Abstract
The objective of the study is to investigate the functional ability, i.e., the physical aspect of the health-related quality of life and its determining factors during therapy with etanercept in children with juvenile idiopathic arthritis (JIA). Assessment of the Child Health Assessment Questionnaire (CHAQ), the number of active joints, duration of morning stiffness, ESR, C-reactive protein, parent’s global assessment of the overall patient’s well-being, physician’s global assessment of the overall disease activity, concomitant treatment with methotrexat, number of aids/devices needed, and calculation of the PedACR-score. Data of 437 children were analyzed for disease severity and impact of the disease on functional abilities. Data of 114 children with a complete data set and a continuous treatment for at least 24 months were used for analysis of the impact of treatment on functional abilities. Before treatment with etanercept, patients with systemic arthritis and seropositive or seronegative polyarthritis were disabled more heavily than those with other subtypes of JIA. There was a correlation between high CHAQ scores and the number of active joints, aids/devices needed, parent’s global assessment of the overall patient’s well-being, morning stiffness, physician’s global assessment of the overall disease activity, and C-reactive protein (P < 0.005). Of the eight areas, “dressing and grooming”, “arising”, “eating”, “walking”, “hygiene”, “reach”, “grip” and “activities”, the latter was most severely affected. 96.5, 93.8, and 90.3% of the patients reached a PedACR-30, -50 and -70 score upon treatment with etanercept for 24 months. The areas of eating and walking were best before therapy and showed highest improvement with therapy. Under therapy with etanercept patients of all JIA subgroups significantly improved their functional ability (P < 0.0001), but patients with polyarthritis less frequently improved their physical functioning. Disease activity and the physical aspect of health-related quality of life including functional ability improved significantly during therapy with etanercept in children with JIA. Duties of everyday life were easier to accomplish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Juvenile idiopathic arthritis (JIA) is the most common chronic autoimmune disease in childhood. It results not only in painful and swollen joints but also in a limitation of functional ability and of everyday life in children [1, 2].
Etanercept, which inhibits TNF-alpha, is licensed for treatment of active, refractory polyarticular chronic arthritis in childhood. The introduction of biologics like etanercept had a major impact on the outcome of patients with polyarticular JIA. The safety and the efficiency of etanercept had been proved in open and controlled studies as well as in long-term studies [3–13].
Several measures for functional ability in children with JIA were introduced in the past years. Among those the Child Health Assessment Questionnaire (CHAQ) is the most common tool used to measure functional ability in children with JIA. The CHAQ is well established for use in children with JIA [14] and is used as one of the six core response variables in clinical drug trials [15–20]. It has been validated and evaluated in the German language [21]. Furthermore, it has been shown to be an useful tool for evaluating outcome in longitudinal studies [22–24].
The outcome of JIA is variable depending on the subgroup of JIA. In some subgroups many children experience spontaneous remission of their joint disease while in other subgroups persistent destructive arthritis frequently occurs. Clinical experience suggests that more children have ongoing disease in adulthood than expected. Though the physical outcome of patients with JIA is relatively good compared to adult patients with rheumatoid arthritis, many patients still have active disease in adulthood, and health status and quality of life are reduced in patients with all types of JIA [25]. Approximately half of the patients with JIA have changes in their body function and structure after disease duration of more than 15 years. Fortunately less than 10% are severely disabled or handicapped [26].
The purpose of the present study was to evaluate the alteration of functional ability in children with JIA before and during treatment with etanercept. Eight areas of everyday life with impact on the functional ability were measured with the CHAQ: “dressing and grooming”, “arising”, “eating”, “walking”, “hygiene”, “reach”,”grip”, and “activities”.
Patients and methods
Study design and patients
Criteria for inclusion in our analysis were diagnosis of JIA subclassified into the six JIA subgroups according to the International League of Associations for Rheumatology (ILAR) criteria and registration in the German etanercept registry from 2001 to 2006 [2]. In order to be eligible for treatment with etanercept, failure to respond or intolerance to methotrexate was required [27]. Patient data were collected at the start of therapy with etanercept and every 6 months during therapy. Data of 437 children were analyzed for disease severity and impact of the disease on functional abilities. Owing to the study character not all criteria were available at every point of time from every patient. Data of 114 children with a complete data set and a continuous treatment for at least 24 months were used for analysis of the impact of treatment on functional abilities.
Functional ability
The patients of at least 13 years of age or parents of patients younger than 13 years were asked to complete the CHAQ. The CHAQ includes 30 questions concerning 8 areas of daily living “dressing and grooming”, “arising”, “eating”, “walking”, “hygiene”, “reach”, “grip”, and “activities”. The response modalities range on a 4-point ordinary scale with 0 for “no difficulty”, 1 for “some difficulties”, 2 for “much difficulties”, and 3 for “unable to do”. An additional response modality, “not applicable for age”, could be used if a child was too young for a certain activity. Besides the 30 items the CHAQ contains 14 questions related to the use of aids/devices plus 8 questions that are related to the child’s need of help from another person. Each of the eight areas is scored as the highest item in that area. If aids/devices or assistance from another person for one of the eight CHAQ areas are required for an activity, the score is further corrected to at least two points for the corresponding area. The eight areas of the CHAQ are averaged to calculate the CHAQ disability index (CHAQ DI) which ranges from 0 (no or minimal physical dysfunction) to 3 (very severe physical dysfunction).
Besides the 30 items, the CHAQ includes a patient’s or parent’s global assessment of the overall patient’s well-being in the previous week on a 0–10 cm visual analogue scale (VAS) (0 = very well, 10 = very poor) and a patient’s or parent’s global assessment of the child’s pain in the previous week on a 0–10 cm VAS (0 = no pain, 10 = very severe pain).
JIA disease activity
Efficacy was assessed with the PedACR30, 50, and 70 criteria including the German CHAQ. These core set parameters consisted of (1) the physician global assessment of disease activity, on a 10-cm visual analogue scale (VAS); (2) the parent/patient global assessment of overall well-being, on a 10-cm VAS; (3) the CHAQ; (4) the number of joints with active arthritis, defined by the presence of swelling or, if no swelling was present, limitation of motion accompanied by pain, tenderness, or both (5) the number of joints with limited range of motion; and (6) the erythrocyte sedimentation rate. The PedACR30 was reached if there was an improvement of ≥30% in at least 3 of 6 core variables, with no more than 1 of the remaining variables worsened by ≥30% [15]. Patients were also evaluated for improvement of ≥50 or ≥70% improvement in at least 3 of 6 core variables, with no more than 1 of the remaining variables worsened by ≥30% (PedACR50 and PedACR70). Other assessments not included in the PedACR score were the duration of morning stiffness, and serum C-reactive protein levels.
Statistical analysis
Data were collected using Microsoft Excel and the statistical analysis was processed with SPSS for windows 14.0.
Continuous variables were evaluated with descriptive statistic analysis using mean values and SD. Categorical variables were evaluated using frequencies and percentages.
Statistical tests included one-way analysis of variance, Chi-square test, Kruskal–Wallis test, ANOVA, and GLM repeated measures. P values less than 0.05 were considered to be significant.
The dimension of functional limitation was categorized by a system proposed by Ruperto et al. (1997). A CHAQ DI 0 implicates no disability, 0.1–0.5 implicates a mild disability, 0.6–1.5 implicates a moderate disability, and >1.5 implicates a severe disability [28].
Results
Until June 1st, 2006 data on 437 patients (70% female) feasible for analysis have been collected. Patients were treated in several pediatric rheumatology centers all over Germany and Austria for 1 to 72 months [mean (± SD) 16 (± 14.6) months, median 12 months]. The main demographic, clinical and laboratory features of the total group of JIA patients are shown in Table 1. According to the ILAR criteria, 76 (17.3%) of the patients had systemic JIA, 138 (31.6%) had seronegative polyarthritis, 53 (12.1%) had seropositive polyarthritis, 15 (3.4%) had persistent oligoarthritis, 73 (16.7%) had extended oligoarthritis, 51 (11.7%) had enthesitis related arthritis, and 31 (7%) had psoriasis arthritis. Concomitant treatment with methotrexate was used in 362 (82.5%) patients.
The mean (±SD) age for disease onset in children with JIA was 6.6 ± 4.6 years. Earliest disease onset was found in children with systemic JIA (3.9 ± 3.4 years). Children with enthesitis-related arthritis and with seropositive polyarthritis were oldest at disease onset (11 ± 3.1 and 10.3 ± 3.5 years). The mean (± SD) disease duration before therapy with etanercept was 5.1 ± 4.0 years.
At the start of therapy with etanercept, the mean (±SD) age of the patients was 12.1 (±4.5) years. As expected, patients of the single JIA subgroups differed in part markedly from patients of other subgroups. Patients with seropositive (14.3 ± 3.1 years) and seronegative polyarthritis (12.3 ± 4.2 years), those with enthesitis related arthritis (15 ± 2.9 years), and those with psoriasis arthritis (13.5 ± 3.8 years) were older than patients with systemic arthritis (9.2 ± 5.1 years) or persistent or extended oligoarthritis (10.0 ± 4.7 years).
High disease activity was reflected by high numbers of active joints, elevated ESR and C-reactive protein, and a prolonged duration of morning stiffness (Table 1). Patients with polyarthritis had the highest numbers of active joints (13 ± 10), and patients with persistent oligoarthritis had the lowest numbers (3 ± 2). Highest inflammatory parameters were observed in patients with systemic JIA (ESR 56 ± 32 mm/h, C-reactive protein 73.2 ± 66.0 mg/l). Morning stiffness was longest in patients with seropositive polyarthritis and in patients with extended oligoarthritis (51 ± 63 and 49 ± 67 min), while patients with persistent oligoarthritis had the shortest duration of morning stiffness (3 ± 5 min). Among patients with JIA, those with systemic arthritis and with seronegative polyarthritis most frequently needed aids/devices (mean number 3 ± 4). Physician’s global assessment of the overall disease activity was worst in patients with systemic arthritis (7.7 ± 2.1 cm on a 10-cm VAS) as well as patients’s/parent’s global assessment of the overall patient’s well-being (6.3 ± 2.7 cm on a 10-cm VAS).
The mean (± SD) CHAQ DI of all patients was 0.9 ± 0.7. This implicates a moderate disability according to the categorization of Ruperto et al. [16]. Higher scores in the CHAQ DI were present in patients with systemic arthritis, seropositive or seronegative polyarthritis and extended oligoarthritis (P < 0,0001 in the Kruskal Wallis test), patients with elevated ESR, C-reactive protein, patients with a large number of active joints, patients experiencing prolonged duration of morning stiffness, those with higher scores in physician’s global assessment of the overall disease activity and parent’s global assessment of the overall patient’s well-being and with the need of many aids/devices. Univariant analysis showed a significant correlation of the parameters of JIA-severity (number of active joints, duration of morning stiffness, parent’s global assessment of the overall patient’s well-being, need of aids/devices, physician’s global assessment of the overall disease activity, CRP) with the CHAQ DI (P < 0.005, Table 1).
At the start of treatment with Etanercept, the scores of the eight functional CHAQ areas varied (Table 2). According to the classification by Ruperto et al., children had a moderate disability in most functional areas before etanercept therapy. The rheumatic disease had the greatest impact on “activities” (1.4 ± 1.0), “reach” (1.1 ± 0.9), “dressing and grooming” (1.0 ± 1.0), and “grip” (1.0 ± 1.0). “Eating” (0.7 ± 0.9) and “walking” (0.7 ± 0.9) were less affected. Patients with systemic arthritis and patients with enthesitis related arthritis had more severe disability while doing “activities” (1.6 ± 1.1 and 1.6 ± 1.0). “Eating” was easiest (“mild disabilities”) for children with persistent oligoarthritis, enthesitis-related arthritis, psoriasis, and arthritis and extended oligoarthritis (0.1 ± 0.4, 0.3 ± 0.6, 0.4 ± 0.8, and 0.5 ± 0.8). Children with persistent oligoarthritis had “mild disability” in all functional areas except for “activities” (0.9 ± 0.9). Significant differences (P < 0.005 in the Kruskal–Wallis test) between the JIA-subtypes were found for the CHAQ-areas “dressing and grooming”, “eating”, “hygiene”, “reach”, and “grip”.
Therapeutic efficacy of the treatment with etanercept
For analysis of the influence of treatment with etanercept on functional ability, patients included were those who had been treated for at least 24 months and in whom a complete set of data was available. By this way, 114 patients could be identified. There was a significant improvement of the CHAQ DI before therapy to the CHAQ DI during therapy in the JIA population in the GLM repeated measures (P < 0.0001). The mean ± SD of the CHAQ DI before therapy was 0.92 ± 0.77 (median 0.75). After 6 months of treatment the CHAQ DI decreased to a mean ± SD of 0.39 ± 0.53 (median 0.13) and after 18 months to a minimum of 0.32 ± 0.5 (median 0; Fig. 1). At the start of therapy the mean CHAQ DI corresponded to “moderate disability”, after 6 months and thereafter to “mild disability” [28].
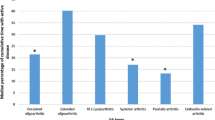
Comparing the subtypes there were markedly higher CHAQ DI in patients with seropositive or seronegative polyarthritis than in other JIA subgroups as outlined in Table 3. Seronegative polyarthritis patients were the largest patient group (n = 33) in this analysis and remained with the highest CHAQ DI during etanercept therapy followed by patients with seropositive polyarticular JIA. Disability disappeared in the majority of patients leading to a decrease of the mean CHAQ DI. At the month 0, 6, 12, 18, and 24 the mean CHAQ DI in seronegative polyarticular JIA patients was 0.96, 0.68, 0.62, 0.53, and 0.57, in seropositive polyarticular JIA it was 1.0, 0.37, 0.36, 0.41, and 0.46. The CHAQ DI for patients with persistent oligoarthritis were the lowest at all points of time (0.25, 0.03, 0.05, 0.05, and 0.03 at 0, 6, 12, 18, and 24 months).
High disease activity was accompanied by large numbers of active joints, elevated ESR and C-reactive protein, high scores in physician’s global assessment of the overall disease activity, high scores in parent’s global assessment of the overall patient’s well-being, and a prolonged duration of morning stiffness and was reflected by a high CHAQ DI at start of therapy (Table 1). Significant improvement in active joints, parent’s global assessment of the overall patient’s well-being, physician’s global assessment of the overall disease activity, duration of morning stiffness, ESR and C-reactive protein was seen after 6, 12, 18, and 24 months (P < 0.0001 for all parameters and all points of time) (Table 4). Functional ability improved though the parameters of disease activity were high before therapy. While high scores in active joints, CRP, ESR, parent’s global assessment of the overall patient’s well-being, physician’s global assessment of the overall disease activity and morning stiffness were attended by high DI before therapy, the patients reached lower levels of CHAQ DI during therapy.
At the start of therapy with etanercept, those children treated with a combined therapy of methotrexate and etanercept had a higher CHAQ DI (0.9 ± 0.79). However, they showed a marked improvement of scores during therapy and a minimum level of 0.33 ± 0.52 was reached after 24 months. Patients treated with etanercept monotherapy at start of therapy had a CHAQ DI of 0.72 ± 0.61 which decreased to 0.34 ± 0.53 after 24 months of treatment.
All functional areas of the CHAQ improved significantly during therapy with etanercept (P < 0.0001) (Fig. 2). Before therapy children had most problems with “activities”, “dressing and grooming”, “reach” and “grip” (mean score of those areas 1.44, 1.12, 1.05, and 0.94). These scores corresponded to “moderate disability”. At 24 months of therapy, the patients still had most problems with “activities”, “reach”, “dressing and grooming” and “grip” (mean score of those areas 0.61, 0.51, 0.44, and 0.38). These levels of disabilities finally corresponded to “mild disability” in all functional areas except for “activities” with a “moderate disability”.
Measuring therapeutic efficiency by the maximal improvement of the PedACR-Score reached during 24 months of therapy, 96.5, 93.8, and 90.3% of the patients reached a PedACR-30, -50 and -70 score. Subtype analysis showed that 90.9, 81.8, and 72.7% of the patients with systemic arthritis reached a PedACR-30, -50 and -70 score which was somewhat lower than in children with non-systemic JIA, who reached a PedACR-30, -50 and -70 score with 97.1, 95.1, and 92.2% of their patient group (Fig. 3).
In our patient population, 8.8% of the patients discontinued the therapy due to inefficiency, 5.1% discontinued due to intolerance. About 7% discontinued treatment due to remission.
Discussion
This study provides data about children who started treatment with etanercept in Germany between 2001 and 2006. Data of 437 children were analyzed for disease severity and impact of the disease on functional abilities. Data of 114 children with a complete data set and a continuous treatment for at least 24 months were used for analysis of the impact of treatment on functional abilities. Only a minority of 8.8% of patients of the registy cohort discontinued due to inefficacy, only 5.1% discontinued due to intolerance. About 7.0% discontinued treatment due to remission. The results confirm previous studies on the German etanercept registry [6, 9]. The major gains of etanercept therapy were as follows:
-
Children with long standing JIA had poor functional ability and high disease activity before the start of treatment with etanercept.
-
Among those, patients with either seronegative or seropositive polyarthritis, extended oligoarthritis, and with systemic JIA suffered from higher disease activity concerning the number of active joints, inflammatory parameters (ESR, C-reactive protein), duration of morning stiffness, parent’s global assessment of the overall patient’s well-being, physician’s global assessment of the overall disease activity, and number of aids/devices needed.
-
JIA patients with higher disease activity parameters also had higher CHAQ DI.
-
There was a high response rate to etanercept in children who previously were unresponsive or intolerant to several antirheumatic drugs.
-
There was a rapid improvement during the first 6 months of therapy concerning the CHAQ DI and the disease activities which remained at lower levels during 24 months of treatment.
-
Combination therapy of etanercept and MTX led to higher improvement rates and led to better abilities in fulfilling everyday’s duties.
Our results indicate that the functional ability improves strongly during 24 months of therapy with etanercept. Clinical improvement was indicated by a decrease of the CHAQ DI and by an improvement according to the PedACR-30, -50 and -70 score. An improvement of the CHAQ DI was already evident after 6 months of treatment and persisted until 24 months with a further decrease of the mean CHAQ DI. A marked decrease of all clinical parameters was noted after 6 months. The results of this long-term study confirm previous findings by Lovell et al. [3–5] on a limited number of patients as well as several other case series that were published thereafter [6–13]. Lovell et al. first started a randomized placebo-controlled study. Later on, patients of this study went in a long-term open-label extension study. 69 patients started the initial study with a mean CHAQ DI 1.5 (± 0.1), 1 year later the mean CHAQ DI was 0.8 (± 0.1) and 4 years later the CHAQ DI was 0.6 (± 0.1). After 8 years of therapy a PedACR 70 was achieved in all patients who continued on treatment.
Functional ability was lowest in patients with either seronegative or seropositive polyarthritis. Patients with polyarthritis remained to have higher CHAQ DI scores during therapy than patients with other JIA-subtypes. This effect suspected that a lower physical ability remains during therapy in patients with higher numbers of affected joints.
Patients with systemic JIA who responded to etanercept and continued treatment for 24 months had an equal improvement of their disease activity as patients with other JIA-subtypes. This finding is similar to a finding of Prince et al. This Dutch long-term follow-up study included 146 JIA-patients who were treated with etanercept for a median of 2.5 years [13].
A combination therapy with etanercept and MTX was well accepted by the patients. Physical ability improved in both groups. However, the extend of improvement of the CHAQ DI was higher in patients with combination therapy of methotrexate and etanercept than in those with etanercept monotherapy. In another analysis of data of the German registry on a patient cohort of 604 patients similar results have been described for the PedACR-Score [9]. Here, the likelihood of achieving a PedACR 70 increased with combination therapy. Earlier studies including fewer patients confirm our results [6, 11, 29].
The eight functional areas of the CHAQ differed before and during therapy. Functional ability improved in all areas and the discrepancy between the areas decreases during therapy. Before and during therapy “activities”, “dressing” and “reach” were hardest for children with JIA, “eating” and “walking” were less affected. Duties of everyday life concerning actions that are indispensable in everyday life like “cutting meat”, “lift up a cup or glass to mouth”, “open a new cereal box”, “walk outdoors on flat ground” and “climb up five steps” were easier to practice and to accomplish than duties that can be avoided or need fine motor skills. Examples are “cut fingernails” or “ride bike or tricycle”. Avoided skills or skills which claim fine motor skills cause higher counts in the respective areas since the question with the highest count is used for calculation the CHAQ. Another aspect was stated by Pouchot et al. They found that for the “run and play” item, it is possible that parents of older children may consider the “run” activity, whereas parents of younger children may concentrate on the “play” activity [30]. If this is correct one can argue that “running” may be a more difficult activity than “playing”.
Oliveira et al. found in their study about health-related quality of life (HRQOL) that children with JIA have a greater impairment in physical well-being than in psychosocial health, and that physical disability and pain are important determinants of HRQOL [23]. These findings indicate that the improvement of physical ability and reduction of pain are the major goals of JIA therapy.
In summary, we found dramatical improvement of functional ability in children with severe and so far treatment-resistant JIA. The major findings of our study were a rapid improvement of functional ability and disease activity parameters already evident after 6 months of therapy which was prolonged and further improved during at least 24 months. Duties of everyday life were easier to accomplish and HRQOL improved.
References
Petty RE, Southwood TR, Baum J, Bhettay E, Glass DN, Manners P, Maldonado-Cocco J, Suarez-Almazor M, Orozco-Alcala J, Prieur AM (1998) Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol 25:1991–1994
Woo P, Wedderburn LR (1998) Juvenile chronic arthritis. Lancet 351:969–973
Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton J, Stein LD, Gedalia A, Ilowite NT, Wallace CA, Whitmore JB, Finck BK (2000) Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med 342:763–769. doi:10.1056/NEJM200003163421103
Lovell DJ, Reiff A, Jones OY, Schneider R, Nocton J, Stein LD, Gedalia A, Ilowite NT, Wallace CA, Whitmore JB, White B, Giannini EH (2006) Long-term safety and efficacy of etanercept in children with polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum 54:1987–1994. doi:10.1002/art.21885
Lovell DJ, Reiff A, Ilowite NT, Wallace CA, Carol A, Chon Y, Lin SL, Baumgartner SW, Giannini EH, Nickeson RW Jr, Jones OY, Schneider R, Nocton J, Stein LD, Gedalia A, Whitmore JB, White B, Bernstein B, Cawkwell GD, Feldman B, Gottlieb B, Graham B, Laxer R, Olson JC, Passo M, Reed A, Shaham B, Sher M, Sherry D, Silverman ED (2008) Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum 58:1496–1504. doi:10.1002/art.23427
Horneff G, Schmeling H, Biedermann T, Foeldvari I, Ganser G, Girschick HJ, Hospach T, Huppertz HI, Keitzer R, Küster RM, Michels H, Moebius D, Rogalski B, Thon A (2004) The German etanercept registry for treatment of juvenile idiopathic arthritis. Ann Rheum Dis 63:1638–1644. doi:10.1136/ard.2003.014886
Kietz DA, Pepmueller PH, Moore TL (2001) Clinical response to etanercept in polyarticular course juvenile rheumatoid arthritis. J Rheumatol 28:360–362
Kietz DA, Pepmueller PH, Moore TL (2002) Therapeutic use of etanercept in polyarticular course juvenile idiopathic arthritis over a two year period. Ann Rheum Dis 61:171–173. doi:10.1136/ard.61.2.171
Horneff G, De Bock F, Foeldvari I, Girschick HJ, Michels H, Moebius D, Schmeling H (2009) Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA registry. Ann Rheum Dis 68(4):519–525
Lahdenne P, Vähäsalo P, Honkanen V (2003) Infliximab or etanercept in the treatment of children with refractory juvenile idiopathic arthritis: an open label study. Ann Rheum Dis 62:245–247. doi:10.1136/ard.62.3.245
Quartier P, Taupin P, Bourdeaut F, Lemelle I, Pillet P, Bost M, Sibilia J, Koné-Paut I, Gandon-Laloum S, LeBideau M, Bader-Meunier B, Mouy R, Debré M, Landais P, Prieur AM (2003) Efficacy of Etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthritis Rheum 48:1093–1101. doi:10.1002/art.10885
Robinson RF, Nahata MC, Hayes JR, Rennebohm R, Higgins G (2007) Quality-of-life measurements in juvenile rheumatoid arthritis patients treated with etanercept. Clin Drug Investig 23:511–518. doi:10.2165/00044011-200323080-00003
Prince FH, Twilt M, Ten Cate R, Van Rossum MA, Armbrust W, Hoppenreijs EP, van Santen-Hoeufft M, Koopman-Keemink Y, Wulffraat NM, van Suijlekom-Smit LW (2009) Long-term follow-up on effectiveness and safety of etanercept in JIA: the Dutch national register. Ann Rheum Dis 68(5):635–641
Ruperto N, Ravelli A, Pistorio A, Malattia C, Cavuto S, Gado-West L, Tortorelli A, Landgraf JM, Singh G, Martini A (2001) Cross-cultural adaptation and psychometric evaluation of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ) in 32 countries. Review of the general methodology. Clin Exp Rheumatol 19:1–9
Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A (1997) Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 40:1202–1209
Ruperto N, Ravelli A, Falcini F, Lepore L, Buoncompagni A, Gerloni V et al (1999) Responsiveness of outcome measures in juvenile chronic arthritis. Rheumatology 38:176–180. doi:10.1093/rheumatology/38.2.176
Ruperto N, Murray KJ, Gerloni V, Wulffraat N, de Oliveira SKF, Falcini F et al (2004) A randomized trial of parenteral methotrexate comparing an intermediate dose with a higher dose in children with juvenile idiopathic arthritis who failed to respond to standard doses of methotrexate. Arthritis Rheum 50:2191–2201. doi:10.1002/art.20288
Van Rossum MA, Fiselier TJ, Franssen MJ, Zwinderman AH, Ten Cate R, Suijlekom-Smit LW et al (1998) Sulfasalazine in the treatment of juvenile chronic arthritis: a randomized, double-blind, placebo-controlled, multicenter study. Dutch Juvenile Chronic Arthritis Study Group. Arthritis Rheum 41:808–816. doi:10.1002/1529-0131(199805)41:5<808::AID-ART6>3.0.CO;2-T
Ruperto N, Nikishina I, Pachanov ED, Shachbazian Y, Prieur AM, Mouy R et al (2005) A randomized, double-blind clinical trial of two doses of meloxicam compared with naproxen in children with juvenile idiopathic arthritis: short- and long-term efficacy and safety results. Arthritis Rheum 52:563–572. doi:10.1002/art.20860
Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G et al (2007) A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular course juvenile rheumatoid arthritis. Arthritis Rheum 56:3096–3106. doi:10.1002/art.22838
Foeldvari I, Ruperto N, Dressler F, Hafner R, Kuster RM, Michels H, Minden K, Schauer-Petrowskaja C, Bullinger M, Landgraf JM, Huppertz HI (2001) The German version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clin Exp Rheumatol 19:71–75
Duffy CM (2005) Measurement of health status, functional status, and quality of life in children with juvenile idiopathic arthritis: clinical science for the pediatrician. Pediatr Clin North Am 52:359–372. doi:10.1016/j.pcl.2005.01.009
Oliveira S, Ravelli A, Pistorio A, Castell E, Malattia C, Prieur AM, Saad-Magalhaes C, Murray KJ, Bae SC, Joos R, Foeldvari I, Duarte-Salazar C, Wulffraat N, Lahdenne P, Dolezalova P, De Inocencio J, Kanakoudi-Tsakalidou F, Hofer M, Nikishina I, Ozdogan H, Hashkes PJ, Landgraf JM, Martini A, Ruperto N (2007) Proxy-reported health-related quality of life of patients with juvenile idiopathic arthritis: the Pediatric Rheumatology International Trials Organization multinational quality of life cohort study. Arthritis Rheum 57:35–43. doi:10.1002/art.22473
Bekkering WP, Ten CR, Van Rossum MA, Vliet Vlieland TP (2007) A comparison of the measurement properties of the Juvenile Arthritis Functional Assessment Scale with the childhood health assessment questionnaire in daily practice. Clin Rheumatol 26:1903–1907. doi:10.1007/s10067-007-0689-8
Foster HE, Marshall N, Myers A, Dunkley P, Griffiths ID (2003) Outcome in adults with juvenile idiopathic arthritis: a quality of life study. Arthritis Rheum 48:767–775. doi:10.1002/art.10863
Minden K, Niewerth M, Listing J, Biedermann T, Bollow M, Schontube M, Zink A (2002) Long-term outcome in patients with juvenile idiopathic arthritis. Arthritis Rheum 46:2392–2401. doi:10.1002/art.10444
Horneff G, Forster J, Seyberth HW, Michels H (2001) Arbeitgemeinschaft Kinder- und Jugendrheumatologie. Recommendations by the Pediatric and Adolescent Rheumatology Study Committee on therapy with Etanercept (p75 TNF-alpha receptor immunoglobulin fusion protein). Pharmacotherapy Committee. Z Rheumatol 59:365–369. doi:10.1007/s003930070043
Ruperto N, Levinson JE, Ravelli A, Shear ES, Link Tague B, Murray K, Martini A, Giannini EH (1997) Long-term health outcomes and quality of life in American and Italian inception cohorts of patients with juvenile rheumatoid arthritis. I. Outcome status. J Rheumatol 24:945–951
Schmeling H, Mathony K, John V, Keyßer G, Burdach S, Horneff G (2001) A combination of etanercept and methotrexate for the treatment of refractory juvenile idiopathic arthritis: a pilot study. Ann Rheum Dis 60:410–412. doi:10.1136/ard.60.4.410
Pouchot J, Ecosse E, Coste J, Guillemin F (2004) Validity of the childhood health assessment questionnaire is independent of age in juvenile idiopathic arthritis. Arthritis Rheum 51:519–526. doi:10.1002/art.20529
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Halbig, M., Horneff, G. Improvement of functional ability in children with juvenile idiopathic arthritis by treatment with etanercept. Rheumatol Int 30, 229–238 (2009). https://doi.org/10.1007/s00296-009-0942-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-009-0942-3