Abstract
To assess longitudinally the course and outcome of juvenile idiopathic arthritis (JIA) in patients diagnosed and followed-up exclusively in the biologic era; also, to define possible predictors of the disease progression and need for early implementation of biologicals. Prospective and retrospective, monocentric cohort study of 120 JIA patients, diagnosed between 2001 and 2010, and followed-up for ≥ 4 years (median 8.04). Disease activity, cumulative articular/extra-articular damage and quality of life were evaluated by the assessment tools Juvenile Arthritis Disease Activity Score (JADAS71), Juvenile Arthritis Damage Index (JADI) and Childhood Health Assessment Questionnaire (CHAQ), respectively. Moreover, potential predictors of the disease progression and their relation to biologic therapy were investigated. High JADAS71 score (> 9) at diagnosis was indicative of progression to polyarticular course and the need for early introduction of biologic treatment. Other independent predictors of progression to polyarthritis, were: involvement of upper limb, hip and ankle within 6 months following JIA diagnosis and percentage of cumulative time with active disease > 35% within the first year. At the end of the study, both the median JADAS71 score and the Disability Index were significantly lower than the initial (p < 0.001) and remission off medication was achieved in 25% of the patients. Articular and extra-articular (only ocular) cumulative damage was demonstrated only in 5 and 7.5% of patients, respectively. Physical functional ability was found normal/mildly restricted in 93.3% and moderately restricted in 6.7% of the patients. We believe that these findings, fit in with a picture of JIA course and outcome under current conditions of objective “disease status” evaluation and of tightly controlled follow-up. Predictors emerged from our study could contribute to the identification of patients who will need early implementation of biologic treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Juvenile idiopathic arthritis (JIA) is the most common chronic rheumatic disease in childhood and one of the most common causes of children’s disability. Over the past 20 years, a revolution has occurred in both, the overtime evaluation of the “disease status” and the therapeutic strategies of the “disease management” [1,2,3,4]. Clinical heterogeneity of JIA as well as the need for an objective assessment of the disease activity during its course, to conduct reliable clinical trials and facilitate comparisons between relevant studies, led to the implementation of quantitative assessment tools [5]. The use of these tools was quickly extended to the routine clinical practice due to the introduction of biologic medications and the implementation of early aggressive treat-to-target (T2T) approach [2, 6,7,8].
The T2T-directed strategy in clinical practice, requires tight control of the disease status over its course, using simple and feasible assessment tools, easily applicable to a busy clinic, to define the: disease activity, response to treatment, disease damage, and health-related quality of life of patients and their families [9,10,11,12].
Publications of the last 15 years regarding the JIA course and outcome, either include patients who had their follow-up assessment over a wide time–space, consisting of periods of different therapeutic achievements or use widely different or poorly standardized clinical criteria for the assessment of the disease status, so that it is difficult to compare their results [13,14,15,16,17,18,19].
Our aim was to study the course and outcome of JIA in a cohort of patients who had been all diagnosed and followed-up at the same referral center, exclusively in the biologic era, their data were recorded systematically in an electronic medical recording program and they all had the same therapeutic options since their diagnosis. We used contemporary and standardized quantitative tools to assess the disease status throughout the study and identify prognostic factors regarding the disease progression and the need for early introduction of biologic therapy. To our knowledge, similar studies, fulfilling the criteria aforementioned, are limited at the time being [3, 20,21,22].
Patients and methods
Patients
This is both a retrospective and prospective study of 120 patients with JIA, who were selected out of the 253 patients who were firstly seen as outpatients between January 2001 and December 2010 at the Pediatric Immunology and Rheumatology Referral Center, 1st Department of Pediatrics, Aristotle University of Thessaloniki. Signed informed consent was obtained from the parents of all enrolled patients. The study was approved by the Research Committee of our University (Reference number 82628) and was in compliance with Helsinki Declaration.
There were two reasons that 2001 was chosen as the first year of patient enrollment in the study. Firstly, it was since 2001 that the validated Greek version of Childhood Health Assessment Questionnaire (CHAQ) [23] had been adapted in our Center’s routine clinical practice and secondly, at that time, the use of biologic medications had already been established [6].
The year 2010 was chosen as the last one of patient enrollment to have a 4-year minimum prospective follow-up. The last snapshot of each patient’s follow-up recorded was the closest one to December 2014.
Additional criteria for patient enrollment were: (1) systematic follow-up at our Center since the time of diagnosis, with an interval between two consecutive visits ≤ 6 months; (2) adequately completed medical records for the needs of this study; (3) age at the last snapshot of follow-up ≤ 18 years.
All patients were diagnosed and classified according to the International League of Associations for Rheumatology (ILAR) classification criteria revised in 2001 [24]. Their data were recorded in an electronic program which was created prior to the start of this study (1/2011), incorporating contemporary, internationally used classification and assessment tools. Clinical data necessary for evaluating the “disease status” using the appropriate “assessment tools” were extracted.
Methods
Evaluation of disease activity, clinical remission, flare/relapse
For quantification of the disease activity level, the assessment tool “JADAS-71” (Juvenile Arthritis Disease Activity Score) was used (range 0–101) [25], at predefined time-points during the disease course. On the other hand, the qualitative assessment of the “disease status” was based on the preliminary criteria for inactive disease (ID), clinical remission (CR) and flare, developed by Wallace et al. [10, 26].
Taking into consideration that the majority of JIA patients experience a chronic disease course displaying active and inactive states over the years, the “picture” of fluctuating disease activity for each patient was given by calculating the cumulative time with active and inactive disease. As “inactive disease state” was defined the period of time starting from the date of visit by which the patient met the Wallace et al. criteria, providing that in the preceding visit, the disease status was defined as “active” on the basis of the same criteria. As ending of this state was defined the date of visit by which the patient did not meet any more the preliminary criteria for ID. An “active disease state” was similarly defined. For each patient, the cumulative time with active disease was calculated and its proportion to the overall duration of the patient’s follow-up time was estimated and expressed as a percentage (%).
The time-interval between the initiation of treatment and the first snapshot of ID recorded was determined as the “time of response to initial treatment” [10, 26].
Between 2001 and 2011 the indications for starting biologic therapy in our center were based on the Greek Rheumatology Society’s “recommendations for the treatment of rheumatic diseases with biologic agents” reported online in 2001 [27]. These were either 3–6 months persistent disease activity under 1–2 conventional DMARDS or drug intolerance to at least one DMARD. Since 2011 indications were adapted to the therapeutic regimens recommended by the American College of Rheumatology (ACR) [28, 29].
Examination of our patients for JIA-associated uveitis (JIA-U) was done according to the guidelines of the American Academy of Pediatrics [30, 31].
Assessment of cumulative damage
Evaluation of the cumulative damage in our JIA patients was enabled by the assessment tool Juvenile Arthritis Damage Index (JADI). Articular damage was assessed by JADI-A (range 0–72), and extra-articular by JADI-E (range 0–17) [32].
Evaluation of the quality of life (QOL)
For evaluating the QOL in our JIA patients, we used the Greek version of CHAQ and the Disability Index (DI range 0–3, where 0 = best and 3 = worst) resulting from CHAQ [23, 33]. The CHAQ score was further divided into 3 categories: 0–0.5 = no disability or mild disability, 0.51–1.5 = moderate disability, and 1.51–3 = severe disability [13, 34].
Laboratory tests
Anti-nuclear antibodies (ANA) were measured in each patient by indirect immunofluorescence and rheumatoid factor (RF) was checked in all patients with polyarticular course by rate nephelometry. ANA were considered as positive if they were present twice, at least 3 months apart, at a titer of ≥ 1/160. RF was considered as positive if it was found above 15 IU/ml twice, at least 3 months apart.
Statistical analysis
Continuous variables were expressed with their median (min, max). Due to the relatively small sample size, nonparametric tests were used for the statistical analysis.
More specifically, comparison of continuous variables between two groups of patients was performed by means of the Mann–Whitney U test and comparison of quantitative variables among ILAR categories was performed by means of the Kruskal–Wallis test.
Dunn’s test was chosen as a posteriori test to assess the statistical significance of differences between pairs of patient groups. The strength of a linear relationship between paired continuous variables was measured by Pearson’s correlation coefficient.
Comparison of categorical data was performed by means of the Chi-square test or Fisher’s exact test, as appropriate. To identify predictive or prognostic factors univariate and multivariate analyses were performed. Statistical significance was assessed at the 0.05 level. Statistical analysis was conducted using SPSS (Statistical Package for Social Sciences, v 0.23.0; IBMSPSS, Armonk, NY, USA).
Results
Demographic, clinical and laboratory characteristics of the patients
Our patient cohort consisted of 120 children with JIA (female/male 103/17) who met the inclusion criteria, with a median age at JIA onset 3.5 years (range 0.8–13.3). Their main demographic, clinical and laboratory characteristics are shown in Table 1.
All patients had at least a 4 years prospective data recording. Of the 120 patients, 12 were diagnosed 1–11 months prior to their enrollment, so, their data were prospectively collected and analyzed and 108 had already been diagnosed 1–10 years before their enrollment and had been followed-up at the same center, meaning that their data were both retrospectively and prospectively collected and analyzed.
The median follow-up time of all patients was 8.04 years (range 4–14.1) and the median number of visits per patient was 27 (range 10–79).
Classification of patients to the disease subtypes [24] is shown in Table 1. Sixty-nine children (57.5%) had oligoarticular type of JIA onset; 42/69 patients (60.9%) remained in the persistent oligoarticular subtype whereas 27 (39.1%) progressed to the extended one. Most of the latter (59.3%) developed extension, at least 2 years after the disease onset, with a median time of extension 43.5 months (range 25–112).
The most commonly involved joints were knees (87.5%) and ankles (71.7%). Of the 120 patients, 26 (21.7%) developed JIA-U (10 unilateral and 16 bilateral). They belonged to oligoarticular extended (n = 11), oligoarticular persistent (n = 10) and polyarticular JIA (n = 5). In all cases the onset of arthritis clearly preceded uveitis, with a median lag time 26.5 months (range 4–99). Comparison between patients with JIA alone and those with additional JIA-U showed that more children with JIA-U were younger than 6 years at JIA diagnosis (25.7 vs 6.9%, p = 0.037), female (24.27 vs 0%, p = 0.022), had oligoarticular type of onset (28.99 vs 9.8%, p = 0.011) and were ANA positive (26.47 vs 13.46%, p = 0.044).
Positive ANA were found in 68/120 (56.7%) patients, 51/68 of the oligoarthritis (75%) and 17/68 of the polyarthritis group (25%). There were no significant differences between patients with ANA (+) oligoarthritis and ANA (+) RF (−) polyarthritis, regarding early age at the disease onset (p = 0.607), female predominance (p = 0.607), high frequency of JIA-U (p = 0.176) and presence of asymmetric arthritis (p = 0.66).
Quantification of the disease activity during the follow-up period
In an attempt to describe the clinical course of JIA in our patient cohort, we analyzed sequential episodes of the disease activity. We found that the median percentage (%) of cumulative time with active disease over the total follow-up period was 29.45% (range 1.54–96.71).
The median JADAS71 score at the disease onset and before initiation of any medication (initial JADAS71 score) was significantly higher than the median JADAS71 score at the last examination [9.5 (range 0.4–73.2) vs. 0 (range 0–26), respectively (p < 0.001)].
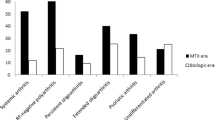
The median percentage of cumulative time with active disease, independently of the JIA type of onset, was significantly higher in patients with polyarticular course compared to those with oligoarticular course [32.01% (range 2.9–96.71) vs. 21.45% (range 1.54–71.91) (p < 0.006)]. Particularly, it differed significantly between patients with extended oligoarthritis [40.16% (range 26.15–77.78)] and those with persistent oligoarthritis [21.45% (range 1.54–71.91)] (p = 0.003), systemic [17.01% (range 2.9–34.51)] (p = 0.012) and psoriatic arthritis [13.33% (range 9.09–21.79)] (p = 0.039) (Fig. 1). In contrast, the median percentage (%) of cumulative time with active disease did not differ significantly between patients with extended oligoarthritis and those with RF (−) polyarthritis [40.16% (range 26.15–77.78) vs. 29.73% (range 4.38–96.71), p = 0.138] (Fig. 1). In addition, patients aged ≤ 6 years at the disease onset (91/120) were found to have a significant higher percentage (%) of cumulative time with active disease than those aged > 6 years [31.48% (range 1.54–96.71) vs. 21.92% (range 4.38–77.78), p = 0.018]. Finally, the percentage (%) of cumulative time with active disease was significantly higher in JIA patients with JIA-U than in those without JIA-U [42.24% (range 18.75–76.56) vs. 26.15% (range 1.54–96.71), p < 0.0001], as well as in patients who had required treatment with biologic agents than in those who had not needed such treatment [37.94% (range 10.16–96.71) vs. 24.57% (range 1.54–71.91), p < 0.0001].
Within the group of patients with oligoarticular type of onset, the initial median JADAS71 score of patients who progressed to extended oligoarthritis was significantly higher than that of patients who remained in the persistent oligoarthritis [8 (range 3–18.5) vs. 6 (range 0.4–14.2), p = 0.032]. Particularly, patients with initial JADAS71 score > 9 were more likely to develop extended oligoarthritis (p = 0.023).
Furthermore, patients with a high initial JADAS71 score, had a longer time of response to initial treatment than those with a lower initial JADAS71 score (p < 0.009). Besides, patients who had required treatment with biologic agents had significantly higher initial JADAS71 score, than those who had not needed such treatment [13.5 (range 0.6–73.2) vs 8.3 (range 0.4–34), p < 0.002]. Finally, a negative correlation was observed between the initial JADAS71 score and the time-interval for introduction of biologic agents (the higher the initial JADAS71 score, the earlier the introduction of biologic agents) (p < 0.015).
Predictors of progression to the extended oligoarticular JIA subtype
Performing univariate and multivariate analyses to identify potential predictors of an extended disease course, we found that the only independent predictors of progression to the extended oligoarticular subtype were: involvement of upper limb, hip and ankle within the first 6 months following diagnosis of JIA, as well as percentage of cumulative time with active disease > 35% within the first year of the disease course (Table 2).
Quantitative assessment of response to treatment
During the study period, 116 of the 120 patients (96.7%) were treated with conventional Disease Modifying Antirheumatic Drugs (DMARDS). Moreover, 49/116 (40.8%) required the introduction of biologic medications. The biologic agents used are shown in Table 3.
Regarding the use of biologic medications within the different disease subtypes (Table 4), it is worth mentioning that the percentage of patients with extended oligoarthritis who required biologic treatment was significantly higher than that of patients with persistent oligoarthritis (48.15 vs 19.05%, p = 0.01). On the other hand, there was no significant difference between the percentages of patients with extended oligoarthritis and those with RF (−) polyarthritis who needed treatment with biologics (48.15 vs 51.43%, p = 0.8).
Patients with at least one time-period of ID within the first 12 months since the disease onset (85.7%) had a significantly lower median percentage (%) of cumulative time with active disease during the overall follow-up, compared to those who had their first time-period of ID after the first 12 months of follow-up [27.27% (range 1.54–75) vs. 48.68% (range 15.96–96.71), p < 0.001].
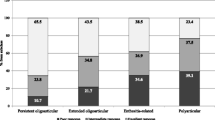
During the study period, “remission on medication” was achieved in 114/120 (95%) patients, with a median duration of 20 months (range 1–80). Remission off medication was achieved in 45/120 (37.5%) patients until the end of the study. There was a significant difference between the percentages of patients with persistent oligoarthritis and those with extended oligoarthritis who ever accomplished remission off medication (52.38 vs 18.52%, p = 0.004). At the last visit 30/120 (25%) patients were still in remission off medication (Table 5).
Assessment of long-term cumulative damage
Among the 120 patients, 6 (5%) had articular damage (JADI-A > 0), with a median score of JADI-A 2 (range 1–38) and 9 (7.5%) had extra-articular damage (JADI-E > 0), with a median score of JADI-E 3.5 (range 2–4). In all cases with JADI-E > 0, ocular damage was the only extra-articular item reported. The median percentage of cumulative time with active disease was significantly higher in patients with JADI-A > 0 and JADI-E > 0 compared to those without damage detected [38.23% (range 14.29–96.71) vs. 28.32% (range 1.54–77.78) (p = 0.033) and 45.07% (range 15.29–75) vs. 28.27% (range 1.54–96.71) (p = 0.035), respectively].
Assessment of quality of life
The DI differed significantly between the first and the last examination in all patients [0.375 (range 0–2.5) vs. 0 (range 0–1), p < 0.001]. Regarding physical functional ability at first presentation, normal or mildly restricted was reported in 73/120 (60.8%), moderately restricted in 39/120 (32.5%) and severely restricted in 8/120 (6.7%) patients. At last examination, physical functional ability was reported as normal or mildly restricted in 112 (93.3%) and as moderately restricted in 8 (6.7%) patients; no patient presented with severely restricted physical functional ability.
Discussion
In literature, most of the authors, who investigated the course and outcome of JIA, refer to the time-periods of different therapeutic options or the use of different clinical criteria for the disease status assessment or the short time-period of total follow-up and long intervals between consecutive visits, as limitations in their studies, so that it is difficult to compare their results [13,14,15,16,17,18,19, 35,36,37]. In this regard, our study has the advantages of including patients who had been all diagnosed and followed-up at the same tertiary center, in a period of time with the same therapeutic options (the biologic era); their follow-up was longitudinal with regular, tightly controlled visits and the disease status was evaluated by cοntemporary and standardized assessment tools. These prerequisites resulted to the low number of patients recruited and this could be a limitation of the study. Finally, patient data were recorded in an appropriate, detailed electronic medical record system. These advantages confer reliability to our findings reflecting the course and outcome of JIA under the same and current conditions of the disease–status evaluation and management. In favor of this argument is a very recent publication of an international task force regarding recommendations for treating JIA to target [38]. Analytically, results of this study support the following issues.
Regarding the demographic, clinical and laboratory data of the study population, it was demonstrated that there was a sixfold female predominance, the median age at the disease onset was < 6 years and in the majority of cases (70%), JIA was diagnosed within the first 3 months after the onset of symptoms.
Classification of our patients showed that the most prevalent group was persistent oligoarthritis (57.5%). The group of enthesitis-related arthritis (ERA) was small (3.3%) and polyarthritis RF+ patients were lacking, meaning that the prevalence of the disease types in our patient cohort is not representative. This is partly due to the limitation of the Greek law according to which, all specialized pediatric centers are authorized to accept children ≤ 14 years of age at first presentation. This prerequisite, along with the criterion to have a 4 year prospective data recording, have led to the low rate of ERA patients and may indeed be a selection bias but it is beyond our means.
Nevertheless, it has also been reported that in European and North American white populations [39] oligoarthritis accounts for 50–80% of all children with chronic arthritis, which is in line with our findings. Regarding the ERA type, a comparable low frequency (2.7%) is mentioned in a recent study [40], whereas in most of the European epidemiological studies a frequency of approximately 10% is reported [41].
Uveitis associated with JIA was demonstrated in 21.7% of our JIA patients and this is consistent with rates mentioned in other recent publications (11.6–30%) [42, 43] suggesting that the early aggressive treatment of the joint disease with DMARDs (conventional or biological) in the last decade, has led to lowering the proportion of patients who develop uveitis [44]. Clinical characteristics of children who developed JIA-U were consistent with relevant literature data [44,45,46].
An overlap in clinical profile was observed between children with ANA (+) oligoarthritis and ANA (+) RF (−) polyarthritis, concerning early age at the disease onset, female predominance, high frequency of JIA-U and presence of asymmetric arthritis. These findings are similar to those of Ravelli et al [47] and justify the debate among investigators who wonder whether ANA positivity and young age at JIA onset, should be determinants in classification or not and propose the definition of a new type of JIA, independent of the number of joints involved or the presence of psoriasis [48, 49].
Assessing the disease activity, we demonstrated that the percentage of cumulative time with active disease during the follow-up period was significantly higher in patients with polyarticular course [extended oligoarthritis and RF (−) polyarthritis] compared to that of the remaining disease groups, including systemic arthritis. This could be attributed to the fact that 2/8 patients with systemic JIA had monocyclic course and the remaining 6 started biologic treatment during the first year of the disease course. Furthermore, the proportions of patients with extended oligoarthritis and RF (−) polyarthritis who required biologic medications were similar. These findings support the view that extended oligoarthritis and RF (−) polyarthritis share common characteristics, as far as the disease course and need for biologic treatment are concerned.
Focusing on patients with oligoarticular JIA onset, it was found, that 39.1% of them progressed to extended oligoarthritis after a mean time of 3 years. This is in agreement with other references [50, 51] mentioning that 10–50% of patients with oligoarticular JIA onset progress to extended oligoarthritis within the first 5 years of follow-up.
Various clinical parameters have been proposed in the literature, as potential predictors of an extended disease course [50, 51]. We have confirmed the upper limb and ankle involvement at the disease onset and have added the hip involvement within 6 months following JIA diagnosis plus a percentage of cumulative time with active disease > 35% within the first year of the disease course.
A worth noting finding was the initial median JADAS71 score in patients with the oligoarticular type of onset who eventually progressed to the extended oligoarthritis. This was found to be significantly higher compared to that of patients who remained with persistent oligoarthritis. Particularly, patients with initial JADAS71 score > 9 were more likely to develop extended oligoarthritis. These findings acquire special meaning, considering that JADAS71 score is an easily feasible assessment tool in the routine clinical practice, ideally suitable to measure the patient disease activity over time and, according to our findings, to predict progression to the extended oligoarticular JIA.
Moreover, a high initial JADAS71 score, was found to be indicative for early introduction of biologic agents in the patients’ treatment. This finding is in accordance with recently published data of Swart et al., who found that clinical JADAS71 (without erythrocyte sedimentation rate) identifies patients in need for anti-tumor necrosis factor (anti-TNF) treatment at 3 and 6 months after start of methotrexate and is a user-friendly tool for treat-to-target in JIA [21].
As for the response to initial treatment and achievement of inactive disease (ID), correlations of JADAS71 score with various disease parameters, showed that, the higher the initial JADAS71 score was, the longer the time-interval between initiation of treatment and succession of inactive disease. These findings lead to the consumption that the initial JADAS71 score may be a good predictor of the disease course and outcome.
Another interesting observation, in this study, was that the majority of patients (85.7%) with at least one time-period of ID within the first year of disease course had a significantly lower mean percentage of cumulative time of active disease than those who had their first time-period of ID after the first year.
During the whole follow-up of the study, 37.5% of the patients succeeded remission off medication. At the last visit, after a median follow-up period of approximately 8 years, 25% of our cohort was still in remission off medication. In the extended oligoarthritis group, a significantly lower percentage of patients (18.52%) succeeded remission off medication, compared to that of the persistent oligoarthritis group (54.76%). These findings are in line with those of Wallace et al. [26], who reported that the proportion of patients with extended and persistent JIA oligoarthritis, who accomplished remission off medication, within 4 years of follow-up, was 31 and 68%, respectively. Our results are also comparable with those of a recently published meta-analysis, including studies in which Wallace criteria were also used [36]. In another relevant meta-analysis Shoop-Worrall et al. conclude that the frequency of current remission increases with increasing disease duration from 7% at 18 months to around 40% after at least 10 years [37].
Results concerning the cumulative long-term disease damage showed low percentages of patients with JADI-A > 0 (5%) or JADI-E > 0 (7.5%) compared to those of relevant studies. Solari et al. [13], analyzing a cohort of 310 patients, with a mean disease duration of 6.7 years, recorded higher proportions of patients with JADI-A > 0 (32.4%) and JADI-E > 0 (26.1%). This difference could be attributed to the fact that most of their patients had their disease onset before the biologic era, so that only 11.3% had received biologicals. Comparison of our findings with those of other relevant studies is difficult, mainly due to the differences among the study populations [52, 53].
Finally, comparing our findings about the patient quality of life with those recorded by Solari et al. [13], we found that the proportion of patients with normal or mildly restricted physical functional ability was high in both studies (93.3 and 80.8%, respectively) whereas the proportion of patients with moderately and severely restricted physical functional ability were lower in our study (6.7 and 0 vs 15.9 and 3.3%, respectively).
Synopsizing, the following factors emerged as early indices of poor prognosis regarding the disease course and need for early implementation of biologic treatment: young age at the disease onset (≤ 6 years), high level of disease activity at first presentation (initial JADAS71 score > 9), presence of uveitis, polyarticular course, failure to accomplish an inactive disease state within the first year of specialized medical care and cumulative time with active disease > 35% within the first year of disease course. To our opinion these findings are representative of JIA course and outcome under current conditions of the “disease status” evaluation and management.
References
Shenoi S (2017) Juvenile idiopathic arthritis—changing times, changing terms, changing treatments. Pediatr Rev 38:221–232
Giancane G, Consolaro A, Lanni S, Davi S, Schiappapietra B, Ravelli A (2016) Juvenile idiopathic arthritis: diagnosis and treatment. Rheumatol Ther 3:187–207
Guzman J, Oen K, Tucker LB et al (2015) The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the reACCh-out cohort. Ann Rheum Dis 74:1854–1860
Cimaz R, Marino A, Martini A (2017) How I treat juvenile idiopathic arthritis: a state of the art review. Autoimmun Rev 16:1008–1015
Consolaro A, Giancane G, Schiappapietra B et al (2016) Clinical outcome measures in juvenile idiopathic arthritis. Pediatr Rheumatol Online J 14:23
Hayward K, Wallace CA (2009) Recent developments in anti-rheumatic drugs in pediatrics: treatment of juvenile idiopathic arthritis. Arthritis Res Ther 11:216
Webb K, Wedderburn L (2015) Advances in the treatment of polyarticular juvenile idiopathic arthritis. Curr Opin Rheumatol 27:505–510
Wallace CA, Giannini EH, Spalding SJ et al (2012) Childhood arthritis and rheumatology research alliance. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheumatol 64:2012–2021
Wallace CA (2010) Developing standards of care for patients with juvenile idiopathic arthritis. Rheumatology 49:1213–1214
Wallace CA, Ruperto N, Giannini EH (2004) Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol 31:2290–2294
Magni-Manzoni S, Ruperto N, Pistorio A et al (2008) Development and validation of a preliminary definition of minimal disease activity in patients with juvenile idiopathic arthritis. Arthritis Rheumatol (Arthritis Care Res) 59:1120–1127
Lovell DJ, Ruperto N, Giannini EH, Martini A (2013) Advances from clinical trials in juvenile idiopathic arthritis. Nat Rev Rheumatol 9:557–563
Solari N, Viola S, Pistorio A et al (2008) Assessing current outcomes of juvenile idiopathic arthritis: a cross-sectional study in a tertiary centre sample. Arthritis Rheumatol 59:1571–1579
Adib N, Silman A, Thomson W (2005) Outcome following onset of juvenile idiopathic inflammatory arthritis. II. Predictors of outcome in juvenile arthritis. Rheumatology 44:1002–1007
Adib N, Silman A, Thomson W (2005) Outcome following onset of juvenile idiopathic inflammatory arthritis. I. Frequency of different outcomes. Rheumatology 44:995–1001
Oen K (2002) Long-term outcomes and predictors of outcomes for patients with juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol 16:347–360
Ravelli A (2004) Toward an understanding of the long-term outcome of juvenile idiopathic arthritis. Clin Exp Rheumatol 22:271–275
Flato B, Lien G, Smerdel A et al (2003) Prognostic factors in juvenile rheumatoid arthritis: a case–control study revealing early predictors and outcome after 14.9 years. J Rheumatol 30:386–393
Arguedas O, Fasth A, Anderson-Gare B (2002) A prospective population based study on outcome of juvenile chronic arthritis in Costa Rica. J Rheumatol 29:174–183
Consolaro A, Van Dijkhuizen EHP, Espada G et al (2017) Development of new JADAS and cJADAS cut-offs for disease activity states in oligoarthritis and RF-negative polyarthritis from a large multinational cohort of children with juvenile idiopathic arthritis. Pediatr Rheumatol 12(Suppl 2):64
Swart JF, Van Dijkhuizen EHP, Wulffraat NM, De Roock S (2018) Clinical Juvenile Arthritis Disease Activity Score proves to be a useful tool in treat-to-target therapy in juvenile idiopathic arthritis. Ann Rheum Dis 77:336–342
Nordal E, Zak M, Aalto K, Berntson L, Fasth A, Herlin T et al (2011) Ongoing disease activity and changing categories in a long-term nordic cohort study of juvenile idiopathic arthritis. Arthritis Rheumatol 63(9):2809–2818
Pratsidou-Gertsi P, Vougiouka O, Tsitsami E et al (2001) Paediatric Rheumatology International Trials Organisation. The Greek version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clin Exp Rheumatol 19:S76–S80
Petty RE, Southwood TR, Manners P et al (2004) International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31:390–392
Consolaro A, Ruperto N, Bazsco A et al (2009) Development and validation of a Composite Disease Activity Score for juvenile idiopathic arthritis. Arthritis Rheumatol (Arthritis Care Res) 61:658–666
Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N, For the Childhood Arthritis and Rheumatology Research Alliance (CARRA), the Pediatric Rheumatology Collaborative Study Group (PRCSG), the Paediatric Rheumatology International Trials Organisation (PRINTO) (2011) American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 63: 929–936
Greek Rheumatology Society (2001) Greek Rheumatology Society’s recommendations for the treatment of rheumatic diseases with biologic agents (article in Greek). http://www.ere.gr/Pdf/eresystaseis.pdf. Accessed in 2001
Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, Morgan DeWitt E et al (2011) 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthitis Care Res 63:465–482
Ringold S, Weiss PF, Beukelman T, DeWitt EM, Ilowite NT, Kimura Y et al (2013) 2013 Update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Care Res 65:1551–1563
Cassidy J, Kivlin J, LindsIey C, Nocton J (2006) Ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics 117:1843–1845
Jabs DA, Nussenblatt RB, Rosenbaum JT (2005) Standardization of uveitis nomenclature for reporting clinical data: results of the first international workshop. Am J Ophthalmol 140:509–516
Duffy CM (2005) Measurement of health status, functional status, and quality of life in children with juvenile idiopathic arthritis: clinical science for the pediatrician. Pediatr Clin N Am 52:359–372
Ruperto N, Ravelli A, Pistorio A, For the Paediatric Rheumatology International Trials Organisation (PRINTO) et al (2001) Cross-cultural adaptation and psychometric evaluation of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ) in 32 countries. Review of the general methodology. Clin Exp Rheumatol 19:S1–S9
Magni-Manzoni S, Pistorio A, Labo E et al (2008) A longitudinal analysis of physical functional disability over the course of juvenile idiopathic arthritis. Ann Rheum Dis 67:1159–1164
Oen K, Tucker L, Huber A et al (2009) Predictors of early inactive disease in a juvenile idiopathic arthritis cohort: results of a Canadian multicenter, prospective inception cohort study. Arthritis Rheum (Arthritis Care Res) 61:1077–1086
Glerup M, Herlin T, Twilt M (2017) Clinical outcome and long-term remission in JIA. Curr Rheumatol Rep 19:75. https://doi.org/10.1007/s11926-017-0702-4
Shoop-Worrall S, Kearsley-Fleet L, Thomson W, Verstappen S, Hyrich K (2017) How common is remission in juvenile idiopathic arthritis: a systematic review. Semin Arthritis Rheumatol 47(3):331–337
Ravelli A, Consolaro A, Horneff G et al (2018) Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis 77(6):819–828
Petty RE, Cassidy JT (2011) Textbook of pediatric rheumatology. Saunders Elsevier, Philadelphia
Marzetti V, Breda L, Miulli E, Filippetti F, Mancini C, Chiarelli F et al (2017) Clinical characteristics of juvenile idiopathic arthritis in an area of central Italy: a population based study. AnIg 29:281–292
Colbert RA (2010) Classification of juvenile spondyloarthritis: enthesitis-related arthritis and beyond. Nat Rev Rheumatol 6:477–485
Angeles-Han ST, Pelajo CF, Vogler LB et al (2013) Risk markers of juvenile idiopathic arthritis-associated uveitis in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry. J Rheumatol 40:2088–2096
Moradi A, Amin RM, Thorne JE (2014) The role of gender in juvenile idiopathic arthritis-associated uveitis. J Ophthalmol 2014:461078
Clarke S, Sen E, Ramanan A (2016) Juvenile idiopathic arthritis-associated uveitis. Pediatr Rheumatol 14:27
Heiligenhaus A, Heinz C, Edelsten C, Kotaniemi K, Minden K (2013) Review for disease of the year: epidemiology of juvenile idiopathic arthritis and its associated uveitis: the probable risk factors. Ocul Immunol Inflamm 21:180–191
Angeles-Han S, McCracken C, Yeh S, Jenkins K, Stryker D, Rouster-Stevens K et al (2015) Characteristics of a cohort of children with juvenile idiopathic arthritis and JIA-associated uveitis. Pediatr Rheumatol 13:19
Ravelli A, Felici E, Magni-Manzoni S et al (2005) Patients with antinuclear antibody-positive juvenile idiopathic arthritis constitute a homogeneous subgroup irrespective of the course of joint disease. Arthritis Rheumatol 52:826–832
Ravelli A, Varnier GC, Oliviera S et al (2011) Antinuclear antibody-positive patients should be grouped as a separate category in the classification of juvenile idiopathic arthritis. Arthritis Rheumatol 63:267–275
Martini A (2012) It is time to rethink juvenile idiopathic arthritis classification and nomenclature. Ann Rheum Dis 71:1437–1439
Guillaume S, Prieur AM, Coste J, Job-Deslandre C (2000) Long-term outcome and prognosis in oligoarticular-onset juvenile idiopathic arthritis. Arthritis Rheumatol 43:1858–1865
Al-Matar MJ, Petty RE, Tucker LB, Malleson PN, Schroeder ML, Cabral DA (2002) The early pattern of joint involvement predicts disease progression in children with oligoarticular (pauciarticular) juvenile rheumatoid arthritis. Arthritis Rheumatol 46:2708–2715
Sarma P, Misra R, Aggarwal A (2008) Physical disability, articular, and extra-articular damage in patients with juvenile idiopathic arthritis. Clin Rheumatol 27:1261–1265
De Oliveira Sato J, Corrente JE, Saadmagalhaes C (2011) Progression of articular and extraarticular damage in oligoarticular juvenile idiopathic arthritis. Clin Exp Rheumatol 29:871–877
Author information
Authors and Affiliations
Contributions
Study design: FKT, MT, PPG, PN. Follow-up and recruitment of patients: FKT, MT, PN, PPG. Patient data recording, electronically: PN. Analysis and interpretation of data: PN, FKT, MT, EF. Statistical analysis: PN, PB. Manuscript preparation: PN, FKT. Manuscript critical review: FKT, FP.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nalbanti, P., Kanakoudi-Tsakalidou, F., Trachana, M. et al. Juvenile idiopathic arthritis in the biologic era: predictors of the disease progression and need for early introduction of biologic treatment. Rheumatol Int 38, 1241–1250 (2018). https://doi.org/10.1007/s00296-018-4062-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-018-4062-9