Abstract
To clarify the clinical significance of anti-cyclic citrullinated peptide antibody (anti-CCP) in the long-term outcome of RA, we established a large observational cohort of RA patients (IORRA) in our institute beginning in 2000. Essentially all RA patients who consulted our institute were registered, and clinical parameters, including disease activity and drug use, were assessed biannually based on patient reports, physician examinations, and laboratory data. In the third phase (October 2001) of the IORRA survey, anti-CCP levels were measured in 1,226 RA patients. In a cross-sectional analysis, clinical variables were compared in anti-CCP-positive versus -negative patients and in RF-positive versus -negative patients. In a longitudinal analysis, subsequent progression of disability was analyzed in anti-CCP-positive versus -negative and in RF-positive versus -negative patients. A verified Japanese version of the Health Assessment Questionnaire (J-HAQ) was used to measure functional disability. In the cross-sectional analysis, anti-CCP-positive patients (84.2%) had a significantly longer disease duration and higher disease activity score and more frequently used corticosteroids and methotrexate compared to anti-CCP-negative patients statistically. Similar phenomena were noted between RF-positive and -negative patients. In contrast, the longitudinal analysis revealed that J-HAQ slopes—a measure of progression of functional disability—were strongly associated with anti-CCP positivity but not with RF positivity. In a linear regression model, J-HAQ scores significantly worsened in anti-CCP-positive patients compared to anti-CCP-negative patients at the third year (annual progression 0.0317, P = 0.001) and the fifth year (annual progression 0.0199, P = 0.0012); however, J-HAQ progression was not influenced by RF status. Anti-CCP is a better predictive and discriminative marker for progression of disability in the long-term outcome of RA patients compared to RF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The autoantibodies rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibody (anti-CCP) are used as serological markers in rheumatoid arthritis (RA) [1]. RF was the first autoantibody to be detected in RA patients, and it has been widely used in screening tests for patients with arthritis. The RF test is the only laboratory test used in the RA classification criteria of the American College of Rheumatology [2]. However, the specificity of RF for RA is not sufficient [3], although it has been recognized as a predictor of more severe structural joint damage [4–6].
Anti-perinuclear factor (APF) and anti-keratin antibody (AKA) have been described as filaggrin-reactive antibodies [7]. APF and AKA can be detected prior to the clinical onset of disease and are considered good diagnostic markers for RA [8, 9]. Schellekens et al. have shown that APF and AKA appear to recognize a similar epitope consisting of a citrullinated peptide [10, 11]. During the development of an enzyme-linked immunosorbent assay (ELISA) using a cyclic citrullinated peptide (CCP), the detected antibody was designated as anti-CCP [12]. Since anti-CCP is highly specific for the diagnosis of RA, particularly for early disease [1, 13], anti-CCP measurement has become widely used in regular RA clinical practice. Many reports have clarified that anti-CCP is associated with the susceptibility and severity of RA [5, 14–16]; however, the association between anti-CCP and long-term functional outcome of RA patients has not been well elucidated.
To clarify the clinical significance of anti-CCP and RF as predictive factors for long-term physical disability in RA patients, we analyzed the IORRA database, a prospective observational cohort study of Japanese RA patients.
Patients and methods
We have established a prospective observational cohort of RA patients who have been treated at the Institute of Rheumatology, Tokyo Women’s Medical University, since October 2000, designated as the IORRA (Institute of Rheumatology, Rheumatoid Arthritis) cohort. This cohort has been previously described in detail [17–20]. Briefly, all RA patients diagnosed using ACR criteria [21] were registered after informed consent was obtained, and their clinical information was collected biannually (in April and in October) when they visited the outpatient clinic. Clinical information consisted of three major components: (1) physician evaluations, which included the number of tender joints and swollen joints and visual analog scale (VAS) score for disease activity (physician-VAS); (2) patient evaluations and information, which included VAS for pain (pain-VAS), VAS for general health (global-VAS), physical disability level using the Japanese version of the Health Assessment Questionnaire (J-HAQ) [22], height, body weight, comorbidities in the last 6 months, and drugs taken during the period; patients were instructed by the attending physician to answer these questions at home and mail responses back in a pre-stamped envelope within 2 weeks after their visit; and (3) patient laboratory data, including C-reactive protein (CRP) level, erythrocyte sedimentation rate (ESR), RF titer, MMP-3, blood cell counts, transaminase levels, creatinine level, cholesterol level, and urinalysis results. Data collected from each component were integrated into one database for analysis. The disease activity score (DAS28) was calculated according to the original method [23].
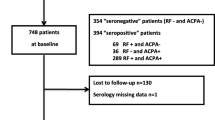
Anti-CCP was measured in 1,226 RA patients randomly selected from 4,338 patients who participated in the third phase (October 2001) of the IORRA survey. Anti-CCP positivity was tested by ELISA (second-generation MESACUP CCP test, MBL, Aichi, Japan) with a cutoff point of 4.5 U/ml.
Statistical analysis
Baseline patient characteristics such as gender, age, and concomitant drugs used were summarized using frequency or median and inter-quartile range (IQR). To cross-sectionally compare the demographic features between anti-CCP-positive and -negative RA patients and between RF-positive and -negative patients, Fisher’s exact test for categorical variables and the Wilcoxon test for continuous variables were performed. To examine the longitudinal progression of J-HAQ scores, we used two statistical models. The first model was a 2-stage model that compared the group difference of each patient’s J-HAQ slope in the anti-CCP-positive group and the anti-CCP-negative group, as well as in the RF-positive group and the RF-negative group. The J-HAQ slope for each patient was calculated using a standard linear regression model without any covariate adjustment, and the group difference of the J-HAQ slope was compared by the ANCOVA model with adjustment. Covariate adjustments were made for gender, age, RA duration, body mass index (BMI), DAS28, J-HAQ, non-steroidal anti-inflammatory drugs’ (NSAIDs) use, corticosteroid use, methotrexate (MTX) use, bucillamine (BUC) use, and salazosulfapyridine (SASP) use. The second longitudinal model was the linear mixed model. Random effect was the intercept, and the functional form of the time trend was assumed to be linear. Differences between anti-CCP-positive and -negative patients and between RF-positive and -negative patients were tested by the interaction of group effect and time. Covariate adjustments were made for the same variables as in the 2-stage model. In this analysis, we transformed J-HAQ to log(J-HAQ+1) due to non-normality. Subgroup analyses using the linear mixed model were performed for MTX users, MTX non-users, corticosteroid users, and corticosteroid non-users, respectively. Statistical significance was set at 0.05, and all reported P-values were not adjusted for multiplicity. All calculations were done by R version 2.9.1 and SAS version 9.2.
Results
Patient characteristics
The baseline characteristics of the 1,226 RA patients are shown in Table 1. Women comprised 81.6% (n = 1,000) of the patient population, the median (IQR) age of patients was 59 (52–67) years, and the mean (IQR) disease duration was 10 (4–16) years. A total of 1,032 (84.2%) patients were anti-CCP-positive and 908 (74.1%) were RF-positive. The median (IQR) J-HAQ score at baseline was 0.6 (0.1–1.3).
Demographic features by anti-CCP or RF status
Differences in clinical features between anti-CCP-positive and -negative RA patients identified using a univariate analysis are shown in Table 2. A similar analysis was performed in RF-positive versus -negative patients (Table 2). Compared to anti-CCP-negative patients, anti-CCP-positive patients had a significantly longer disease duration, higher CRP level, higher ESR, higher MMP-3 level, higher RF positivity (81.6%), higher DAS28, and higher J-HAQ score. Similar phenomena were observed in RF-positive versus RF-negative patients. In RF-positive patients, the prevalence of anti-CCP positivity (92.7%) was quite high. The frequencies of corticosteroid use and MTX use were higher in patients who were anti-CCP- or RF-positive compared to patients who were anti-CCP- or RF-negative.
Predictive factors for progression of physical disability
Trends in J-HAQ score over the 5 years after baseline (the third phase) without any adjustment are shown in Fig. 1. Figure 1 showed the trend in J-HAQ. Although J-HAQ scores increased constantly in RA patients regardless of anti-CCP or RF status, baseline J-HAQ scores in patients who were anti-CCP- or RF-positive were significantly higher than those in patients who were anti-CCP- or RF-negative. Differences in J-HAQ scores in patients who were anti-CCP-positive versus -negative were much higher than in those who were RF-positive versus -negative; thus, anti-CCP appears to be more discriminative than RF with respect to J-HAQ progression.
We next compared the differences in progressive J-HAQ scores between RA patients who were anti-CCP-positive and -negative, as well as between those who were RF-positive and -negative, using statistical models. Using the 2-stage model, J-HAQ slopes were associated with anti-CCP positivity, and annual J-HAQ progression was 0.0317 (P = 0.001) at the third year and 0.0199 (P = 0.012) at the fifth year after adjustment of several factors, while J-HAQ slopes were not associated with RF status, even following adjustment of several factors (Table 3). These results indicate that anti-CCP-positive patients are most likely to be disabled compared to anti-CCP-negative patients at the third year and the fifth year after baseline (the third phase), whereas RF status does not have an impact on disability.
Using the linear mixed model, the trend of log(J-HAQ+1) was significantly worse in anti-CCP-positive patients compared to anti-CCP-negative patients (P = 0.032). However, no differences in log(J-HAQ+1) were identified between RF-positive and -negative patients (P = 0.971) (Table 4). This result indicates that anti-CCP is a better marker of subsequent progression of physical disability than RF. In a similar analysis conducted in RA patients treated with MTX, neither anti-CCP nor RF status was associated with subsequent progression of disability; however, in patients who did not receive MTX, anti-CCP positivity, but not RF positivity, was indicative of subsequent progression of disability (P = 0.012). In contrast, in patients treated with corticosteroids, anti-CCP, but not RF, predicted progression of disability (P = 0.0193), while neither anti-CCP nor RF status was associated with disability progression in patients who did not receive corticosteroids.
Discussion
The objective of our study was to investigate the clinical significance of anti-CCP and RF, particularly with respect to the prediction of progression of physical disability. Using J-HAQ score as a marker of physical disability, we successfully demonstrated that anti-CCP status is superior to RF status as a predictive marker for long-term disability in RA patients.
Many reports have demonstrated that anti-CCP-positive RA patients have higher disease activity and have disease that is more progressive with respect to joint destruction compared to anti-CCP-negative RA patients [16, 24, 25]. Among these studies, Kastbom A et al. demonstrated that DMARDs were more frequently prescribed to anti-CCP-positive RA patients than to anti-CCP-negative patients [26]. Our data also demonstrated that anti-CCP-positive RA patients have significantly higher disease activity and worse disability and are more frequently treated with DMARDs, particularly MTX, compared to anti-CCP-negative RA patients. It is noteworthy that anti-CCP was measured in a situation blinded to attending rheumatologists; thus, anti-CCP data did not influence treatment decisions in this study. We believe that DMARDs were prescribed significantly more frequently to anti-CCP-positive patients due to the higher disease activity in these patients. RF is a widely accepted, established predictive marker of RA outcome [27, 28]. RF-positive patients were also found to have significantly higher disease activity and worse disability and were more likely to have received MTX compared to RF-negative patients.
In this report, we analyzed whether anti-CCP and RF could predict subsequent functional disability in a longitudinal analysis. Using linear regression analysis, we successfully demonstrated that anti-CCP positivity was a significant indicator of disability at the third year and at the fifth year after baseline (the third phase), whereas RF status was not. In the mixed-effect model, J-HAQ scores significantly progressed in anti-CCP-positive patients compared to anti-CCP-negative patients, but a similar phenomenon was not identified between RF-positive and -negative patients. These results indicate that subsequent progression of disability can be predicted by anti-CCP positivity but not by RF positivity. Despite several reports that have shown that anti-CCP-positive patients experienced more severe disease activity than anti-CCP-negative patients, many studies have failed to demonstrate a relationship between anti-CCP and HAQ scores, even in cross-sectional analysis [29], with the exception of one analysis conducted by Inanc N et al. [24]. To date, no other longitudinal analyses have demonstrated a relationship between anti-CCP and progression of functional disability [26, 29, 30], presumably due to a lack of statistical power in such analyses, since the percentage of anti-CCP-negative patients is relatively small, particularly in the long-standing RA population. By taking advantage of the available data from a large number of patients who comprise the IORRA cohort, we believe that the present report provides important evidence about the significance of anti-CCP in the long-term outcome of RA patients.
Our subgroup analyses suggested that MTX use, but not corticosteroid use, could potentially overcome disability progression in anti-CCP-positive RA patients compared to anti-CCP-negative patients. These results strongly indicate that even in anti-CCP-positive RA patients who are likely to be more disabled, progression of physical disability may be prevented by active treatment with MTX, but not with corticosteroids. It is notable that this study was conducted before the frequent use of biologics; thus, further analysis of the ability of biologics to prevent long-term disability in anti-CCP-positive RA patients is required.
In conclusion, we have demonstrated that anti-CCP-positive RA patients tend to have more severe disease and a worse prognosis with respect to functional disability compared to anti-CCP-negative patients. Moreover, the poor prognosis of anti-CCP-positive patients may be improved by treatment with MTX, but not with corticosteroids. Thus, treatment of anti-CCP-positive RA patients must adhere to stricter guidelines to prevent worse functional outcomes of this chronic progressive disease.
References
Lee AN, Beck CE, Hall M (2008) Rheumatoid factor and anti-CCP autoantibodies in rheumatoid arthritis: a review. Clin Lab Sci 21:15–18
Vander Cruyssen B, Peene I, Cantaert T et al (2005) Anti-citrullinated protein/peptide antibodies (ACPA) in rheumatoid arthritis: specificity and relation with rheumatoid factor. Autoimmun Rev 4:468–474
Nijenhuis S, Zendman AJ, Vossenaar ER et al (2004) Autoantibodies to citrullinated proteins in rheumatoid arthritis: clinical performance and biochemical aspects of an RA-specific marker. Clin Chim Acta 350:17–34
Möttönen T, Paimela L, Leirisalo-Repo M et al (1998) Only high disease activity and positive rheumatoid factor indicate poor prognosis in patients with early rheumatoid arthritis treated with “sawtooth” strategy. Ann Rheum Dis 57:533–539
Guler H, Turhanoglu AD, Ozer B et al (2008) The relationship between anti-cyclic citrullinated peptide and bone mineral density and radiographic damage in patients with rheumatoid arthritis. Scand J Rheumatol 37:337–342
Combe B, Eliaou JF, Daurès JP et al (1995) Prognostic factors in rheumatoid arthritis. Comparative study of two subsets of patients according to severity of articular damage. Br J Rheumatol 34:529–534
Sebbag M, Simon M, Vincent C et al (1995) The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies. J Clin Invest 95:2672–2679
Young BJ, Mallya RK, Leslie RD et al (1979) Anti-keratin antibodies in rheumatoid arthritis. Br Med J 2:97–99
Gan SQ, McBride OW, Idler WW et al (1990) Organization, structure, and polymorphisms of the human profilaggrin gene. Biochemistry 29:9432–9440
Schellekens GA, de Jong BA, van den Hoogen FH et al (1998) Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest 101:273–281
Girbal-Neuhauser E, Durieux JJ, Arnaud M et al (1999) The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol 162:585–594
Schellekens GA, Visser H, de Jong BA et al (2000) The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum 43:155–163
Meyer O, Labarre C, Dougados M et al (2003) Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis 62:120–126
Vallbracht I, Rieber J, Oppermann M et al (2004) Diagnostic and clinical value of anti-cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Ann Rheum Dis 63:1079–1084
Kim SK, Park SH, Shin IH et al (2008) Anti-cyclic citrullinated peptide antibody, smoking, alcohol consumption, and disease duration as risk factors for extraarticular manifestations in Korean patients with rheumatoid arthritis. J Rheumatol 35:995–1001
del Val del Amo N, Ibanez Bosch R, Fito Manteca C et al (2006) Anti-cyclic citrullinated peptide antibody in rheumatoid arthritis: relation with disease aggressiveness. Clin Exp Rheumatol 24:281–286
Tanaka E, Mannalithara A, Inoue E et al (2008) Efficient management of rheumatoid arthritis significantly reduces long-term functional disability. Ann Rheum Dis 67:1153–1158
Yamanaka H, Inoue E, Singh G et al (2007) Improvement of disease activity of rheumatoid arthritis patients from 2000 to 2006 in a large observational cohort study IORRA in Japan. Mod Rheumatol 17:283–289
Iikuni N, Sato E, Hoshi M et al (2009) The influence of sex on patients with rheumatoid arthritis in a large observational cohort. J Rheumatol 36:508–511
Momohara S, Inoue E, Ikari K et al (2008) Risk factors for wrist surgery in rheumatoid arthritis. Clin Rheumatol 27:1387–1391
Arnett FC, Edworthy SM, Bloch DA et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Matsuda Y, Singh G, Yamanaka H et al (2003) Validation of a Japanese version of the stanford health assessment questionnaire in 3,763 patients with rheumatoid arthritis. Arthritis Rheum 49:784–788
Prevoo ML, van ‘t Hof MA, Kuper HH et al (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38:44–48
Inanc N, Dalkilic E, Kamali S et al (2007) Anti-CCP antibodies in rheumatoid arthritis and psoriatic arthritis. Clin Rheumatol 26:17–23
Forslind K, Ahlmén M, Eberhardt K et al (2004) Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibodies to citrullinated peptides (anti-CCP). Ann Rheum Dis 63:1090–1095
Kastbom A, Strandberg G, Lindroos A et al (2004) Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis 63:1085–1089
Nell VP, Machold KP, Stamm TA et al (2005) Autoantibody profiling as early diagnostic and prognostic tool for rheumatoid arthritis. Ann Rheum Dis 64:1731–1736
Scott DL, Symmons DP, Coulton BL et al (1987) Long-term outcome of treating rheumatoid arthritis: results after 20 years. Lancet 1:1108–1111
van der Helm-van Mil AH, Verpoort KN, Breedveld FC et al (2005) Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther 7:R949–R958
Kroot EJ, de Jong BA, van Leeuwen MA et al (2000) The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 43:1831–1835
Acknowledgments
The IORRA cohort was supported by non-restricted research grants from 34 pharmaceutical companies; Abbott Japan Co., Ltd., Asahikasei Kuraray Medical Co., Ltd., Asahikasei Pharma Corporation, Astellas Pharma Inc., AstraZeneca K. K., Banyu Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Fine Chemical Co., Ltd., Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Eisai Co., Ltd., GlaxoSmithKline K. K., Janssen Pharmaceutical K. K., Japan Tobacco Inc., Kaken Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Mitsubishi Chemical Medience Corporation, Mitsuibishi Tanabe Pharma Corporation, Nippon Chemiphar Co., Ltd., Nippon Shinyaku Co., Ltd., Novartis Pharma K. K., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Sanofi-aventis K. K, Santen Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Sekisui Medical Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, Teijin Pharma Limited, Torii Pharmaceutical Co., Ltd., UCB Japan Co., Ltd., Wyeth K. K. and Zeria Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shidara, K., Inoue, E., Hoshi, D. et al. Anti-cyclic citrullinated peptide antibody predicts functional disability in patients with rheumatoid arthritis in a large prospective observational cohort in Japan. Rheumatol Int 32, 361–366 (2012). https://doi.org/10.1007/s00296-010-1671-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-010-1671-3