Abstract
The purpose of this study was to evaluate the reliability of Chinese version of the ankylosing spondylitis quality of life questionnaire (ASQoL) for AS patients. All the enrolled AS patients should fulfill five questionnaires (BASDAI, BASFI, DFI, BAS-G and ASQoL) by himself, then the investigators did physical examination of the patients, fulfilling BASMI. Physical function has a strong correlation with QoL in patients with AS. In different disease activity groups, ASQoL had a correlation with BASFI, especially in the moderate activity group (γ = 0.66, P < 0.0001). All four questionnaires in entanacept treatment group improved distinctly on week 6 and 12 comparing to baseline. There were significantly correlations of changing between ASQoL and BAS-G, BASDAI and BASFI after treatment with etanercept in AS patients. The Chinese ASQoL questionnaire is valuable to evaluate the activity of AS patients and effect of biologic agent treatment in patients with AS. It is a good generic instrument to measure QoL in patients with AS in China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disorder of the spine that affects skeletal and extra-skeletal tissues. AS occurs particularly among young men in the workforce, and the patients have pain, morning stiffness and disability, which increase with duration of the disease. The main results of the disease are functional disability and decrease in the QoL. Chamberlain reported that two-third of male patients had difficulty at work; one-third had social problems, and up to two-third reported having difficulty with sexual activity. Reactive depression and frustration were noted, together with impaired self-esteem and social skills. Energy related problems are also widely reported. All these features demote significant effects the disease on lifestyle [1]. Determination of disability and QoL in AS can help the clinician to assess illness-related suffering and to develop management strategies.
Health status is a measure of how a person feels and functions, and includes assessment of the severity of symptoms, the impact of symptoms and activity limitations on functioning, and the impact of illness on a person’s ability to participate in life. There are many different kinds of instruments to evaluate the QoL in patients with ankylosing spondylitis, such as short form-36 (SF-36), the Dougados Gunctional index (DFI) [2], the health assessment questionnaire for the spondylarthropathies (HAQ-S) [3], the arthritis impact measurement scales 2 specific to AS (AS-AIMS2) [4]. But all of them do not inform on the impact of the condition on QoL. This study adopts the instrument—ankylosing spondylitis quality of life questionnaire (ASQoL), which developed by Doward et al. [5]. This measure adopted the needs-based model that postulates that QoL is dependent on an individual’s ability to meet his or her needs. It is a fixed-response questionnaire that asks endorsement (yes/no) of 18 items related to symptoms, functioning, and disease-related concern. The ASQoL questionnaire has been validated in UK and The Netherlands.
The aim of this study was first to evaluate which factors could affect the physical function and ASQoL in patients with AS, to determine the relationship between functional status and ASQoL in a Chinese population, and to study the reliability and serviceability of Chinese version of the ankylosing spondylitis quality of life questionnaire (ASQoL).
Materials and methods
This study was carried out at the third hospital of Sun Yat-sen University in Guangzhou, People's Republic of China between January 2004 and June 2004. Hundred and sixteen AS patients (102 male, 14 female) who fulfilled the modified New York criteria [6] were included. Patients with severe concomitant medial illness were excluded. The trained rheumatologist evaluated all patients. Demographics and disease-related variables were collected.
The physician global assessments of the patients were done on a visual analog scale (VAS) from 0 (very well) to 10 (very bad). The global pain was also assessed on a VAS from 0 (no pain) to 10 (maximum pain). Disease activity was assessed by the BASDAI (from 0 = no activity to 10 = maximum) [7]. We defined three levels of disease activity. A score of <4 meant mildly active disease, a score of 4–6 indicated moderate disease activity, and a score of >6 defined severe disease activity [8, 9]. A metrology index, BASMI (from 0 = the best metrology to 10 = the worst) was also applied [10]. The same researcher evaluated all patients.
Physical function was assessed using BASFI (from 0 = very well to 10 = very bad) [11]. Quality of life was assessed with ASQoL, which contained 18 items. The original English version of the ASQoL was translated and adapted to a Chinese version by one bilingual rheumatologists and one bilingual general specialist. Then the Chinese version ASQoL was back translated into English by two other bilingual specialists who had corrected the mistakes. The translation and back-translation was done twice. Then the test–retest evaluation was done in 20 randomly selected patients. So the Chinese ASQoL was validated. Each item on the ASQoL was given a score of “1” or “0”. A score of “1” was given where the item was affirmed, indicating adverse QoL. All item scores were summed to give a total score or index. Scores could range from 0 (good QoL) to 18 (bad QoL).
A randomized, double-blind, placebo controlled trial was designed to investigate whether our Chinese ASQoL had the similar value in evaluating the result after treatment with etanercept, an anti-tumor necrosis factor-αfusion receptor protein, compare to other indexes such as BASDAI, BASFI and BAS-G, etc. Only patients fulfilling the 1984s modified New York criteria for AS with positive HLA-B27 were included. These patients were those who had severe disease activity that was defined by a BASDAI of 4 or greater and spinal pain of 4 or greater on a 10 cm visual analogue scale, failed to DMARDs or NSAIDs therapy for at least 3 months. If the patient was treated with NSAIDs, the dose should be stable for at least 4 weeks. DMARDs treatment was forbidden during the trial and should be stopped for at least 4 weeks before baseline. Patients were excluded if they had current tuberculosis confirmed by chest plain or positive PPD test, history of malignant disease or multiple sclerosis, current infection, positive result of HBsAg, anti-HCV antibody or anti-HIV antibody. Patients were also excluded if they had signs and symptoms of severe renal, hepatic, hematological, pulmonary, cardiac, gastrointestinal, endocrine or neurological diseases. We also excluded patients if they had anti-TNF-αtreatment before. Pregnant and lactation women were forbidden in this trial. All patients provided written informed consent and the trial were approved by local ethics committees.
Patients received etanercept 25 mg or placebo twice weekly by subcutaneous injection during the first 6 weeks and then all patients received etanercept 25 mg (manufactured by Shanghai Zhongxin Parmacautical) injection twice a week at the following 6 weeks. The following questionnaires were filled out at baseline, week 6 and 12: ASQoL, BASDAI, BASFI and BAS-G.
With the patients’ permission, the blood samples were taken to determine ESR and hs-CRP. The ESR was assessed in mm/h using the Westergren method (normal range 0–20) and hs-CRP in mg/l by the enzyme-linked immunosorbent assay method (normal range 0–6.8).
We performed multiple correlations and multiple regression analysis to study the data. All statistical analysis was performed using the SAS 6.12 software package. Statistical significance was determined at P < 0.05.
Results
-
1.
The study included 102 males (89%) and 14 (11%) females with a mean age (mean ± SD) of 31.8 ± 8.8 years. The mean disease duration (mean ± SD) is 8.8 ± 7.0 years. All the patients fulfilled the questionnaires by themselves, and the mean scores of clinical, functional and laboratory measures, including BASDAI, BASFI, BASMI, BAS-G, Pain-VAS, ASQoL, ESR and hs-CRP, are listed in Table 1.
-
2.
Positive response to each item of the ASQoL was listed in Table 2. Among the 18 items of ASQoL, fatigue, pain and depression got the highest scores. The positive of them are 94 (81.0%), 81 (69.8%) and 69 (59.5%), respectively.
-
3.
The correlation between these indexes.
Both of the scores of BASFI and ASQoL correlated significantly with BASDAI, BASMI, BAS-G, Pain-VAS as well as with laboratory parameters including ESR and hs-CRP (Table 3). Pearson’s correlation analysis demonstrated that the strongest factors correlating with physical function were the score of ASQoL, BASDAI and Pain-VAS, and the strongest factors correlating with QoL were the score of BASFI, Pain-VAS and BASDAI.
Multiple regression models were constructed to identify variables associated with physical function and ASQoL. In the first model, the BASFI as dependent variable and the clinical and laboratory measures as explanatory variables revealed three contributing variables (ASQoL, BASMI and BASDAI), significantly explaining 66% of the total variance of the physical function (Table 4). The strongest predictive variable was the ASQoL score. In the second model, the ASQoL as dependent variable and the clinical and laboratory measures as explanatory variables revealed only one variable (BASFI), significantly explaining 59% of the total variance of the physical function (Table 5).
-
4.
To evaluate the correlation between ASQoL and other indexes in different active AS patients
According to the BASDAI scores, we divided the patients into three groups: mildly active group (n = 61), moderate disease group (n = 36) and severe disease group (n = 19). We performed multiple correlations to the variables (Table 5). In the first group, the ASQoL had a correlation with ESR, BAS-G, BASDAI, and BASFI. In the second group, the ASQoL only had a correlation with BASFI. In the last group, ASQoL had a correlation with BASFI, Pain-VAS, and BASMI. It showed no matter what extent of the diseases activities were, ASQoL had a correlation with BASFI.
-
5.
Value of ASQoL on evaluating the effect after 3 months treatment with etanercept.
In this trial 43 AS patients, 40 males and 3 females, were included. Twenty-one patients were distributed to entanercept group and 22 to placebo group randomizedly. Forty-one of them completed the trial and two were lost in the last visit because of compliance.
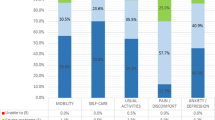
Figure 1 compared the change of the questionnaires after treatment with entanercept between the entanercept and placebo groups. We could see that on the beginning of treatment the questionnaires between groups had no significant difference. And on week 6 the scores of entanercept group were markedly better than those of placebo group in all four questionnaires. On week 12 BAS-G and BASFI scores of entanercept group were still better than those of placebo group.
Figure 2a, b showed changes of questionnaires within individual group after treatment with etanercept or placebo. In entanercept group all four questionnaires improved distinctly on week 6 and 12 comparing to baseline. In placebo group BAS-G and BASDAI score improved significantly after 6-week placebo therapy but BASFI and ASQoL score did not, and on week 12 all four questionnaires improved markedly comparing to baseline.
The correlation of changing between ASQoL and BAS-G, BASDAI and BASFI three indexes after treatment with etanercept or placebo in AS patients (Table 6).
Discussion
Patients suffering from chronic pain syndromes may have distress, negative feelings, and dissatisfaction in many aspects of life. According to our results of patients with AS, fatigue (81.0%), pain (69.8%) and depression (59.5%) get the highest score among the eighteen questions in ASQoL questionnaire (Table 2). In another study, Ward MM identified aspects of health-related quality of life in 175 patients with AS. Of the 23 quality of items, the most prevalent concerns involved stiffness, pain, fatigue and poor sleep. The scores of fatigue, pain and distress are 62.4, 83.1 and 28.7%, respectively [15]. The aspect of fatigue and pain also got high scores. Pain and fatigue were recognized as major health concerns for patients with AS, and our study confirms that almost 70% patents are affected by this symptoms. In addition, more than 80% patients have problem with fatigue. Depression has been established as a common reaction to rheumatoid arthritis and has been investigated among people with other forms of arthritis. No evidence was found to support the stereotype of the “typical” ankylosing spondylitis patient as being less depressed than people with other forms of arthritis [12]. So the depression in patients with AS is not uncommon. Whether these patients in depression will have problems with social interactions need further studies.
In this study, the wide ranges of the scores of the disease-related variables reflect the broad spectrum of our population with AS. The regression modes suggest that QoL(ASQoL score), activity of the disease (BASDAI score) and metrology (BASMI score) are the main factors associated with physical function in AS, and physical function is the main factor associated with QoL (Table 4). The study in Spanish also showed activity of the disease and metrology are main factors associated with physical function [13]. The same conclusion can get from these two studies: metrology index associated with physical function in AS. BASMI can assess accurately axial status that was regarded as cervical, dorsal and lumbar spine, hips and pelvic soft tissue, and define clinical significant changes in spinal movement. And it usually takes several minutes to ask the patients to answer the questionnaires when clinicians see patients. So it should be first recommended to adopt to reflect indirectly the physical function status in AS in China mainland.
There have been few studies suggesting a relationship between QoL and functional status in AS patients [14–16]. The study in Turkey showed the most important determinants in self-reported QoL were the levels of functional disability and disease activity [17]. In our study, no matter what the extent of the diseases activity are, ASQoL has a correlation with BASFI (Table 7), which is accordant to the former study in Turkey. But our results also showed that ASQoL was less influenced by disease activity. It is inconsistent with the former results. Some factors should be considered: firstly, quality of life status was assessed with the Turkish version of Nottingham health profile (NHP) in the Turkey study. We adopted the ASQoL as an index. The NHP focuses predominantly on symptoms (impairment) and functioning (disability). It does not inform on the impact of the condition on QoL. Our results also showed functional disability had a correlation with disease activity. Quality of life takes account of the effect of impairments and disability on the patient in addition to other influences including personality, social and physical environment, economic resources, and cultures. The ASQOL is specific to AS and adopts the needs-based model, which postulates that QoL is dependent on an individual’s ability to meet his or her needs. The measure is shown to be superior to the NHP in terms of relevance of its content to patients and its psychometric properties. Secondly, a selection bias must be considered. There were a small proportion of patients with concomitant peripheral arthritis comparing with the number of the patients who only have axial involvement. One study recently reported that disease activity measured by the BASDAI was higher in patients with concomitant peripheral disease compared with patients with disease restricted to the axial skeleton [18]. And if we take the total patients as a whole integrity, the results showed that BASFI, Pain-VAS and BASDAI were more significantly related QoL (Table 3). So whether the QoL is influenced by the disease activity is not sure. It needs further study.
Levels of acute-phase reactants (erythrocyte sedimentation rate or C-reactive protein level) are elevated in only a limited number of patients with AS. A normal value for acute phase reactants does not rule out active disease. The discriminative power of ESR and Hs-CRP does not comprehensively represent the disease process in AS. There was some evidence for an association of ESR and CRP with disease activity in AS [19]. And recent studies concluded that neither CRP nor ESR was superior in assessing disease activity [9, 19]. Another study in Turkey showed clinical measures of disease activity and functional disability correlated more with CRP than with ESR [17]. In our study, although we used the high sensitive method that could detect lower lever concentration of CRP, both physical function and QoL correlated more with ESR than with HS-CRP. It still needs further study by increasing the sample numbers.
There have been only two reports studying the value of ASQoL on evaluate the efficacy after treating with biologic agents in AS patients, one about etanercept [20] and the other about anakinra [21 ], an interleukin 1 receptor antagonist. In the two open studies, there were significant improvements of ASQoL after treating with biologic agents, as well as other functional and disease activity indexes, such as BASFI and BASDAI, laboratory measures reflecting inflammation (ESR and CRP), and radiographic evidence of enthesitis such as MRI of spine and sacroiliac joints. In our randomized controlled trial, ASQoL improved significantly in etanercept group compare to placebo group at week 6. And at week 12 ASQoL improved significantly in both groups. That proved the above conclusions. Besides, BASDAI and BAS-G showed significant difference at week 6 in placebo group while ASQoL and BASFI did not. It suggested that the two front indexes were influenced by other subjective factors more and maybe ASQoL was better to evaluate the efficacy.
In conclusion, physical function and ASQoL of patients with AS are damaged in a significant way. Quality of life, physical function and disease activity improved markedly after biologic agent therapy and the physical function index and QoL index were influenced by subjective factors less than disease activity indexes. Trying to maintain physical activity can increase QoL in patients suffering from AS. If the clinicians can pay more attention on maintaining physical activity, the ultimate goal of health care that is to increase QoL in patients suffering from AS can be made. The ASQoL may be used as generic instruments to measure health-related QoL in patients with AS. Our translated Chinese ASQoL questionnaire is a reliable and useful instrument to evaluate the physical function and the quality of life (QoL) in patients with AS in China.
References
Chamberlain MA (1983) Soci-economic effects of ankylosing spondylitis in females: a comparison of 25 female with 25 male subjects. Int Rehabil Med 5:149–153
Dougados M, Gueugen A, Nakache JP et al (1988) Evaluation of the functional index and an articular index in ankylosing spondylitis. J Rheumatol 15:302–307
Daltroy LH, Larson MG, Liang MH (1990) A modification of the health assessment questionnaire for the spondyloarthropathies. J Rheumatic 17:946–950
Guillemin F, Challier B, Uriacher F et al (1999) Quality of life in ankylosing spondylitis: validation of the ankylosing spondylitis arthritis impact measurement scales2, a modified arthritis impact measurement scales questionnaire. Arthritis Care Res 12:157–162
Doward LC, Spoorenberg A, Cook SA et al (2003) Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis 62:20–26
Van der Linden S,Valkenburg HA et al (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27:361–368
Garrett S, Jenkinson T, Kennedy LG et al (1994) A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). J Rheumatic 21:2286–2291
Borman P, Bodur H, Bingol N et al (2001) Bone mineral density and bone turnover markers in a group of male ankylosing spondylitis: relationship to disease activity. J Clin Rheumatic 7:315–321
Spoorenberg A, van der Heijde D, Klerk E et al (1999) Relative value of erythrocyte sedimentation rate and C-reactive protein in assessment of disease activity in ankylosing spondylitis. J Rheumatol 26:980–984
Jenkinson TR, Mallorie PA, Whitelock HC et al (1994) Defining spinal mobility in ankylosing spondylitis (AS): the Bath AS Metrology Index. J Rheumatic 21:1694–698
Calin A, S Garrett et al (1994) A new approach defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Guncrional Index. J Rheumatol 21:2281–2285
Barlow JH, Macey SJ, Struthers GR et al (1993) Gender, depression, and ankylosing spondylitis. Arthritis Care Res 6:45–51
Ariza-Ariza R, Hernandez-Cruz B, Navarro-Sarabia F (2003) Physical function and health-related quality of life of Spanish patients with ankylosing spondylitis. Arthritis Rheum 49:483–487
Challier B, Urlacher F, Vancon G et al (1999) Is quality of life affected by season and weather conditions in ankylosing spondylitis? Clin Exp Theumatol 12:247–255
Ward MM (1998) Quality of life in patients with ankylosing spondylitis. Rheum Dis Clin North Am 24:815–824
Ward MM (1999) Health-related quality of life in ankylosing spondylitis: a survey of 175 patients. Arthritis Care Res 12:247–255
Bostan EE, Borman P, Bodur H (2003) Functional disability and quality of life in patients with ankylosing spondylitis. Rheumatol Int 23:121–6
Heuft-Dorenbosch L, van Tubergen A, Spoorenberg A et al (2004) The influence of peripheral arthritis on disease activity in ankylosing spondylitis patients as measured with the Bath Ankylosing Spondylitis Disease Activity Index. Arthritis Rheum 51:154–159
Ruof J, Stucki G (1999) Balidity aspects of erythrocyte sedimentation rate and C-reactive protein in ankylosing spondylitis: a literature review. J Rheumatol 26:966–970
Marzo-Ortega H, McGonagle D, O’Connor P, Emery P (2001) Efficacy of etanercept in the treatment of the entheseal pathology in resistant spondylarthropathy: a clinical and magnetic resonance imaging study. Arthritis Rheum 44:2112–2117
Tan AL, Marzo-Ortega H, O’Connor P, Fraser A, Emery P, McGonagle D (2004) Efficacy of anakinra in active ankylosing spondylitis: a clinical and magnetic resonance imaging study. Ann Rheum Dis 63:1041–1045
Acknowledgments
Dr. Gu JR’s work is supported by National science grant 30471611 in China and the Distinguished Young Scientist research Grant 30325019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhao LK and Liao ZT contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhao, L.K., Liao, Z.T., Li, C.H. et al. Evaluation of quality of life using ASQoL questionnaire in patients with ankylosing spondylitis in a Chinese population. Rheumatol Int 27, 605–611 (2007). https://doi.org/10.1007/s00296-006-0267-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-006-0267-4