Abstract
Introduction
Antibody against cyclic citrullinated protein (ACPA) is counted as one of the most important biomarkers in diagnosis, classification, and prognosis of rheumatoid arthritis (RA). We examined the evolution of ACPA during disease course and assess predictive value of time-weighted cumulative ACPA titer on radiographic progression in RA patients.
Method
A group of 734 patients with RA was followed longitudinally over 2 years, with annual measurements of ACPA. The cumulative titers of ACPA were calculated using the trapezoidal rule and were divided into three categories: negative, low-to-moderate, and high. Radiographs of the hands were scored with the modified Sharp score (SHS). Multivariable logistic regression models were performed to identify independent predictors over follow-up for individual patients with different combinations of risk factors. The effect size was computed by Cohen’s d method.
Results
The patients with radiographic progression had a higher SHS at baseline; and smoking status, diabetes, RF positivity, and use of biologic DMARDs were independently associated with radiographic progression (all P < 0.05). As for ACPA, reversion happened more commonly in men and was associated with younger onset age and lower titer at baseline, but it had no direct relevance to radiographic outcome. In multivariable regression analysis, only high cumulative or baseline titer of ACPA had a predictive power for rapid radiographic progression (all P < 0.05), and cumulative ACPA titer was superior in terms of statistical significance (Cohen’s d, 0.637 versus 0.583).
Conclusions
High cumulative ACPA titer was independently associated with accelerated radiographic progression, especially with initiation of joint damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease characterized by synovial inflammation and hyperplasia, autoantibody production, and joint destruction, which can eventually lead to structural and functional impairments and a decrease in the quality of life [1]. Antibodies against cyclic citrullinated protein (ACPAs), the key components of RA classification criteria [2], are found in more than 70% of patients with RA and appear several months or years before the clinical onset of RA [3, 4]. They not only show an excellent diagnostic performance of 98% specificity and 68% sensitivity [5] but also have a predictable power for radiographic progression and bone erosions [6,7,8,9,10]. It is noteworthy that a high titer of ACPA has been associated with more severe radiographic progression [7, 11]. Several provocative lines of evidences support the ACPA’s direct involvement in the pathogenesis of disease and joint destruction, as well as its role as a biomarker [12,13,14]. Administration of ACPAs into mice with experimental arthritis significantly enhanced inflammation when mild synovitis was already present [12], and this was mediated partly by specifically binding cartilage via cross-recognition of a flexible epitope on type II collagen that mimicked the citrulline-containing structure [13]. Clinically, B cell-depleting therapy using rituximab has proven to be more effective, particularly in patients with RA carrying the ACPA [15]. Based on these findings, it is assumed that the concentration of ACPA and duration of exposure to it would have a substantial impact on the development and progress of the disease.
Levels of ACPA can fluctuate with the course of disease, and seroconversion (from negative to positive) or seroreversion (from positive to negative) is observed on occasion in a subset of patients with RA in clinical practice. However, the significance of this change remains controversial [16,17,18,19,20,21,22,23,24,25,26]. Decrease of ACPA titer correlated with clinical response after treatment with methotrexate or tumor necrosis factor (TNF) inhibitors such as etanercept, adalimumab, and infliximab in several studies [16,17,18,19, 26], but not in others [20, 21]. Inconsistent results might be ascribed partly to differences in the study design (prospective or retrospective), characteristics of study subjects (size, ethnicity, ages, gender ratio, disease activity and duration, comorbidity), treatment agents and duration, interval between baseline and follow-up, and type of antibody assay.
Evolution of ACPA titer and its cumulative effect in terms of exposure time to radiographic progression is not well defined. To tackle these questions, we examined annual measurements of titers of IgM rheumatoid factor (RF) and ACPA in a group of RA patients and examined the changes of antibody levels over time. We also calculated the time-weighted cumulative volume of antibody and investigated its association with radiographic progression.
Patients and methods
Patients
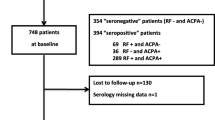
A total of 1208 RA patients who fulfilled the 2010 RA classification criteria [2] and had received care at St. Vincent’s Hospital, the Catholic University of Korea (Suwon, Republic of Korea) between 2003 and 2017 were identified. Clinical, laboratory data and radiographic images were retrieved from the medical records. Of these, 756 had X-rays of the hands available for scoring from baseline and over 2 years of follow-up, and 962 had annual records of RF and ACPA test over a 2-year period, which were aimed at inspecting the change of antibodies during the treatment. A total of 734 had both criteria and were enrolled in this study. The study group was maximally followed up to 175 months. The study was carried out in accordance with the Helsinki Declaration and approved by the Institutional Review Board of St. Vincent’s Hospital, the Catholic University of Korea (No. VC18RESI0136).
Assay of RA-associated antibodies
ACPA was analyzed by chemiluminescent microparticle immunoassay (Abbott Laboratories, IL, USA), and a positive reading was defined with a cutoff value of 5 U/mL. The antibody concentration maximum was defined as 340 U/mL, and for statistical purposes, the value of 340 U/mL was assigned to all measurements > 340 U/mL. ACPA was divided into three categories: < 5 U/mL (negative), 5–200 U/mL (low to moderate level), and > 200 U/mL (high level) in conformity with a previous study [7]. RF titers were measured with a latex agglutination test (Beckman Coulter, CA, USA) with a cutoff value of 14 U/mL. RF levels were also divided into three categories: < 14 U/mL (negative), 14–100 U/mL (low to moderate level), and > 100 U/mL (high level) in conformity with a previous study [9]. The evolution of antibodies between baseline and follow-up visits was stratified into four trajectories: remain negative, remain positive, conversion (negative at baseline to positive ever once during follow-up), and reversion (positive at baseline to negative ever once during follow-up).
Calculation of time-weighted cumulative volume of antibodies
The cumulative volume of antibodies was calculated using the trapezoidal rule [27]. If we have measurements y1 and y2 at times t1 and t2, then the area under the curve (AUC) between those two times is the product of the time difference and the average of the two measurements. If we have n + 1 measurements yi at times t (i = 0, …, n), then the AUC is calculated as:
The unit of time difference was set to months, and the integrated amount of antibody was further adjusted by total duration of follow-up (months) to facilitate comparison with the baseline value.
Radiographic evaluation
Anteroposterior radiographs of the hands were scored by two experienced readers by van der Heijde modified Sharp score (SHS) [28]. The films were scored in chronological order, and the readers were blinded for all patient data. The potential maximum total score for both hands is 280 (16 areas scored for erosions [score 0–5] and 15 areas for joint space narrowing [score 0–4] in each hand). A change in the hand SHS at a rate of > 1 unit/year during a follow-up period over 2 years was regarded as radiographic progression in conformity with a previous study [7]. The interobserver reliability was assessed by calculating the intraclass correlation coefficient, and it was 0.860 (95% confidence interval [CI], 0.779 to 0.922).
Statistical analyses
For continuous distributed data, the results are shown as means with standard deviation (SD) or medians with interquartile ranges (IQRs); between-group comparisons were performed using the Student’s t test or Mann–Whitney U test. Categorical or dichotomous variables are expressed as frequencies and percentages and were compared using the chi-squared test or Fisher’s exact test. Multivariable logistic regression models were performed to identify independent predictors and to calculate the probability of having radiographic progression over follow-up for individual patients with different combinations of risk factors. The independent variables were selected from univariate analyses if their P value was < 0.10. The effect size was computed by Cohen’s d method [29]. Radiographic outcome in the three antibody level categories was depicted as a cumulative probability plot. A two-sided P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using R (version 3.5.0, The R Project for Statistical Computing, www.r-project.org).
Results
Characteristics of the study population
The baseline characteristics of the study subjects (n = 734) and separate baseline values for patients with and without radiographic progression are given in Table 1. The median age of the patients was 52 [44, 61] years and 82.7% (n = 607) were female. The patients were followed up for a median of 8 [5, 10] years. At baseline, 87.3% (n = 641) were seropositive for RF and 89.0% (n = 653) for ACPA, respectively. At baseline 34.2% (n = 251) had erosive disease, and after follow-up 52.0% (n = 382). The median SHS at baseline was 0 [0, 10] and 3 [0, 15] at follow-up. Radiographic progression developed in 20.3% (n = 149) of patients (> 1 unit/year). The median yearly progression rate (SHS/follow-up period) was 0.14 [0.0, 0.75] unit/years.
As compared with the patients without radiographic progression, the patients with radiographic progression had a higher SHS at baseline (P < 0.001), higher levels of ESR and CRP (all P < 0.001), and a longer disease duration (P = 0.020). Taking into account all of the DMARDs prescribed during the follow-up period, biologic DMARDs were more often used in patients with radiographic progression (52.3% versus 24.3%, P < 0.001). There was a higher proportion of patients with RF positivity at baseline among the patients with radiographic progression (P = 0.021), while there was no difference in the proportion of patients with ACPA positivity between the two groups (89.3% versus 88.9%, P = 1.000). Among patients with radiographic progression, the frequency of smoking was lower, and the rate of diabetes was higher by only a marginal significance (P = 0.052 and P = 0.067, respectively).
Cumulative titer and evolution of RF and ACPA
RF and ACPA were measured on average 6.67 ± 3.60 SD times per person at a mean interval of 11.26 ± 5.20 SD months. The variation of RF and ACPA titer over time divided into three categories at baseline, and along with radiographic progression, were plotted in Fig. 1. The time-adjusted cumulative titer of antibodies was calculated using the trapezoidal rule, taking into account the values of serial measurement and intervals between time points [27], and is summarized in Table 2. The time-adjusted cumulative titer of IgM and ACPA did not differ in the distribution of values or category assignment (negative, low to moderate, and high) between the two groups (all P > 0.05) (Table 2). The cumulative ACPA titer was strongly correlated with the baseline ACPA titer (γ = 0.792, P < 0.001).
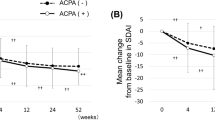
Changes of IgM RF and ACPA titer over the follow-up period. Plots were separated by baseline three-level categories and radiographic progression status. Each point is the titer at a specific time point and is linked by a single patient. A group of the linked points reflects the transition of RF or ACPA in a single patient. a Variation of IgM RF titer. The value was imposed a ceiling on 1000 IU/ml for better visualization. b Variation of ACPA titer. ACPA was assayed up to 340 U/mL at maximum
In terms of RF evolution, most of RF conversion and reversion occurred in low-to-moderate or negative RF titer groups (26.8% versus 0.4%, P < 0.001). Conversion was less frequent, and reversion was more frequent in patients with radiographic progression, but this difference failed to attain statistical significance (P = 0.056). No discernible difference was found in the evolution of ACPA between the two groups (P = 0.623). However, on the whole, ACPA positivity or negativity changed little (84.2% versus 72.8%) and conversion or reversion was relatively rare (15.8% versus 27.2%) as compared with RF (P < 0.001 for both). As for ACPA, reversion (from positive at baseline to negative during follow-up) (n = 96) happened more commonly in men (26.0% versus 16.9%, P = 0.045), and at a younger age of onset (51[44, 57] versus 53 [46, 62] years, P = 0.048), and lower titer at baseline (24.1[11.6, 96.0] versus 100.0[49.3, 154.3] U/mL, P < 0.001) as compared with the “remain positive” subgroup (n = 557). In 2.7% (n = 20) of patients, ACPA was absent at baseline but appeared during follow up and the titer was variable, from low to high (range 5.3–340 U/mL). Conversion of ACPA took place more frequently in progressors as compared with non-progressors (37.5% [6/16] versus 21.5% [14/65]), but the difference was statistically insignificant (P = 0.316).
Independent predictors of radiographic progression
Baseline radiographic score, erythrocyte sedimentation rate (ESR) or C-reactive protein level, presence of RF and ACPA, smoking status, and body mass index (BMI) are known to have predictive power for radiographic progression in RA [7, 30,31,32]. In order to identify the predictive factor for radiographic progression in the present study population, we performed multivariable logistic regression model analysis, including the baseline variables with a P < 0.1 in addition to known predictable factors (Table 3). Smoking status, diabetes, positive RF, and use of biologic DMARDs are independently associated with radiographic progression (all P < 0.05). Use of biologic DMARDs had the strongest association (OR [95% CI] 3.39 [2.29, 5.01], P < 0.001), followed by the presence of RF (OR [95% CI] 2.75 [1.29, 5.85], P = 0.009). In principle, biologic DMARDs were used when methotrexate-based double or triple combinations of DMARDs over 6 months did not effectively control the patient’s disease activity in this study group. BMI and baseline ESR were marginally associated with radiographic progression (all P < 0.1), and positive ACPA was not an independent factor (P = 0.507). Titer and stratified subgroups by levels of ACPA did not attain the statistical significance as an independent variable (data not shown).
Independent factor for radiographic progression rate
Next, in order to identify the factors for severity of radiographic progression in progressors (n = 149), we performed a multivariable linear regression model analysis on the progression rate of SHS (units/year) separately adjusted for baseline or cumulative RF and ACPA (Table 4). Only high baseline or cumulative titer of ACPA was significantly associated with the radiographic progression rate (all P < 0.05), and the effect size of cumulative titer was stronger than that of baseline titer (Cohen’s d, 0.637 versus 0.583). The radiographic progression rate with high titer of ACPA was significantly more rapid than rate with negative or low-to-moderate titer (all P < 0.05) (Fig. 2a). The change in SHS over time in the three ACPA level categories is depicted in a probability plot (Fig. 2b).
Discussion
Joint damage and the resultant deformation accounts for a substantial number of cases of disability and poor quality of life in RA, in both the earlier and established phases of the disease [33, 34]. The importance of identifying the predictive factor for rapid radiographic progression, and that of early and aggressive treatment of the high-risk patients, can hardly be emphasized enough. In this study, we showed that a high cumulative titer of ACPA was closely associated with more rapid radiographic progression, although it was not significantly different between the progressors and non-progressors. However, RF status at baseline was helpful for predicting radiographic progression. In a subset of patients, conversion or reversion of RF and ACPA was observed during the follow-up period, but the change had no direct relationship with the radiographic outcome.
In this study, use of biologic DMARDs, RF positivity, smoking status, and diabetes were independent predictors of radiographic progression. The patients who received biologic DMARDs were regarded as having had a higher disease activity or having suffered severe disease for a considerable time because they showed inadequate response at least over 6 months to methotrexate-based DMARDs combination treatment. The longitudinal level of disease activity is reported to have a close relationship with radiographic progression [35, 36]. The relationship of RF with radiographic progression was confirmed in a series of studies [9, 30, 35, 37]. However, the clinical usefulness of RF in making an accurate estimate of radiographic progression might be weak because the proportion of patients with positive RF was greater than 85% both in progressors and non-progressors. Index event bias may explain the paradoxical negative association between smoking on radiographic progression (OR 0.47, P = 0.015) [38]. Index event bias means that all recurrent risk analyses are prone to a particular bias that leads to such paradoxical results. It has been reported that development of diabetes is associated with poor clinical response in RA [39]. Contrary to previous studies [6,7,8,9, 11, 30], ACPA status at baseline did not differ significantly between progressors and non-progressors. The rate of positive ACPA in this study group was 89.0%, which is remarkably higher compared with that of the RA population in previous studies (50–70%). This establishes the study subjects as having true RA, but the high disproportion between patients with and without ACPA, specifically, less effective contribution by a small proportion of ACPA-negative patients, probably would dilute their differential effect on radiographic progression according to ACPA status. Recent trend in early and aggressive use of biologic DMARDs before discernible joint damage begins might be instrumental to this result as well.
However, we corroborated that high levels of ACPA are related to accelerated radiographic progression, especially in patients whose joint damage had just begun. A higher SHS at baseline in progressors might be ascribed partly to longer disease duration (Table 1), but this confounding effect was adjusted by multivariable analysis (Table 3). This finding is in good agreement with the experimental observation that administration of ACPA promoted the development and severity of inflammation in mice when submaximal arthritis was instigated, although the antibody administration alone was insufficient for development of the arthritis [12]. However, in a recent study by Jonsson et al., radiographic progression was not associated with baseline levels or changes of ACPA reactivity in DMARD-naïve early RA patients [40]. As in our result, ACPA positivity did not have an independent predictive power for radiographic progression. Taken together, high titer of ACPA and the presence of baseline joint damage are the more highly weighted factors than ACPA positivity itself. Some RA patients develop radiographic progression despite the achievement of remission or low disease activity [41]. In a recent study by Koga et al., ACPA positivity was also the strongest independent predictor of clinically relevant radiographic progression in those patients [42]. Cumulative ACPA titer was a good marker for the effect, because dose and exposure time are important for a toxic substance to have a pathogenic effect. However, the baseline ACPA titer has a close correlation with the cumulative ACPA titer and was not so inferior for predicting the radiographic progression. This is probably because conversion and reversion are relatively rare, and with a small width of variation [23, 26].
One clinical question is whether seroconversion or seroreversion can take place, and their clinical implications. Guzian et al. reported that conversion and reversion of RF and ACPA blurred the prognostic significance of initial RF and ACPA positivity, and anti-Sa predicted severe outcome even if it disappeared afterward [23]. Kastbom et al. suggested that early disappearance of a particular subtype of ACPAs may be associated with better radiological outcomes [24]. We confirmed that seroconversion or seroreversion takes place in a small proportion of patients; and in particular, reversion of ACPA is more common in men and is associated with a younger onset age and low titer at baseline, which is consistent with the previous result [23]. However, it had no direct relation with radiographic outcome. It is difficult to claim that disappearance of ACPA, especially at a certain time point, is associated with clinical response or radiographic outcome because it can fluctuate over time irrespective of treatment response and cannot represent rheumatoid inflammation on its own [22, 24, 43]. It is noteworthy that a small proportion of patients (2.7%) were seronegative for ACPA at baseline and converted to seropositive status later. It would be interesting to investigate the difference is between the consistently seronegative and converted RA patients in terms of clinical features, treatment choice, and radiographic prognosis, and whether subsequent emergence of ACPA can predict radiographic progression similar to pre-existing ACPA at baseline, although we failed to verify it due to the small sample size.
There are some limitations to be addressed in this study. First, radiographic images evaluations and antibody assays were prospectively executed, but data were retrospectively collected. Retrospective data collection is inherently susceptible to bias, including both misclassification, information, and selection bias. However, we thoroughly reviewed the fulfillment of RA classification criteria, and images and antibody records are objective data without bias. High frequency of seropositivity might be ascribed to selection bias, but this facilitated the comparison between the ACPA subgroups by titer. Second, we did not analyze the association with the disease activity index because records of disease activity index were incomplete and were mainly from patients receiving biologic DMARDs. Third, the maximal limits of ACPA measurement was set to 340 U/mL, so that accurate estimate of cumulative titer were not possible. Fourth, radiographs of the feet were not available for all patients.
The discovery of the ACPA over the last decades has greatly enhanced our understanding of the pathogenesis of RA and has changed the paradigm of diagnosis and classification in RA [4]. Serial measurement of ACPA can provide information about its dynamics over the clinical course of RA and can facilitate additional estimates of radiographic progression. However, detailed clinical implications largely remain undefined. Further investigation and research may allow refinement in our understanding of the ACPA in RA and its beneficial application in clinical practice.
References
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, Yamamoto K (2018) Rheumatoid arthritis. Nat Rev Dis Primers 4:18001. https://doi.org/10.1038/nrdp.2018.1
Neogi T, Aletaha D, Silman AJ, Naden RL, Felson DT, Aggarwal R, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Khanna D, Kvien TK, Laing T, Liao K, Mease P, Menard HA, Moreland LW, Nair R, Pincus T, Ringold S, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G (2010) The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: Phase 2 methodological report. Arthritis Rheum 62:2582–2591. https://doi.org/10.1002/art.27580
Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA (2004) Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 50:380–386. https://doi.org/10.1002/art.20018
van Venrooij WJ, van Beers JJ, Pruijn GJ (2011) Anti-CCP antibodies: the past, the present and the future. Nat Rev Rheumatol 7:391–398. https://doi.org/10.1038/nrrheum.2011.76
Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, van Venrooij WJ (2000) The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum 43:155–163. https://doi.org/10.1002/1529-0131(200001)43:1<155::Aid-anr20>3.0.Co;2-3
Kroot EJ, de Jong BA, van Leeuwen MA, Swinkels H, van den Hoogen FH, van't Hof M, van de Putte LB, van Rijswijk MH, van Venrooij WJ, van Riel PL (2000) The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 43:1831–1835. https://doi.org/10.1002/1529-0131(200008)43:8<1831::Aid-anr19>3.0.Co;2-6
Syversen SW, Gaarder PI, Goll GL, Odegard S, Haavardsholm EA, Mowinckel P, van der Heijde D, Landewe R, Kvien TK (2008) High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis 67:212–217. https://doi.org/10.1136/ard.2006.068247
Hetland ML, Stengaard-Pedersen K, Junker P, Ostergaard M, Ejbjerg BJ, Jacobsen S, Lottenburger T, Hansen I, Tarp U, Andersen LS, Svendsen A, Pedersen JK, Lauridsen UB, Ellingsen T, Lindegaard H, Podenphant J, Vestergaard A, Jurik AG, Horslev-Petersen K (2010) Radiographic progression and remission rates in early rheumatoid arthritis - MRI bone oedema and anti-CCP predicted radiographic progression in the 5-year extension of the double-blind randomised CIMESTRA trial. Ann Rheum Dis 69:1789–1795. https://doi.org/10.1136/ard.2009.125534
Hecht C, Englbrecht M, Rech J, Schmidt S, Araujo E, Engelke K, Finzel S, Schett G (2015) Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA. Ann Rheum Dis 74:2151–2156. https://doi.org/10.1136/annrheumdis-2014-205428
Jilani AA, Mackworth-Young CG (2015) The role of citrullinated protein antibodies in predicting erosive disease in rheumatoid arthritis: a systematic literature review and meta-analysis. Int J Rheumatol 2015:728610–728618. https://doi.org/10.1155/2015/728610
Berglin E, Johansson T, Sundin U, Jidell E, Wadell G, Hallmans G, Rantapaa-Dahlqvist S (2006) Radiological outcome in rheumatoid arthritis is predicted by presence of antibodies against cyclic citrullinated peptide before and at disease onset, and by IgA-RF at disease onset. Ann Rheum Dis 65:453–458. https://doi.org/10.1136/ard.2005.041376
Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, Holers VM (2006) Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest 116:961–973. https://doi.org/10.1172/jci25422
Ge C, Tong D, Liang B, Lonnblom E, Schneider N, Hagert C, Viljanen J, Ayoglu B, Stawikowska R, Nilsson P, Fields GB, Skogh T, Kastbom A, Kihlberg J, Burkhardt H, Dobritzsch D, Holmdahl R (2017) Anti-citrullinated protein antibodies cause arthritis by cross-reactivity to joint cartilage. JCI Insight 2. https://doi.org/10.1172/jci.insight.93688
England BR, Thiele GM, Mikuls TR (2017) Anticitrullinated protein antibodies: origin and role in the pathogenesis of rheumatoid arthritis. Curr Opin Rheumatol 29:57–64. https://doi.org/10.1097/bor.0000000000000356
Chatzidionysiou K, Lie E, Nasonov E, Lukina G, Hetland ML, Tarp U, Gabay C, van Riel PL, Nordstrom DC, Gomez-Reino J, Pavelka K, Tomsic M, Kvien TK, van Vollenhoven RF (2011) Highest clinical effectiveness of rituximab in autoantibody-positive patients with rheumatoid arthritis and in those for whom no more than one previous TNF antagonist has failed: pooled data from 10 European registries. Ann Rheum Dis 70:1575–1580. https://doi.org/10.1136/ard.2010.148759
Alessandri C, Bombardieri M, Papa N, Cinquini M, Magrini L, Tincani A, Valesini G (2004) Decrease of anti-cyclic citrullinated peptide antibodies and rheumatoid factor following anti-TNFalpha therapy (infliximab) in rheumatoid arthritis is associated with clinical improvement. Ann Rheum Dis 63:1218–1221. https://doi.org/10.1136/ard.2003.014647
Atzeni F, Sarzi-Puttini P, Dell’ Acqua D, de Portu S, Cecchini G, Cruini C, Carrabba M, Meroni PL (2006) Adalimumab clinical efficacy is associated with rheumatoid factor and anti-cyclic citrullinated peptide antibody titer reduction: a one-year prospective study. Arthritis Res Ther 8:R3. https://doi.org/10.1186/ar1851
Bos WH, Bartelds GM, Wolbink GJ, de Koning MH, van de Stadt RJ, van Schaardenburg D, Dijkmans BA, Nurmohamed MT (2008) Differential response of the rheumatoid factor and anticitrullinated protein antibodies during adalimumab treatment in patients with rheumatoid arthritis. J Rheumatol 35:1972–1977
Chen HA, Lin KC, Chen CH, Liao HT, Wang HP, Chang HN, Tsai CY, Chou CT (2006) The effect of etanercept on anti-cyclic citrullinated peptide antibodies and rheumatoid factor in patients with rheumatoid arthritis. Ann Rheum Dis 65:35–39. https://doi.org/10.1136/ard.2005.038851
Iannone F, Tampoia M, Giannini M, Lopalco G, Cantarini L, Villalta CD, Galeazzi M, Lapadula G (2016) Changes in anti-cyclic citrullinated peptide antibodies and rheumatoid factor isotypes serum levels in patients with rheumatoid arthritis following treatment with different biological drugs. Clin Exp Rheumatol 34:424–429
Bruns A, Nicaise-Roland P, Hayem G, Palazzo E, Dieude P, Grootenboer-Mignot S, Chollet-Martin S, Meyer O (2009) Prospective cohort study of effects of infliximab on rheumatoid factor, anti-cyclic citrullinated peptide antibodies and antinuclear antibodies in patients with long-standing rheumatoid arthritis. Joint Bone Spine 76:248–253. https://doi.org/10.1016/j.jbspin.2008.09.010
Mikuls TR, O'Dell JR, Stoner JA, Parrish LA, Arend WP, Norris JM, Holers VM (2004) Association of rheumatoid arthritis treatment response and disease duration with declines in serum levels of IgM rheumatoid factor and anti-cyclic citrullinated peptide antibody. Arthritis Rheum 50:3776–3782. https://doi.org/10.1002/art.20659
Guzian MC, Carrier N, Cossette P, de Brum-Fernandes AJ, Liang P, Menard HA, Boire G (2010) Outcomes in recent-onset inflammatory polyarthritis differ according to initial titers, persistence over time, and specificity of the autoantibodies. Arthritis Care Res 62:1624–1632. https://doi.org/10.1002/acr.20288
Kastbom A, Forslind K, Ernestam S, Geborek P, Karlsson JA, Petersson IF, Saevarsdottir S, Klareskog L, van Vollenhoven RF, Lundberg K (2016) Changes in the anticitrullinated peptide antibody response in relation to therapeutic outcome in early rheumatoid arthritis: results from the SWEFOT trial. Ann Rheum Dis 75:356–361. https://doi.org/10.1136/annrheumdis-2014-205698
Cambridge G, Leandro MJ, Edwards JC, Ehrenstein MR, Salden M, Bodman-Smith M, Webster AD (2003) Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum 48:2146–2154. https://doi.org/10.1002/art.11181
Bohler C, Radner H, Smolen JS, Aletaha D (2013) Serological changes in the course of traditional and biological disease modifying therapy of rheumatoid arthritis. Ann Rheum Dis 72:241–244. https://doi.org/10.1136/annrheumdis-2012-202297
Matthews JN, Altman DG, Campbell MJ, Royston P (1990) Analysis of serial measurements in medical research. BMJ 300:230–235
van der Heijde D (2000) How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 27:261–263
Lee DK (2016) Alternatives to P value: confidence interval and effect size. Korean J Anesthesiol 69:555–562. https://doi.org/10.4097/kjae.2016.69.6.555
Courvoisier N, Dougados M, Cantagrel A, Goupille P, Meyer O, Sibilia J, Daures JP, Combe B (2008) Prognostic factors of 10-year radiographic outcome in early rheumatoid arthritis: a prospective study. Arthritis Res Ther 10:R106. https://doi.org/10.1186/ar2498
Joo YB, Bang SY, Ryu JA, Lee S, Lee HS, Bae SC (2017) Predictors of severe radiographic progression in patients with early rheumatoid arthritis: a prospective observational cohort study. Int J Rheum Dis 20:1437–1446. https://doi.org/10.1111/1756-185x.13054
Rydell E, Forslind K, Nilsson JA, Jacobsson LTH, Turesson C (2018) Smoking, body mass index, disease activity, and the risk of rapid radiographic progression in patients with early rheumatoid arthritis. Arthritis Res Ther 20:82. https://doi.org/10.1186/s13075-018-1575-2
Odegard S, Landewe R, van der Heijde D, Kvien TK, Mowinckel P, Uhlig T (2006) Association of early radiographic damage with impaired physical function in rheumatoid arthritis: a ten-year, longitudinal observational study in 238 patients. Arthritis Rheum 54:68–75. https://doi.org/10.1002/art.21548
Scott DL, Pugner K, Kaarela K, Doyle DV, Woolf A, Holmes J, Hieke K (2000) The links between joint damage and disability in rheumatoid arthritis. Rheumatology (Oxford) 39:122–132
Welsing PM, Landewe RB, van Riel PL, Boers M, van Gestel AM, van der Linden S, Swinkels HL, van der Heijde DM (2004) The relationship between disease activity and radiologic progression in patients with rheumatoid arthritis: a longitudinal analysis. Arthritis Rheum 50:2082–2093. https://doi.org/10.1002/art.20350
Tsuji H, Yano K, Furu M, Yamakawa N, Ikari K, Hashimoto M, Ito H, Fujii T, Yamamoto W, Ohmura K, Taniguchi A, Momohara S, Matsuda F, Allaart CF, Yamanaka H, Mimori T, Terao C (2017) Time-averaged disease activity fits better joint destruction in rheumatoid arthritis. Sci Rep 7:5856. https://doi.org/10.1038/s41598-017-05581-w
Aletaha D, Alasti F, Smolen JS (2013) Rheumatoid factor determines structural progression of rheumatoid arthritis dependent and independent of disease activity. Ann Rheum Dis 72:875–880. https://doi.org/10.1136/annrheumdis-2012-201517
Choi HK, Nguyen US, Niu J, Danaei G, Zhang Y (2014) Selection bias in rheumatic disease research. Nat Rev Rheumatol 10:403–412. https://doi.org/10.1038/nrrheum.2014.36
Ruscitti P, Ursini F, Cipriani P, Liakouli V, Carubbi F, Berardicurti O, De Sarro G, Giacomelli R (2017) Poor clinical response in rheumatoid arthritis is the main risk factor for diabetes development in the short-term: a 1-year, single-Centre, longitudinal study. PLoS One 12:e0181203. https://doi.org/10.1371/journal.pone.0181203
Jonsson MK, Hensvold AH, Hansson M, Aga AB, Sexton J, Mathsson-Alm L, Cornillet M, Serre G, Lillegraven S, Fevang BS, Catrina AI, Haavardsholm EA (2018) The role of anti-citrullinated protein antibody reactivities in an inception cohort of patients with rheumatoid arthritis receiving treat-to-target therapy. Arthritis Res Ther 20:146. https://doi.org/10.1186/s13075-018-1635-7
Cohen G, Gossec L, Dougados M, Cantagrel A, Goupille P, Daures JP, Rincheval N, Combe B (2007) Radiological damage in patients with rheumatoid arthritis on sustained remission. Ann Rheum Dis 66:358–363. https://doi.org/10.1136/ard.2006.057497
Koga T, Okada A, Fukuda T, Hidaka T, Ishii T, Ueki Y, Kodera T, Nakashima M, Takahashi Y, Honda S, Horai Y, Watanabe R, Okuno H, Aramaki T, Izumiyama T, Takai O, Miyashita T, Sato S, Kawashiri SY, Iwamoto N, Ichinose K, Tamai M, Origuchi T, Nakamura H, Aoyagi K, Eguchi K, Kawakami A (2017) Anti-citrullinated peptide antibodies are the strongest predictor of clinically relevant radiographic progression in rheumatoid arthritis patients achieving remission or low disease activity: a post hoc analysis of a nationwide cohort in Japan. PLoS One 12:e0175281. https://doi.org/10.1371/journal.pone.0175281
Ronnelid J, Wick MC, Lampa J, Lindblad S, Nordmark B, Klareskog L, van Vollenhoven RF (2005) Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis 64:1744–1749. https://doi.org/10.1136/ard.2004.033571
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was carried out in accordance with the Helsinki Declaration and approved by the Institutional Review Board of St. Vincent’s Hospital, the Catholic University of Korea (No. VC18RESI0136)
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joo, Y.B., Park, YJ., Park, KS. et al. Association of cumulative anti-cyclic citrullinated protein antibodies with radiographic progression in patients with rheumatoid arthritis. Clin Rheumatol 38, 2423–2432 (2019). https://doi.org/10.1007/s10067-019-04554-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04554-w