease activity assessment following rituximab therapy
Six studies assessed rituximab’s effectiveness using the Safety of Estrogens in Lupus National Assessment-SLE Disease Activity Index (SELENA-SLEDAI) [18, 21, 24, 26, 27]. AlE’ed et al. [21], found a significant decrease at six months. Lehman et al. and Hogan et al. reported decreases at 12 and 60 months and 12 months, respectively [24, 27]. Sawhney et al. reported significant decreases at six, 12, 24, and 36 months [26]. Nwobi et al. found improvement at their follow-up endpoint [18]. Kostik et al. had an average of eight months follow-up and reported a 60% decrease in SLEADI scores by last visit [31]. Tambralli et al. used the Physician Global Assessment (PGA) score, and the authors reported a significant improvement at 12 months [22]. The British Isles Lupus Assessment Group (BILAG) global scores were used in two studies. Marks et al. reported a decline at 18 months [22], while Podolskaya et al. showed a significant improvement in the score at one, six, and 12 months [25].
Disease activity assessment following belimumab therapy
Six studies used different response parameters to assess SLE disease activity following belimumab treatment [10, 29, 30, 32,33,34]. SLE Responder Index 4 (SRI4) was used by Brunner et al. as the main outcome measure, but at week 52, there was no statistically significant difference between the belimumab and placebo groups [29]. Hui-Yuen et al. used a simplified version of SRI, reporting 65% of cSLE patients showing at least 50% improvement in clinical manifestations by six months [30]. Akbar et al.’s retrospective study included multiple response parameters and showed notable improvement in the SLEDAI score at six months for all patients [10]. Three additional studies showed improvement in SLEDAI scores by six and/or 12 months follow-up [31, 32, 35]. Only two studies showed a statistical significance in the SLEDAI scores [30, 32]. PGA was used by Akbar et al., with overall score improvement at the 6-month follow-up and at the last visit; however, timing was not specified [10]. Meanwhile, Wang et al. reported a 75% and 85% decrease in PGA at six- and 12-months follow-up [32].
Erythrocyte sedimentation rate
ESR was measured in five studies: three retrospectives [22, 25, 31]. one prospective [20] and one pilot study [24]. Tambralli et al., and Podolskaya et al. showed significant ESR improvement at 12 months [22, 25]. The prospective study demonstrated significant improvement at 6 months [20], while in the pilot study significant improvement was reported at 12 and 60 months [24]. Kostik et al. showed a 29% decrease in ESR with a significant p-value [33].
Hemoglobin
Six studies assessed bDMARDs treatment impact on hemoglobin levels [19, 20, 22,23,24,25]. All showed statistically significant increase in hemoglobin levels. Various researchers reported significant increase at various time intervals, including six, 12, 24, and 60 months [22,23,24,25]. Marks et al. also observed significant hemoglobin improvement [23]. In Kumar et al.’s study, all four patients with autoimmune hemolytic anemia achieved complete remission, with stable hemoglobin levels of more than 120 g/L for at least 12 weeks, persisting up to 24 months of follow-up [15].
Platelets
Podolskaya et al. and Marks et al. showed significant platelet count increase during follow-ups [23, 25]. Kumar et al., achieved complete remission in all five patients with autoimmune thrombocytopenia [19]. Jansson et al., reported overall platelet level improvement; but without statistical significance [20]. Additionally, Wang et al. noted a marked reduction in proportion of patients with thrombocytopenia at baseline compared to six- and 12- months follow-up [31].
Renal profile
Eight studies assessed bDMARDs’ impact on renal improvement [18, 22, 25,26,27, 29, 31]. All six studies reported a reduction in urine protein/creatinine ratio after three-12 months of biologic therapy. Akbar et al. showed a 44.6% decrease at six months and 51.2% at the last follow-up [10]. Nwobi et al. and Hogan et al. reported an 85% and 93% decrease from baseline [18, 27]. Podolskaya et al. demonstrated significant reduction at three, six and 12, and 18 months [25]. Sawhney et al. observed an 81.6% decrease at six months, sustained at12 months [26]. However, Tambralli et al. and Brunner et al. found a non-significant (21.9% and 2% respectively) decrease from baseline [22, 29]. Wang et al. and Kostik et al. measured the 24-h urine protein to evaluate renal outcome and showed a significant decrease in proteinuria by 12-month follow-up and last visit respectively [32, 33].
The change in eGFR was evaluated in three studies [18, 25, 27]. Hogan et al. reported eGFR increases of 40.3%, 36.1% and 45.3% at three, six and 12 months, respectively [27]. Nwobi et al. showed a 67.4% increase from baseline [18]. Podolskaya et al. demonstrated a 25.9% increase at one month, with significant improvements at three, six, and 12 months [25]. Kostik et al. briefly mentioned that eGFR improved following rituximab treatment, however, did not provide data to assess the degree of change [33].
Four studies assessed serum albumin levels [18, 22, 24, 25]. Lehman et al. reported increases of 21.1% and 26.4% at 12 and 60 months respectively [24]. Nwobi et al. study showed a 34.62% increase from baseline [18]. Podolskaya et al. demonstrated significant improvement at three months, sustained at six, 12, and 24- months [25]. Tambralli et al. reported a 13.5% increase at 12 months [22].
Daily corticosteroids dose
bDMARDs resulted in overall corticosteroid dose reduction [10, 18,19,20,21,22, 24,25,26,27,28, 30,31,32,33,34,35]. AlE’ed et al. and Podolskaya et al. reported significant decreases at six months, with percentage reductions of 53.3% and 60%, respectively [21, 25]. Kostik et al. showed a 60% decrease in the average corticosteroids dose over the duration of follow-up from six months to three years after the addition of rituximab [33]. Roberts et al. reported a 20% and 60% decrease in corticosteroids dose after six and 12 months of belimumab treatment [35]. Meanwhile, Wang et al. showed 60% decrease in corticosteroids dose at six months and more than 78% decrease at 12 months [31]. Nwobi et al. demonstrated a significant reduction of 83.5% [18]. Three other studies reported reductions at six months without providing statistical data [19, 31, 32]. Sawhney et al. found an 82.1% reduction at six months and 90.7% at 12 months [26]. Hogan et al. reported reduced corticosteroid dose at six and 12 months [27]. At 12 months, Lehman et al. and Tambralli et al. demonstrated statistically significant reductions by 57.2% and 72.2% from baseline, respectively [22, 24]. In Kumar et al. only one out of nine patients remained on low-dose prednisone [19]. Jansson et al. showed 75% of the sample had dose reduction by six months, and in 33% of patients, corticosteroids were discontinued [20]. Willems et al. reported that 9.1% of patients maintained a low dosage, and in 45.5% (5/11) of patients, the dose was tapered to 25% to 50% of the baseline dosage [28].
Relapse
The average percentage of patients experiencing a flare and needing an additional cycle in all studies is 18% [10, 18, 19, 21, 23,24,25,26,27,28,29,30]. Hui-yuen et al. and Roberts et al. had the lowest incidence of severe disease flare at 4% and 4.7% respectively [30, 35]. Akbar et al. reported 16.7% of patients experiencing a flare, while AlE’ed et al. reported 33.3% requiring multiple bDMARDs cycles [10, 21]. Brunner et al.’s clinical trial showed 17% of patients in the bDMARDs group experienced disease flare, compared to 35% in the placebo group [29]. Hogan et al. found that 8.3% and 33.3% needing additional bDMARDs cycles at one and six months respectively [27]. Kumar et al., Lehman et al., Marks et al., and Podolskaya et al. reported 22.2%, 16.7%, 14.3% and 10.5% of patients, respectively, experienced disease flare and needed further treatment [19, 23,24,25]. Nwobi et al.’s study indicated 27.8% experienced clinical relapse, with all responding well except one patient [18]. Sawhney et al. reported 11.8% of patients had a clinical flare and required three additional cycles [26]. Willems et al. noted 9% of their patients had a relapse; but achieved remission after the second bDMARDs course [28]. Kostik et al. reported no significant flares while Wang et al. and Wang D et al. did not report the incidence of flares [31,32,33].
Anti-dsDNA
AlE’ed A et al., Marks et al. and Tambralli et al. studies showed non-statistically significant reductions in anti-dsDNA levels [21,22,23]. Podolskaya et al. demonstrated significant reductions at one, six and 12-month follow-ups [25]. Hogan et al., Willems et al., Hui-yuen et al. and Lehman et al. reported that 41.7%, 71.4%, 50% and 50% of their sample had reduced anti-dsDNA titers [24, 27, 28, 30]. In the Nwobi et al. and Kostik et al. studies, there was a significant decrease in anti-dsDNA titers [18, 31]. Akbar et al. also showed decreased levels at six-month follow-up [10]. Brunner et al.’s bDMARDs group experienced a 44.9% decrease in anti-dsDNA levels [29]. Wang et al. noted 29.4% and 66.3% decrease in the proportion of patients with positive anti-dsDNA at six- and 12-months follow-up [31]. Wang D et al. showed a 46.6% decrease in the anti-dsDNA positive rate after six months which was sustained at 12-months follow-up [32].
Complement levels
Jansson et al. observed a 64.7% increase in C3 levels, and Hui-Yuen et al. reported a 25% increase from baseline in 18% of patients [21, 30]. Wang D et al. showed more than 58% increase in C4 levels and more than 50% in C3 levels at six- and 12-months [32]. AlE’ed et al. demonstrated 49.3% increase in C3 levels and a 54.6% increase in C4 levels [21]. Brunner et al. reported 17.3% increase in C3 levels in the bDMARDs group at week 52 compared to a 6% in the placebo group, and 50% increase in C4 levels in the bDMARDs group compared to 18.1% in the placebo group [29]. Marks et al. showed improvement in both C3 and C4 levels, but it did not achieve statistical significance [23]. Nwobi et al. demonstrated a significant increase in C3 levels but not significant in C4 levels [18]. Podolskaya et al. reported significant increase in C3 levels at one month, maintained at six- and 12-months follow-up, and significant increase in C4 levels at six- and 12-months follow-up [25]. Tambralli et al. reported significant improvement in both C3 and C4 levels at 12-months follow-up, with 63.4%-120,7% increase from baseline respectively [21]. In Willems et al. study, C3 and C4 levels were normalized in 40% of patients by the end of their follow-up period [27]. Kostik et al. showed a 23% increase in C4 levels over the duration of follow-up but did not report the change in C3 levels [33]. Lehman et al. showed twofold and 95.5% increases in C3 at 12 and 60 months [23].
Safety
Several studies reported mild and severe adverse effects in patients receiving a certain treatment. Figure 3 summarizes the adverse effects that have been reported.
Mild adverse effects were reported in ten studies, primarily related to infusion and infection [18, 19, 21, 22, 24, 27,28,29, 32, 33]. In one clinical trial, 79.2% of patients experienced mild adverse effects, while the placebo group had 82.5% [29]. However, three retrospective cohort studies and one prospective study reported no mild adverse effects [10, 20, 22, 30]. One study reported no infections nor infusion reactions except for one case of appendicitis within a week of the first dose of belimumab [35]. Severe adverse effects were reported in six studies [10, 18, 21, 29,30,31,32,33]. In a randomized clinical trial, 17% of patients had severe adverse effects compared to 35% in the placebo group [29]. One retrospective study reported 16% of patients with sepsis, while another retrospective study reported 6.25% with multiorgan failure [10, 21]. A prospective study showed 3.1% of patients had group A streptococcal bacteremia [30]. Another retrospective study reported varying percentages of patients experiencing different severe adverse effects, including rash, thrombocytopenia, septicemia, lymphopenia, and impetigo [28]. One of the retrospective studies showed two deaths secondary to macrophage activation syndrome and complicated by a severe infection [33]. That study also reported three cases of serious adverse events including pneumonia, transient agranulocytosis, and meningitis due to Listeria monocytogenes [33]. Lastly, in a different retrospective study, one patient had endocarditis, and unfortunately died after open heart surgery [18].
Discussion
The goal of treating cSLE is to control inflammation, prevent disease damage, avoid comorbidities, minimize drug-related toxicities, and improve overall well-being and development [31]. Current recommendations include systemic corticosteroids, hydroxychloroquine, and cDMARDs [8, 13, 35]. Treatment plans and recommendations for cSLE are being modeled after aSLE; most of the pediatric studies, including dosages and regimens, are performed on the basis of existing adult trials, although it is challenging to compete with them [36].
In order to ascertain the overall reported efficacy and safety of these medications, we thoroughly analyzed the literature on the use of bDMARDs, specifically belimumab and rituximab, in cSLE. It is worth mentioning that discussion of the pharmacokinetics of bDMARDs is beyond the scope of this review. Therefore, these aspects will not be highlighted here. This review indicates that there is a scarcity of reported data from randomized controlled trials that evaluate the efficacy and safety of bDMARDs in cSLE. As a result, pediatric rheumatologists have started using bDMARDs off-label for cSLE due to their successful use in various autoimmune and inflammatory diseases, particularly in aSLE. Our search demonstrated that only one pediatric clinical trial exists on the use of a biologic, belimumab, which currently stands as the sole FDA-approved biologic for treating cSLE patients [29]. The remaining studies on belimumab and rituximab were retrospective cohorts, and case series. These studies used standardized outcome measures like SLEDAI and BILAG to assess the disease activity, which are validated for cSLE and considered useful for treatment response evaluation. Monitoring disease progression and organ damage in cSLE also involved measuring lupus-related autoantibodies and complement levels. Our inclusion criteria required reporting these outcome measures after six and/or 12 months of follow-up, providing some consistency in the data, although not all patients reported data at these time points.
Our search found six studies with data on belimumab; as well as two systematic reviews on its use in cSLE [10, 16, 29, 30, 37, 38]. One of the studies included patients with monogenic lupus, a rare, hereditary form of childhood lupus that is caused by single-gene defects [10, 39]. Despite the lack of high-quality clinical trials of belimumab in cSLE, the available randomized clinical studies and observational data suggest that when used in conjunction with cDMARDs, belimumab is well-tolerated and provides clinical benefits. It helps reduce corticosteroid dosages and controls disease flare and activity, which aligns with results from aSLE [29, 40]. Overall, the results showed that long-term belimumab use for more than six months is recommended for better disease control [10, 25,26,27, 31, 32].
The efficacy of rituximab in SLE is not supported by several randomized clinical studies in aSLE [41, 42]. Despite the lack of approved use for children with cSLE, it has been used off-label. In real practice, adding rituximab to standard therapy led to more responders, with improved disease activity, delay renal flares, and remarkable improvement in renal and hematologic parameters. Additionally, rituximab treatment successfully reduced daily corticosteroid dosages in most studies [21, 24,25,26,27,28, 43,44,45].
Both rituximab and belimumab have demonstrated a favorable safety profile in cSLE. The most frequently reported adverse events were mild infusion reactions and infections [19, 28, 46].
Recently, in 2021 a new biologic, anifrolumab, has been approved. Despite its novelty and proven efficacy, it has been tested on aSLE patients only. According to the search done, it had not been tested on cSLE yet [47]. Similarly, studies emerged that showed efficacy with combining both rituximab and belimumab for severe aSLE but not cSLE [48]. This further emphasizes the need for future clinical trials tailored for children with SLE.
Limitations
This review has certain limitations, primarily stemming from the inconsistencies in the data extracted from the included studies. Statistical analysis was not possible due to the variations in outcome measures and follow-up intervals. Additionally, some studies included patients with other autoimmune diseases, and one study involved both adult and pediatric patients, further adding to the complexity of the analysis. Moreover, another limitation to our study encompassed the fact that the study was not registered in the International Prospective Register of Systematic Reviews.
Conclusion
In summary, treatment plans and recommendations for cSLE are being based on those used for aSLE. However, cSLE exhibit more severe manifestations and organ involvement compared to aSLE. The data on the efficacy and safety of bDMARDs in cSLE remains limited. Nevertheless, current evidence suggests that belimumab and rituximab, can be considered as treatment options for refractory and severe cases of cSLE. substantial efficacy noticed after months of using the biologic treatments. Overall, disease activity decreased, and many patients achieved either complete or partial remission with a reasonable safety profile. Nonetheless, further studies are needed to draw more definitive conclusions, especially in patients with major organ involvement.
References
Harry O, Yasin S, Brunner H (2018) Childhood-onset systemic lupus erythematosus: a review and update. J Pediatr 196:22-30.e2. https://doi.org/10.1016/j.jpeds.2018.01.045
Pan L, Lu M, Wang J, Xu M, Yang S (2020) Immunological pathogenesis and treatment of systemic lupus erythematosus. World J Pediatr 16:19–30. https://doi.org/10.1007/s12519-019-00229-3
Morgan T, Watson L, McCann L, Beresford M (2013) Children and adolescents with SLE: not just little adults. Lupus 22:1309–1319
Hedrich C, Smith E, Beresford M (2017) Juvenile-onset systemic lupus erythematosus (jSLE)- Pathophysiological concepts and treatments options. Best Pract Res Clin Rheumatol 31:488–504
Bundhun P, Kumari A (2017) Huang F (2017) Differences in clinical features observed between childhood-onset versus adult-onset systemic lupus erythematosus: A systematic review and meta-analysis. Medicine (Baltimore) 96(37):e8086. https://doi.org/10.1097/MD.0000000000008086
Rodriguez-Smith J, Brunner H (2019) Update on the treatment and outcome of systemic lupus erythematous in children. Curr Opin Rheumatol 31(5):464–470. https://doi.org/10.1097/BOR.0000000000000621
Wenderfer S, Ruth N, Brunner H (2017) Advances in the care of children with lupus nephritis. Pediatr Res 81(3):406–414
Smith E, Sen E, Pain C (2019) Diagnosis and treatment of childhood-onset systemic lupus erythematosus (European evidence-based recommendations from the SHARE initiative). Arch Dis Child Educ Pract Ed 104(5):259–264. https://doi.org/10.1136/archdischild-2017-314049
Lei L, Muhammad S, Al-Obaidi M, Sebire N, Cheng I, Eleftheriou D et al (2018) Successful use of ofatumumab in two cases of early-onset juvenile SLE with thrombocytopenia caused by a mutation in protein kinase C δ. Pediatr Rheumatol Online J 16(1):61. https://doi.org/10.1186/s12969-018-0278-1
Akbar L, Alsagheir R, Al-Mayouf SM (2020) Efficacy of a sequential treatment by belimumab in monogenic systemic lupus erythematosus. Eur J Rheumatol 7(4):184–189. https://doi.org/10.5152/eurjrheum.2020.20087
Bernal C, Zamora L, Navarra S (2015) Biologic therapies in systemic lupus erythematosus. Int J Rheum Dis 18:146–153
Murphy G, Isenberg D (2019) New therapies for systemic lupus erythematosus - past imperfect, future tense. Nat Rev Rheumatol 15(7):403–412. https://doi.org/10.1038/s41584-019-0235-5
Trindade V, Carneiro-Sampaio M, Bonfa E, Silva C (2021) An update on the management of childhood-onset systemic lupus erythematosus. Paediatr Drugs 23(4):331–347
Gatto M, Zen M, Iaccarino L, Doria A (2019) New therapeutic strategies in systemic lupus erythematosus management. Nat Rev Rheumatol 15(1):30–48. https://doi.org/10.1038/s41584-018-0133-2
Samotij D, Reich A (2019) Biologics in the treatment of lupus erythematosus: a critical literature review. Biomed Res Int 2019:8142368. https://doi.org/10.1155/2019/8142368
Peterknecht E, Keasey M, Beresford M (2018) The effectiveness and safety of biological therapeutics in juvenile-onset systemic lupus erythematosus (JSLE): a systematic review. Lupus 27(13):2135–2145. https://doi.org/10.1177/0961203318804879
Richardson W, Wislon M, Nishikawa J, Hayward R (1995) The well-built clinical question: a key to evidence-based decisions. ACP J Club 123(3):A12-13
Nwobi O, Abitbol CL, Chandar J, Seeherunvong W, Zilleruelo G (2008) Rituximab therapy for juvenile-onset systemic lupus erythematosus. Pediatr Nephrol 23(3):413–419
Kumar S, Benseler SM, Kirby-Allen M, Silverman E (2009) B-cell depletion for autoimmune thrombocytopenia and autoimmune hemolytic anemia in pediatric systemic lupus erythematosus. Pediatrics 123(1):e159–e163
Jansson A, Sengler C, Kuemmerle-Deschner J, Gruhn B, Kranz A, Lehmann H et al (2011) B cell depletion for autoimmune diseases in paediatric patients. Clin Rheumatol 30(1):87–97
AlE’ed A, AlSonbul A, Al-Mayouf SM (2014) Safety and efficacy of combined cyclophosphamide and rituximab treatment in recalcitrant childhood lupus. Rheumatol Int 34(4):529–533
Tambralli A, Beukelman T, Cron R, Stoll M (2015) Safety and efficacy of rituximab in childhood-onset systemic lupus erythematosus and other rheumatic diseases. J Rheumatol 42(3):541–546
Marks S, Patey S, Brogan P, Hasson N, Pilkington C, Woo P et al (2005) B lymphocyte depletion therapy in children with refractory systemic lupus erythematosus. Arthritis Rheum 52(10):3168–3174
Lehman T, Singh C, Ramanathan A, Alperin R, Adams A, Barinstein L et al (2014) Prolonged improvement of childhood onset systemic lupus erythematosus following systematic administration of rituximab and cyclophosphamide. Pediatr Rheumatol 12(1):3
Podolskaya A, Stadermann M, Pilkington C, Marks S, Tullus K (2008) B cell depletion therapy for 19 patients with refractory systemic lupus erythematosus. Arch Dis Child 93(5):401–406
Sawhney S, Agarwal M (2021) Rituximab use in pediatric systemic lupus erythematosus: indications, efficacy and safety in an Indian cohort. Lupus 30(11):1829–1836
Hogan J, Godron A, Baudouin V, Kwon T, Harambat J, Deschênes G et al (2018) Combination therapy of rituximab and mycophenolate mofetil in childhood lupus nephritis. Pediatr Nephrol 33(1):111–116
Willems M, Haddad E, Niaudet P, Koné-Paut I, Bensman A, Cochat P et al (2006) Rituximab therapy for childhood-onset systemic lupus erythematosus. J Pediatr 148(5):623-627.e3
Brunner H, Abud-Mendoza C, Viola D, Calvo Penades I, Levy D, Anton J et al (2020) Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial. Ann Rheum Dis 79(10):1340–1348
Hui-Yuen J, Reddy A, Taylor J, Li X, Eichenfield A, Bermudez L et al (2015) Safety and efficacy of Belimumab to treat systemic lupus erythematosus in academic clinical practices. J Rheumatol 42(12):2288–2295
Wang L, Liang X, Cao Z, Wang D, Luo Y, Feng Y et al (2023) Evaluation of belimumab in treatment of Chinese childhood-onset systemic lupus erythematosus: a prospective analysis from multicenter study. Rheumatology (Oxford) 22:kead406
Wang D, Shan C, Liu J, Zhang R, Zhu G, Gao T et al (2022) Efficacy and safety of belimumab for the treatment of refractory childhood-onset systemic lupus erythematosus: a single-center, real-world, retrospective study. Front Immunol 13:1067721
Kostik M, Kalashnikova E, Rinat R, Isupova EE, Gaidar E, Soloviev A et al (2023) Rituximab biosimilar BCD20 shows superior efficiacy above conventional non-biologics treatment in pediatric nephritis: the data of retrospective cohort study. Biomedicines 11(5):1503
Luijten K, Tekstra J, Bijlsma J, Bijl M (2012) The Systemic Lupus Erythematosus Responder Index (SRI); a new SLE disease activity assessment. Autoimmun Rev 11(5):326–329
Roberts J, Burn C, Sadun R, Smitherman E, Wenderfer S, Son M (2023) Real-world use and outcomes of belimumab in childhood-onset lupus: a single-center retrospective study. Lupus 32(9):1111–1116
Chalhoub N, Wenderfer S, Levy D, Rouster-Stevens K, Aggarwal A, Savani S et al (2022) International consensus for the dosing of corticosteroids in childhood-onset systemic lupus erythematosus with proliferative lupus nephritis. Arthritis Rheumatol 74(2):263–273. https://doi.org/10.1002/art.41930
Athanassiou P, Athanassiou L (2023) Current treatment approach, emerging therapies and new horizons in systemic lupus erythematosus. Life (Basel) 13(7):1496. https://doi.org/10.3390/life13071496
Chen F, Zheng Y, Chen X, Wen Z, Xu Y, Yang J, Xu K (2022) Belimumab in childhood systemic lupus erythematosus: a review of available data. Front Immunol 13:940416. https://doi.org/10.3389/fimmu.2022.940416
Navarra S, Guzmán R, Gallacher A, Hall S, Levy R, Jimenez R et al (2011) Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377(9767):721–731. https://doi.org/10.1016/S0140-6736(10)61354-2
Al-Mayouf SM, Akbar L, Abdwani R, Ginesi G, Volpi S, Gattorno M et al (2022) Performance of the EULAR/ACR 2019 classification criteria for systemic lupus erythematous in monogenic lupus. Clin Rheumatol 41(9):2721–2727. https://doi.org/10.1007/s10067-022-06209-9
Levy R, Gonzalez-Rivera T, Khamashta M, Fox N, Jones-Leone A, Rubin B et al (2021) 10 Years of belimumab experience: What have we learnt? Lupus 30(11):1705–1721. https://doi.org/10.1177/09612033211028653
Rovin B, Furie R, Latinis K, Looney R, Fervenza F, Sanchez-Guerrero J et al (2012) Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 64(4):1215–1226. https://doi.org/10.1002/art.34359
Merrill J, Neuwelt C, Wallace D, Shanahan J, Latinis K, Oates J et al (2010) Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 62(1):222–233. https://doi.org/10.1002/art.27233
Olfat M, Silverman E, Levy D (2015) Rituximab therapy has a rapid and durable response for refractory cytopenia in childhood-onset systemic lupus erythematosus. Lupus 24(9):966–972. https://doi.org/10.1177/0961203315578764
Watson L, Beresford M, Maynes C, Pilkington C, Marks D, Glackin Y et al (2015) The indications, efficacy and adverse events of rituximab in a large cohort of patients with juvenile-onset SLE. Lupus 24(1):10–17. https://doi.org/10.1177/0961203314547793
Mahmoud I, Jellouli M, Boukhris I, Charfi R, Ben Tekaya A, Saidane O et al (2017) Efficacy and safety of rituximab in the management of pediatric systemic lupus erythematosus: a systematic review. J Pediatr 187:213-219.e2. https://doi.org/10.1016/j.jpeds.2017.05.002
Ahmed A, Osman N, Furie R (2022) An evaluation of anifrolumab for use in adults with systemic lupus erythematosus. Expert Rev Clin Immunol 18(11):1095–1106
van Schaik M, Arends E, Soonawala D, van Ommen E, de Leeuw K, Limper M et al (2022) Efficacy of belimumab combined with rituximab in severe systemic lupus erythematosus: study protocol for the phase 3, multicenter, randomized, open-label Synbiose 2 trial. Trials 23(1):939
Funding
This study was not funded by any agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conduct of this manuscript. The search, data extraction and manuscript writing were all divided among the authors. All co-authors take full responsibility for the integrity and accuracy of all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
Ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethics committee of the Research Affairs Council at king Faisal Specialist Hospital & Research Center approved the study protocol. Also, Ethical approval was also obtained by ethics committee of the collaborative centers.
Conflicts of interest/competing interest
All authors declare that that they have neither competing financial nor non-financial interests in relation to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elshaer, R., Jaber, S., Odeh, N. et al. Safety and efficacy of biologics in childhood systemic lupus erythematosus: a critical systematic review. Clin Rheumatol 43, 863–877 (2024). https://doi.org/10.1007/s10067-023-06833-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-023-06833-z