Abstract
We have investigated the role of the Th17-related cytokines interleukin-17A (IL-17A), IL-21, and IL-23 in spondyloarthritis (SpA) by examining their association with disease activity and magnetic resonance imaging (MRI) findings in patients with SpA (n = 80). Furthermore, to investigate the cellular origins of the cytokines, paired mononuclear cells from blood and synovial fluid were examined for the expression of IL-17A, IL-21, and IL-23R using multicolor flow cytometry. Both IL-21 and IL-23 levels were increased in plasma from SpA patients compared with healthy volunteers (P < 0.05), whereas IL-17A was not. A significant correlation was observed between individual levels of IL-21 and IL-23 (r = 0.7, P < 0.001). No association between individual levels of IL-17A, IL-21, and IL-23 with C-reactive protein (CRP), MRI changes, and clinical scoring (BASMI, BASFI, and BASDAI) were observed. The frequency of CD4+CD45RO+ T cells expressing IL-21 and IL-23R was increased in the inflamed SpA joint compared to peripheral blood (P < 0.05). This study demonstrate that the plasma levels of the Th17-related cytokines IL-21 and IL-23, but not IL-17A, are increased in SpA patients, but we did not find evidence that the level of these cytokines reflect disease activity in SpA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spondyloarthritides (SpA) assemble a heterogeneous group of related chronic inflammatory diseases, including ankylosing spondylitis, psoriatic arthritis, reactive arthritis, enteropathic arthritis, and undifferentiated seronegative arthritis [1]. The etiology of SpA is unknown, but different genetic factors have been shown to be associated with the pathogenesis, including polymorphisms in the gene encoding the interleukin-23 receptor (IL-23R) [2]. Furthermore, the newly recognized subset of IL-17A producing CD4+ T cells (Th17), which have been associated with a wide range of chronic inflammatory diseases including SpA [3], have the capacity to produce IL-17A, IL-17F, and IL-22 and are reported to express surface IL-23R [4].
The cytokines IL-21 and IL-23 are important factors in the development and expansion of the Th17 cell subset. IL-21, together with transforming growth factor β (TGF-β), induces the development of human naïve CD4+ T cells into Th17 cells, while both IL-21 and IL-23 function by sustaining and expanding the Th17 cells [5, 6]. IL-21 is produced by activated CD4+ T cells and is reported to be produced in high amounts by the Th17 subset, although this association has lately been questioned [5, 6]. The IL-21 receptor (IL-21R) is constitutively expressed on resting B-, NK-, and T cells and is potently upregulated on T cells upon T-cell receptor activation [7]. IL-21 stimulates proliferation and activation of NK cells, T cells, and B cells and can induce the production of various pro-inflammatory cytokines including IL-21 itself [8, 9]. Its ability to initiate the development of Th17 cells makes it particularly interesting in chronic inflammatory diseases like SpA. IL-23 is a heterodimeric protein, consisting of a unique p19 subunit and a p40 subunit shared with IL-12 [10]. The major source of IL-23 is activated antigen-presenting cells including dendritic cells [10]. The IL-23 receptor complex is composed of a subunit shared with IL-12, IL-12Rβ1, and a specific subunit, IL-23R [11]. Cells expressing IL-23R are primarily of the CD45RO+ memory T-cell subset, while only a limited number of naïve CD45RA+ T cells express IL-23R [4]. IL-23 seems to occupy a central role in SpA. This is supported by the presence of IL-23 in serum and increased amounts of Th17 cells and IL-23R-positive cells in serum of patients with these diseases. Moreover, recent studies report beneficial effects of anti-IL-23 treatment of psoriatic skin and joint disease [12, 13].
The purpose of this study was to investigate the levels of the Th17-related cytokines IL-21, IL-23, and IL-17A in a large group of SpA patients with axial involvement and their possible association with disease activity measured by relevant clinical and biochemical parameters [14] in addition to disease activity and chronicity measured by magnetic resonance imaging (MRI). Finally, to investigate IL-17A, IL-21, and IL-23R expression on CD4+ T cells from peripheral blood mononuclear cells (PBMC) and synovial fluid mononuclear cells (SFMC) from SpA patients.
Materials and methods
Collection of samples
Plasma from 80 SpA patients with symptoms restricted to the axial skeleton was obtained, together with clinical and radiological measurements. The patients all met the European Spondyloarthropathy Study Group (ESSG) criteria, and 48 patients met the modified New York criteria for ankylosing spondylitis (Table 1) [1, 15]. The clinical scorings comprise Bath Ankylosing Spondylitis Metrology Index (BASMI), Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), physicians’ global score on a 100-mm visual analog scale (VAS), and C-reactive protein (CRP). The radiological examinations included radiography and MRI of the SIJ and spine [16, 17]. Plasma from age- and gender-matched healthy volunteers (HV) (n = 35) served as controls (Table 1). All plasma samples were collected in heparinized tubes and kept at −80°C until use.
A second study population consisting of SpA patients with peripheral arthritis were included for flow cytometric analyses on leukocyte populations (n = 8). All patients contacted the clinic because of a knee joint effusion, none received biological treatment, but all received standard disease-modifying antirheumatic drugs (DMARD). PBMCs and SFMCs were isolated by conventional Ficoll-Paque (GE Healthcare, Waukesha, WI) density-gradient centrifugation and cryopreserved at −135°C until time of analysis. Samples from both study groups were collected at the outpatient clinic at Aarhus University Hospital. All samples were obtained after informed written consent according to the Declaration of Helsinki and approved by the Local Ethics Committee (project numbers 20050046 and 20058432) and the Danish Data Protection Agency.
ELISA
Quantification of IL-17A, IL-21, and IL-23 levels in plasma and SF from patients with SpA was performed using Human IL-17A ELISA (eBioscience, San Diego, CA), Human IL-21 ELISA (eBioscience), and Human IL-23 ELISA (p19/p40) (eBioscience) in accordance with the manufacturers’ guidelines. Heat inactivated immunoglobulin of the same species as detection antibody was added, showing no changes in optical density (OD) readings. All samples were analyzed in duplicates using the average of the OD values to calculate concentrations. The minimum detection limit after optimization was 4 pg/ml for IL-17A, 16 pg/ml for IL-21, and 2.5 pg/ml for IL-23, defined as the average of the blanks plus two standard deviations. The IL-23 ELISA consists of antibodies that captures the p19 subunit and develops by detecting the p40 subunit. No extrapolated OD values outside the standard curve range were used. OD values below the cutoff were assigned the cutoff value.
MRI examination and grading
All MR examinations were performed with a 1.5 T Siemens Magnetom Symphony scanner (Siemens, Erlangen, Germany). MRI of the SIJ encompassed the following sequences: semi-coronal T1-weighted spin echo (T1), semi-coronal T1 fat saturated (T1FS), semi-axial short tau inversion recovery (STIR) as well as semi-coronal and semi-axial T1FS sequence after gadolinium contrast (Gd.) [0.2 ml/kg (0.1 mmol/kg); maximum 15 ml]. The spinal MRI encompassed sagittal STIR and T1 sequences of the entire spine. All MR examinations were evaluated by a senior radiologist (AGJ) blinded to clinical and biochemical findings. The recently published Danish quantification methods for SIJ and spinal changes in SpA were used [16, 17]. The methods have proven a reliable inter- and intra-observer agreement with kappa values in the range of 0.62–1.0.
Signs of SIJ disease activity were assessed corresponding to the cartilaginous and ligamentous portion of each SIJ (four osseous locations) and encompassed the following: (1) bone marrow edema (BME) visualized on STIR images and (2) enhancement in the bone marrow visualized on post-contrast T1FS images. Both parameters were graded according to extent in the subcortical bone using the following division: (0) normal; (1) slight: <25% of the subcortical bone area; (2) moderate: 25–50%, and (3) severe: ≥50% of the subcortical area. An additional score value of one was added for signal intensity comparable to that of the spinal fluid or great vessels and for depth >1 cm, respectively. This resulted in a total maximum activity score value of 20 for a joint and 40 for a patient by STIR and post-contrast T1FS, respectively. The mean values of the grading by STIR and Gd-enhanced images were used as activity scores [17]. The chronic SIJ score encompassed (1) fatty marrow deposition (FMD) in the same four osseous positions analyzed for activity and (2) erosions in the cartilaginous part of the joint, similarly divided into four grades: (0) no FMD or erosion; (1) <25%; (2) 25–50%; and (3) ≥50% of the subcortical area or joint facet. An additional score value of 1 was added for FMD depth >1 cm, resulting in a total maximum chronic score of 24 for a joint and 48 for a patient [17].
The spine was assessed corresponding to the 23 disco-vertebral units (DVU) from the segment C2/C3 to the segment L5/S1 and scored for activity and chronic changes. The activity score encompassed BME corresponding to the vertebral plates, graded according to extent from 0 to 3. In addition, BME at the costovertebral joints was assessed dichotomously using a score of one for involvement, resulting in a total maximum activity score of 81. The chronic changes comprised FMD, erosions of vertebral plates and syndesmophytes or vertebral fusions graded according to extent from 0 to 3; total maximum score 207 [16].
Flow cytometric analysis
PBMCs and SFMCs were stimulated for 4 h using 50 ng/ml phorbol 12-myristate 13-acetate (Sigma, St. Louis, MO) and 1 μg/ml ionomycin (Sigma) in culture medium (RPMI-1640 supplemented by 10% heat-inactivated fetal calf serum (FCS), 0.8 mg/ml penicillin/streptomycin and 2 mM l-glutamine) at 37°C and 5% CO2 in the presence of brefeldin A (Sigma) in a concentration of 10 μg/ml.
Prior to surface staining, PBMCs and SFMCs were blocked for 15 min with 10% normal goat serum (DAKO, Glostrup, Denmark) to prevent unspecific binding of the goat antibody. IL-23R-biotin (BAF1400, R&D Systems, UK), CD45RO ECD (Beckman Coulter, Fullerton, CA, clone UCHL1), CD4 APC, or CD4 PE (DAKO, clone MT310) was added and incubated for 15 min. After the cells had been washed in PBS/BSA/Azid (PBS pH 7.4 supplemented with 0.5% BSA and 0.05% sodium azid), streptavidin-FITC (DAKO) was added and incubated for 15 min to detect IL-23R. 7-AAD Via-Probe™ (BD Biosciences, San Jose, CA) was used to identify dead cells. For intracellular staining, cells were fixed and permeabilized using FACS lysing solution (BD Biosciences) and FACS permeabilization solution 2 (BD Biosciences) and then blocked for 15 min with 10% normal mouse serum to prevent unspecific binding of the antibodies. Cells were stained with IL-17A FITC or IL-17A Alexa647 (eBioscience) and IL-21 PE (eBioscience). Subsequently, the cells were fixed in 0.9% (w/v) formaldehyde and analyzed on an FC500 flow cytometer (Beckman Coulter). Fluorescence minus one (FMO) was used as controls and to set the quadrant gates using FlowJo software version 8.8.3 (Tree Star Inc., USA) for data analysis.
Statistical analysis
Statistical analyses were performed using Prism 5.0 for Macintosh (GraphPad software, La Jolla, CA). The Mann–Whitney rank-sum test was used to analyze differences between groups with unpaired data, the Wilcoxon matched-pairs test for paired data, and the Spearman rank-order correlation to analyze correlations of individual levels of IL-17A, IL-21, and IL-23 to the clinical and radiological measurements. Unless otherwise stated, all data in the text are expressed as medians with interquartile range (IQR) in parentheses. The level of significance was a two-sided P value of less than 0.05.
Results
Increased levels of IL-21 and IL-23 but not IL-17A in SpA patients
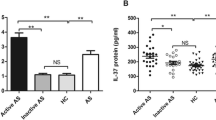
The Th17-related cytokines IL-17A, IL-21, and IL-23 have been proposed to play a major role in autoinflammatory diseases. In SpA patients, we measured significantly increased plasma levels of both IL-21 and IL-23 compared with HV (P < 0.05 and P < 0.01, respectively; Fig. 1), whereas IL-17A levels were below the detection limit and therefore not quantified (not shown). When correlating the individual plasma levels of IL-21 and IL-23 in SpA, we observed a strong intercorrelation (r = 0.7, P < 0.001).
Cytokine contents in SpA plasma samples. IL-21 (a) and IL-23 (b) were measured by sandwich ELISA in plasma from spondyloarthritis patients (n = 80) and healthy volunteers (n = 37). The two groups were compared using the nonparametric Mann–Whitney rank-sum test. Bars represent medians, and whiskers represent 25 and 75 percentile. * Statistical significance of P < 0.05 and ** P < 0.01. Values below cutoff were assigned the cutoff value
We performed a subgroup analysis of the plasma levels of IL-21 and IL-23 in the 48 patients with ankylosing spondylitis comparing them to the SpA patients who met the ESSG criteria, but not the modified New York criteria. However, no differences were observed.
Relation of IL-21 and IL-23 to clinical and MR findings
We then investigated whether the IL-21 or IL-23 plasma levels correlated to age, gender, HLAB27, disease duration, clinical disease activity score (BASMI, BASFI, BASDAI, Global VAS, CRP), and pharmacological treatment (Table 1), but no correlations were observed.
Because activity judged by MRI plays a major role in the evaluation of inflammatory activity in SpA, we correlated the IL-21 and IL-23 plasma levels with the MR score values (for details, please see “Materials and methods”). No significant correlations between individual levels of IL-21 or IL-23 and signs of SIJ and/or spinal inflammation or chronic changes by MRI were observed. Significant correlations between levels of IL-21 or IL-23 and MRI changes were also lacking when grouping the patients according to activity in the different SIJ portions and when evaluating the ankylosing spondylitis patients fulfilling the modified New York criteria for ankylosing spondylitis separately [1].
Increased frequency of IL-21 and IL-23R-positive CD4+ T cells in the inflamed joints
The spine and SIJ are the major sites of inflammation in SpA patients, but due to difficulties in obtaining samples from these areas, we instead examined cells from synovial fluid extracted from inflamed joints as a surrogate marker. The vast majority SF CD4+ T cells express the surface antigen CD45RO (>95%), in contrast to only approximately 40% of the PB CD4+ T cells. Within the CD4+CD45RO+ T-cell subset, we observed that the number of IL-21-positive cells was significantly increased in the inflamed joint to 4.5% (3.8–6.8%) when compared with 2.7% (2.3–3.0%; P < 0.05) in PB. The number of CD4+CD45RO+ T cells expressing IL-23R was also elevated in the inflamed joint to 34.1% (30.0–51.2) compared with peripheral blood 33.3% (18.3–39.2; P < 0.05). In contrast, no significant increase was observed in IL-17A-positive CD4+ T cells (2.2% (1.4–7.1%)) in the joint compared with PB [1.9% (1.5–4.6%); Fig. 2].
Cytokine expression in paired peripheral blood mononuclear cells (PBMC) and synovial fluid mononuclear cells (SFMC) from spondyloarthritis patients (n = 8) was analyzed for IL-17A (a), IL-21 (b), and IL-23R (c) by flow cytometry. The staining was performed following 4 h of stimulation with PMA and ionomycin in the presence of brefeldin A. Bars represent the median percentages of the CD4+CD45RO+ T cells. Whiskers represent 25 and 75 percentiles. * Represents statistical significance P < 0.05 by Wilcoxon matched-pairs test
We investigated the connection between the CD4+CD45RO+ T cells producing IL-17A and IL-21 in further detail by examining the co-expression of IL-17A, IL-21, and IL-23R in the CD4+CD45RO+ subpopulation (Fig. 3). In the PB CD4+CD45RO+ T-cell subset, 10% (8–14%) of the IL-21-producing cells co-expressed IL-17A and 31% (19–40%) expressed the IL-23R. In the SF CD4+CD45RO+ T-cell subset, 5% (3–24%) of the IL-21-producing cells were co-expressing IL-17A and 30% (18–34%) were expressing IL-23R. None of these differences between PB and SF were statistically significant. Of the IL-17A-producing PB CD4+CD45RO+ T cells, 14% (7–23%) co-expressed IL-21 and 29% (21–42%) co-expressed IL-23R. In the SF CD4+CD45RO+ IL-17A-producing T cells, 12% (8–17%) co-expressed IL-21 and 27% (25–47%) co-expressed IL-23R. Neither here did we observe any statistically significant differences between PB and SF. Thus, the majority of both IL-21- and IL-17A-positive CD4+CD45RO+ T cells were single positives for either IL-17A or IL-21, and about one-third of both IL-21- and IL-17A-producing cells co-expressed IL-23R (Fig. 3).
Co-expression of IL-17A, IL-21, and IL-23R in paired peripheral blood (PB) and synovial fluid (SF) from spondyloarthritis patients. Cytokine expression in CD4+CD45RO+ T cells was measured by flow cytometry. Staining was performed following 4 h of stimulation with PMA and ionomycin in the presence of brefeldin A. Values in each quadrant in the diagrams represent median values in each quadrant of the gate from experiments on eight patients. Diagrams show data from one representative SF from a patient with spondylarthritis
Discussion
This is the first study to report that patients with axial SpA have increased levels of IL-21 in plasma compared with HVs. We also report increased levels of IL-23, whereas we were unable to detect IL-17A in plasma from SpA patients. We did not observe association with cytokine levels and disease activity clinically nor with disease activity and chronicity measured by MRI, despite a detailed MR scoring of the SIJ and the entire spine [16, 17].
The strong intercorrelation between plasma levels of IL-21 and IL-23 together with the increased frequencies of both IL-21- and IL-23R-positive cells in the inflamed joints of SpA patients advocate that the functions of these cytokines are closely connected. Both IL-21 and IL-23 are strongly associated with the development of Th17 cells [4, 5]. Because IL-17A levels were not increased and both IL-21R and IL-23R are expressed on a wide variety of cell types associated with chronic inflammatory lesions, this points to multiple target cells for IL-21 and IL-23, suggesting that these cytokines have effects beyond the Th17 system [8, 11, 18–20].
In our study, 48 of the 80 SpA patients examined fulfilled the modified New York criteria for ankylosing spondylitis [1], and the group in general was also not highly active using standard clinical scoring systems for SpA (Table 1). Rudwaleit et al. have shown that signs of active inflammation by MRI in SpA do not correlate to clinical measurements, using the Berlin MRI spine score method [21]. The present and previous results showed that the use of a more extensive scoring system did not associate MRI findings to the clinical parameters [17, 22]. Adding to this, we did not find an association between MRI and IL-17A, IL-21, and IL-23 plasma levels. This is in line with studies by Wang et al., where levels of the p19 part of IL-23 did not correlate with biochemical signs of activity in SpA [23, 24]. Thus, neither IL-17A, IL-21 nor IL-23 seems to be suitable markers of clinical disease or MRI activity in SpA. Although none of the cytokines correlated with clinical evaluations, their elevated levels of IL-21 and IL-23 revealed that SpA patients are immunologically active even in this group of SpA patients with a low clinical activity.
IL-23R has been suggested as a defining marker for the Th17 cells, while IL-21 has been reported as part of the Th17 cytokine repertoire [4]. In this study, we investigated the cellular co-expression of IL-17A, IL-21, and IL-23R in mononuclear cells from both peripheral blood and synovial fluid of SpA patients. We found that only a third of the IL-17A-positive T cells co-expressed IL-23R. Furthermore, we observed that only a minor fraction of the IL-21-producing CD4+ T cells also expressed IL-17A, suggesting that in SpA these two cytokines are not primarily produced by the same cellular subset. We also observed that only a few IL-17A-producing cells expressed surface IL-23R. However, only limited data exist on how Th17 cells behave in the chronic situation. The missing IL-23R expression observed in the present study could be due to receptor downregulation provoked by specific conditions that exist in the micro-environment of chronic inflammation.
In the present study, we have demonstrated that patients with SpA have increased plasma levels of IL-21 and IL-23, but not IL-17A. This increase in IL-21 and IL-23 levels was not correlated to BASMI, BASFI, BASDAI, Global VAS, CRP nor to MRI signs of disease activity and chronic changes. That IL-21 and IL-23 could be of relevance for the pathogenesis of SpA is, however, supported by the findings of increased numbers of CD4+CD45RO+ T cells expressing IL-21 or IL-23R in inflamed SpA peripheral joints.
References
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27:361–368
Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI et al (2007) Association scan of 14, 500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet 39:1329–1337
Layh-Schmitt G, Colbert RA (2008) The interleukin-23/interleukin-17 axis in spondyloarthritis. Curr Opin Rheumatol 20:392–397
Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K et al (2007) Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 8:950–957
Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL (2003) Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 278:1910–1914
Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA (2008) IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 454:350–352
Leonard WJ, Zeng R, Spolski R (2008) Interleukin 21: a cytokine/cytokine receptor system that has come of age. J Leukoc Biol 84:348–356
Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K et al (2000) Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408:57–63
Li J, Shen W, Kong K, Liu Z (2006) Interleukin-21 induces T-cell activation and proinflammatory cytokine secretion in rheumatoid arthritis. Scand J Immunol 64:515–522
Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N et al (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715–725
Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R et al (2002) A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol 168:5699–5708
Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, Li S, Dooley LT et al (2008) Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371:1665–1674
Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P (2008) Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum 58:2307–2317
Sidiropoulos PI, Hatemi G, Song IH, Avouac J, Collantes E, Hamuryudan V, Herold M, Kvien TK et al (2008) Evidence-based recommendations for the management of ankylosing spondylitis: systematic literature search of the 3E Initiative in Rheumatology involving a broad panel of experts and practising rheumatologists. Rheumatology (Oxford) 47:355–361
Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, Cats A, Dijkmans B et al (1991) The European Spondylarthropathy study group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 34:1218–1227
Madsen KB, Jurik AG (2010) MRI grading method for active and chronic spinal changes in spondyloarthritis. Clin Radiol 65:6–14
Madsen KB, Jurik AG (2010) Magnetic resonance imaging grading system for active and chronic spondylarthritis changes in the sacroiliac joint. Arthritis Care Res (Hoboken) 62:11–18
Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR (2008) Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol 180:5167–5171
Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W et al (2003) Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744–748
Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF Jr, Cheever AW, Young DA, Collins M et al (2006) The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest 116:2044–2055
Rudwaleit M, Schwarzlose S, Hilgert ES, Listing J, Braun J, Sieper J (2008) MRI in predicting a major clinical response to anti-tumour necrosis factor treatment in ankylosing spondylitis. Ann Rheum Dis 67:1276–1281
Madsen KB S-CB, Jurik AG (2010) Prognostic significance of MR changes of the sacroiliac joints in spondyloarthritis. A follow-up study. J Rheumatol 37:1718–1727
Melis L, Vandooren B, Kruithof E, Jacques P, De Vos M, Mielants H, Verbruggen G, De Keyser F et al (2009) Systemic levels of IL-23 are strongly associated with disease activity in rheumatoid arthritis but not spondyloarthritis. Ann Rheum Dis 69:618–623
Wang X, Lin Z, Wei Q, Jiang Y, Gu J (2009) Expression of IL-23 and IL-17 and effect of IL-23 on IL-17 production in ankylosing spondylitis. Rheumatol Int 29:1343–1347
Acknowledgments
The authors are grateful for the support from the Danish Agency for Science, Novo Nordisk, Leo Pharma, the Danish Rheumatoid Association, Danish Psoriasis Association, and The Institute of Clinical Medicine at the University of Aarhus.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Thomas Andersen and Tue Kruse Rasmussen shared first authorship.
Rights and permissions
About this article
Cite this article
Andersen, T., Rasmussen, T.K., Hvid, M. et al. Increased plasma levels of IL-21 and IL-23 in spondyloarthritis are not associated with clinical and MRI findings. Rheumatol Int 32, 387–393 (2012). https://doi.org/10.1007/s00296-010-1655-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-010-1655-3