Abstract
Color-coded sonography is an interesting option for the diagnosis of temporal arteritis. We present our experiences regarding examination technique and diagnostic accuracy, comparing biopsy and clinical results in a series of 83 patients with suspected temporal arteritis. A dark halo around the vessel wall (representing inflammatory oedema), reduced or absent vessel wall pulsations (demonstrated by M mode), and vessel occlusions were used as diagnostic criteria. Forty-eight patients underwent biopsy of the temporal artery following ultrasound examination. Comparing these findings with biopsy yielded a sensitivity of 73%, specificity of 93%, positive predictive value (PPV) of 96%, and negative predictive value (NPV) of 58%. The halo sign alone had a lower sensitivity (67%). Comparison with overall clinical assessment (n=83) yielded a sensitivity of 65%, specificity of 100%, PPV of 100%, and NPV of 73%. Irregular atherosclerotic vessel wall changes were the main differential diagnosis. Important pitfalls were false focus setting, too much/less color gain, and ‘bifurcation halo’. In conclusion, a positive sonographic result in combination with typical clinical signs might replace the need for biopsy, while a negative result should not be used for exclusion of temporal arteritis. Considering the low PPV and high NPV of the clinical criteria defined by the American College of Rheumatology, color-coded sonography is a useful tool in the noninvasive diagnostic workup of temporal arteritis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temporal or giant-cell arteritis is the most common form of vasculitis in older Caucasians. It frequently affects the superficial temporal artery but also larger, mainly supra-aortal arteries. Its clinical presentation is manifold, varying from the typical temporal headache and vision loss to atypical forms with e.g. isolated jaw claudication or diplopia. In many cases, it starts with nonspecific systemic symptoms, and the decision of when to perform a biopsy for confirmation of diagnosis is often difficult.
In 1990, the American College of Rheumatology (ACR) proposed five criteria (age ≥50 years, ESR >50 mm, new onset of headache, abnormal palpation of the temporal artery, and positive biopsy), of which three have to be fulfilled for the diagnosis of temporal arteritis. Despite good sensitivity and specificity and an excellent negative predictive value (NPV) of 99%, the positive predictive value (PPV) of these criteria has been reported to be as low as 29% [1]. This low positive predictive value is due to the less specific noninvasive ACR criteria 1–4, which alone could lead to a positive result. In fact, biopsy is usually performed to confirm the diagnosis of temporal arteritis. However, it is not tolerated by all patients, and rebiopsy in equivocal cases is problematic. Rarely, in patients with skin necrosis or retrograde flow in the ophthalmic artery, biopsy is even contraindicated.
Over the last few years, color-coded (duplex) sonography has attracted increasing interest as a noninvasive diagnostic tool for patients suspected of having temporal arteritis [2]. This approach evolved with the development of high frequency, high-resolution ultrasound scanners. As a main sign, a dark (hypoechoic) so-called halo around the vessel wall was found to be pathognomonic for acute arteritis [3]. However, very few studies reporting on larger collectives of patients have been published so far [3, 4, 5]. Furthermore, ultrasonographic examination techniques and possible pitfalls, which might have influenced the somewhat contradictory findings of recent studies [5, 6], have not been extensively delineated and discussed.
In the following material, we present our experiences using color-coded sonography in the diagnostic workup of suspected temporal arteritis. We address questions of examination technique, possible misinterpretations, differential diagnoses on imaging, and the diagnostic accuracy of our series by comparing ultrasound with biopsy and clinical results.
Materials and methods
Patients
Between July 1999 and July 2002, 83 patients were examined using color-coded sonography (49 women, mean age 68±11 years). They were mostly referred from the Departments of Ophthalmology or Rheumatology of the University of Freiburg in Germany, where they received complete clinical examination. No treatment longer than 6 days with steroids had been done in 79 patients; the remaining four had received steroids for more than 15 days. In each patient, the ACR criteria for temporal arteritis (TA) were assessed at the time of presentation, and the clinical diagnosis of TA was suspected or at least possible. Prior to the temporal artery investigation, all patients underwent extracranial Doppler/duplex sonography and, if necessary, transcranial Doppler and/or duplex sonography. This was done to (1) assess atherosclerotic disease, (2) check for possible arteritic involvement of larger arteries, and (3) exclude retrograde flow in the supratrochlear artery prior to ipsilateral biopsy (the superficial temporal artery would be a brain-supplying vessel in this situation).
Ultrasound technique and settings
We used a modern ultrasound scanner (HDI 3500/5000, ATL, Bothell, Wash., USA) with a small, high-frequency linear transducer (5–10 MHz, ATL, length 26 mm) (Fig. 1). System settings were as follows: color scale 5, chrome scale 3, color gain approximately 85% (adjusted), color sensitivity low, color wall filter <20 Hz, color persistence medium, PRF 350–1000 Hz, and focus point adjusted to vessel depth typically 5 mm. The power mode was used in special cases but usually not of advantage in comparison with the color mode. For assessment of vessel wall pulsations, the M mode was used.
Examination technique
All parts of the vessel were illustrated in two different planes (axial, longitudinal) and as continuously as possible. The examination conditions are illustrated in Fig. 1. Examinations were performed by one investigator and, in complex or equivocal cases, by two (MR and/or AH). In general, the examination started by identifying the main stem of the superficial temporal artery in front of the tragus in the axial plane. The proximal main stem is usually not visible, and often one can see an elongated or curled course as it ascends from behind the neck of the mandible. Usually the main stem divides into the frontal and parietal branches on a level with the top of the auricle. Considerable anatomic variety has been described previously [7]: the most common form (94%) is a bifurcation into a frontal and parietal ramus. The frontal ramus regularly runs straight in the frontal direction, while the parietal ramus follows the direction of the main stem. Less frequent are trifurcation (2.5%) and a single strong frontal ramus with recurrent parietal branches (2.5%). If the patient complains of occipital or nuchal headache, it is necessary to examine additionally the occipital artery, which is usually found over the mastoid processus. However, imaging the distal parts of the often tortuous vessel about 4 cm laterally to the external occipital protuberance is difficult because of patients’ hairs. Thorough bilateral examination takes 30 min on average.
Biopsy was performed unilaterally in 48 patients. Usually, a sample of approximately 2 cm was excised from the distal frontal branch. If a unilateral halo was present, then the biopsy was performed on this side. In 35 cases, biopsies were not performed: this was due to establishment of other clinical diagnoses in 31 cases and refusal to undergo biopsy in four. Biopsy was regarded as positive if vasculitis of the temporal artery with predominantly mononuclear or granulomatous cell infiltrations were found, while the presence of giant cells was not a prerequisite [8, 9].
For validation of ultrasound findings in all patients, overall assessment based on clinical ACR criteria and results of clinical workup (leading to other diagnoses) was made in those who did not undergo biopsy regarding the likelihood of temporal arteritis, and patients were assigned to ‘positive’ (n=43) or ‘negative’ (n=40) groups. Patients revealing positive biopsies were always defined as ‘positive’ (regardless of other ACR criteria), and those with negative biopsies were regarded as clinically positive when all other ACR criteria were clearly fulfilled and no other diagnosis was established (n=3).
Results
Typical signs on ultrasound
The incidence of the following main ultrasound signs for all patients as well as for the subgroup undergoing biopsy is given in Table 1.
Hypoechoic vessel wall thickenings (halo sign)
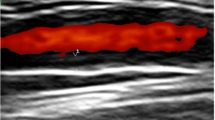
Typical illustrations are shown in Fig. 2. Usually the halo appeared totally anechoic (i.e., black). With modern, high-resolution scanners, we also observed sporadically clearly delineated hypoechoic halos. Less hypoechoic or even hyperechoic segmental vessel wall thickenings were observed in 17 of the patients who did not fulfill the ultrasound criteria. These were interpreted as being due to atherosclerosis. Looking at the extracranial carotid arteries, clear atherosclerotic changes (plaques, stenosis) were present in all of these patients (100%), in only 19 (50%) of those without atherosclerosis of the temporal artery, and in 16 (55%) of those who fulfilled the ultrasound criteria.
Hypoechoic vessel wall changes in acute arteritis (halo sign) of an 80-year old man with biopsy-proven, acute temporal arteritis. a Main stem of the common superficial artery. Typical halo effect in the axial plane. b The same vessel segment with halo in the longitudinal plane (long arrows). Note the less hypoechoic reflexes in the edematous vessel wall (short arrows). c Distal frontal branch showing pseudo-occlusion due to vessel wall edema. Using a very high color gain, only few pixels light up within the lumen (short arrows), while the rest of the vessel has no detectable color flow signal (long arrows)
Occlusion or pseudo-occlusion
Occlusion or pseudo-occlusion was assumed only if a nonperfused vessel was visible, not in case of a nondetectable branch. The latter was observed in three cases (in two of which the parietal branch was not detectable), all with negative biopsy. Mostly, the occlusions were present in the distal frontal branch. In one patient with a positive biopsy, we observed the preceding stage of pseudo-occlusion: a massive halo with a filiform or only spot-like color signal in combination with segments without a visible color signal (Fig. 2c). The Doppler signal showed only a small systolic peak in this vessel. In another patient, occlusion of the main stem of the temporal artery was observed. The examination was performed 6 days after starting treatment with high-dose prednisolone; biopsy was positive and showed an intraluminal thrombus. Only one of the biopsy-negative patients had an occlusion (distal frontal branch). This was presumably due to arterial embolism from a high-grade stenosis of the external carotid artery on the same side.
Reduced or missing vessel wall pulsations
M mode illustrations are shown in Fig. 3. Segmentally reduced pulsations were also observed in patients with presumed arteriosclerosis who did not undergo biopsy, while total absence of pulsations was more likely to indicate positive histology. Overall, it tended to be the less specific than the other criteria.
Comparison with biopsy results and overall clinical assessment
Sensitivity, specificity, and predictive values in comparison with biopsy are shown in Table 2. Furthermore, to include patients who did not undergo biopsy, values are also given in comparison with overall assessment of temporal arteritis as positive (n=43) or negative (n=40). For ultrasonographic diagnosis of temporal arteritis, the presence of a true halo in one or more vessel segments was regarded as sufficient. If no halo was present, the two other, ‘minor’ criteria (i.e., abnormal pulsation and occlusions) had to be fulfilled.
Discussion
Color-coded sonography is a valuable, noninvasive approach to diagnosis of temporal arteritis. Despite promising initial results, only a few studies have been published so far on this issue. A dark halo around the vessel wall is accepted to be the main sign of temporal arteritis [2, 4, 5]. It is most probably due to vessel wall edema in the acute stage of inflammation.
Sensitivity and specificity values of the halo in our study were in the range of that reported by Schmidt et al. [2], with a trend toward lower sensitivity (67% vs 80% for histology, 60% vs 71% for clinical diagnosis). Other, recent studies comparing ultrasound with biopsy reported sensitivity values of 40–86% and specificity values of 78–100% [4, 5, 10]. Predictive values might be more meaningful in individual cases. In accordance with other studies [2, 3], we found very high PPV of ultrasound, while our NPV was considerably lower. In contrast, two recent studies found poor PPV (≤50%) of the halo sign [4, 5].
Thus, the individual examination technique and diagnostic acceptance of a halo is obviously different between the latter studies [4, 5] and others, including the present work [2, 3, 10]. A number of issues have to be addressed in this context:
-
1.
There are important misinterpretations and possible pitfalls when looking for a halo (Table 3).
Table 3 Possible pitfalls -
2.
The main differential diagnostic findings in our study were less hypoechoic or even hyperechoic, irregular, segmental vessel wall thickenings (Fig. 4). Given the age of the patients, we interpreted these changes as being due to atherosclerosis. As known from sonography of the carotid arteries, it is not uncommon that atheromatous plaques are hypoechoic [11]. Usually, however, they are eccentric, and differentiation to a local halo should be made by this criterion. On the other hand, if these changes are hyperechoic, they can not be reliably differentiated from fibrotic postarteritic vessel wall changes. Even biopsy is not able to differentiate between these two states [12].
Fig. 4 Atherosclerotic vessel wall changes, here of the temporal artery, can be hypoechoic (atheromatous, long arrows) or hyperechoic (atherosclerotic, short arrows). They often appear irregular and are not circular. No relevant stenosis was present in this case. The image shows the example of a 69-year-old man who also had marked atherosclerotic plaques at the carotid bifurcation with bilateral severe stenosis of the external carotid artery
-
3.
There are presently no commonly accepted criteria for exact definition of a halo. Its thickness has been reported to lie between 0.3 mm and 1.2 mm, while a recent publication postulated a thickness of ≥1 mm for diagnosing a halo [3, 5]. A number of other studies including ours did not assess the thickness of the halo but interpreted it as a qualitative criterion [4, 10]. In our experience, it is sufficient to observe a circumferential dark hypoechoic zone around the perfused lumen with a thickness of at least one quarter that of the perfused lumen.
-
4.
In some cases, the inflammations could be segmentally located in distal parts of the vessel, which are more difficult to assess by ultrasonography because of their small diameter and the limited resolution of ultrasound. In these cases, halos may be hard to detect.
-
5.
Finally, there might actually be arteritis without any halo. In a previous study exactly comparing histological and sonographic results, we found that four of eight patients with false negative ultrasound had only minor inflammatory infiltration confined to one vessel layer or the vasa vasorum. This probably indicates a very early stage of inflammation, which might lack vessel wall edema [13].
When discussing the sensitivity, specificity, and predictive value of ultrasound, the applied reference standard has to be critically assessed. Even for biopsy, false negative results have been reported in about 10–20% due to segmental manifestation of arteritis [14, 15]. Thus the presence of a halo despite negative biopsy results might be due to skip lesions and different sites of ultrasonographic signs and biopsy. Another reference point is the comprehensive clinical assessment of the presence of temporal arteritis. This approach has been used in a number of previous studies, especially when biopsy was not performed regularly [3, 4]. In our study, only the NPV increased significantly when comparing the overall clinical assessment with biopsy as a reference. This can be explained by the fact that most patients without biopsy (who were additionally considered in the overall clinical assessment group) were assessed as not having temporal arteritis. On the other hand, patients with negative ultrasonographic results undergoing biopsy usually had clear clinical signs and were thus more likely to have positive biopsy results. This bias is a limitation of the present study.
In occlusions, halos are no longer present in the sense of color signals surrounded by dark edema. We found low sensitivity but high PPV for this sign. In two cases with occlusions, halos might well have been present in preceding stages up to pseudo-occlusion. Therefore, the occlusion criterion is important, in our view, as it might represent the climax of a halo and restriction to the halo sign alone might be an oversimplification in some cases. Occlusions, however, can also occur with fibrotic changes of chronic inflammation and be of atherosclerotic origin. A differentiation to the latter might be possible by concomitant atherosclerotic changes of nonoccluded vessel segments and examination of the carotid bifurcation. It is important to emphasize that occlusions should be diagnosed only if the vessel can be clearly delineated on ultrasound, not in case of undetectable vessels, which are more likely due to anatomical variability of the ramification [7].
Reduced, asymmetric, or missing pulsations of the temporal artery have been described as a sensitive sign on clinical examination [16]. We therefore introduced the semiquantitative assessment of vessel wall pulsations in the M mode as a further diagnostic criterion. Usually, the presence or absence of pulsations can already be roughly assessed in the B mode image. The main pitfall when assessing pulsations is loss of the exact plane of the vessel when switching to the M mode, leading to a false positive diagnosis. Of the single criteria, it had the best sensitivity and the lowest specificity. It might thus mainly be helpful in combination with the more specific halo and occlusion sign. Overall, inclusion of conspicuous pulsations and the occlusion sign as second-order criteria led to increased sensitivity in our study.
A limitation of our study might be that we did not regularly record flow velocities and thus stenoses of the temporal artery. A pure Doppler flow study using a complex score rating stenosis and occlusion of the temporal, facial, and ophthalmic artery found a PPV of 70% and an NPV of 85% for detection of temporal arteritis [17]. The lower PPV might be explained by the low specificity of stenosis. Also, in the study of Schmidt et al., introduction of the criterion ‘stenosis or occlusion’ increased the sensitivity (but decreased the specificity) [3]. However, it should be noted that isolateḋ stenosis occurred only in six of 24 patients fulfilling the ‘stenosis/occlusion’ criteria in this study, while the remaining patients also had occlusion [3]. Stenoses due to active arteritis are likely to be due to inflammatory vessel wall thickenings which are already reflected by halos. Thus, we believe that—in contrast to the occlusion criterion—inclusion of the Doppler sonographic stenosis criterion does not considerably increase the diagnostic value of color-coded ultrasound.
In conclusion, different examination techniques and definitions of positive sonographic signs might explain the divergent results of previous studies. We outlined a number of differential diagnoses and pitfalls. In our hands, ultrasound had excellent PPV and moderate NPV. Therefore, a negative result should not be used for exclusion of temporal arteritis. However, a positive result in combination with typical clinical signs might also replace the need for biopsy. Also, considering the low PPV but high NPV of the clinical ACR criteria, ultrasound is a useful additional tool in the noninvasive diagnostic workup of temporal arteritis. A commonly accepted consensus and standard on ultrasound criteria and examination technique is strongly needed before further studies on larger patient populations are performed.
References
Rao JK, Allen NB, Pincus T (1998) Limitations of the 1990 American College of Rheumatology classification criteria in the diagnosis of vasculitis. Ann Intern Med 129:345–352
Schmidt WA (2000) Doppler ultrasonography in the diagnosis of giant cell arteritis. Clin Exp Rheumatol 18:S40–S42
Schmidt WA, Kraft HE, Vorpahl K, Volker L, Gromnica-Ihle EJ (1997) Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 337:1336–1342
Nesher G, Shemesh D, Mates M, Sonnenblick M, Abramowitz HB (2002) The predictive value of the halo sign in color Doppler ultrasonography of the temporal arteries for diagnosing giant cell arteritis. J Rheumatol 29:1224–1226
Salvarani C, Silingardi M, Ghirarduzzi A, Lo SG, Macchioni P, Bajocchi G, Vinceti M, Cantini F, Iori I, Boiardi L (2002) Is duplex ultrasonography useful for the diagnosis of giant-cell arteritis? Ann Intern Med 137:232–238
Schmidt WA, Gromnica-Ihle E (2002). Incidence of temporal arteritis in patients with polymyalgia rheumatica: a prospective study using colour Doppler ultrasonography of the temporal arteries. Rheumatology (Oxford) 41:46–52
Daumann C, Putz R, Schmidt D (1989) The course of the superficial temporal artery. Anatomic studies as a prerequisite to arterial biopsy [German]. Klin Monatsbl Augenheilkd 194:37–41
Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, Lie JT (1990) The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 33:1122–1128
Schmidt D, Loffler KU (1994) Temporal arteritis. Comparison of histological and clinical findings. Acta Ophthalmol (Copenh) 72:319–325
Schmid R, Hermann M, Yannar A, Baumgartner RW (2002) Color duplex ultrasound of the temporal artery: replacement for biopsy in temporal arteritis [German]. Ophthalmologica 216:16–21
Tegos TJ, Kalomiris KJ, Sabetai MM, Kalodiki E, Nicolaides AN (2001) Significance of sonographic tissue and surface characteristics of carotid plaques. AJNR Am J Neuroradiol 22:1605–1612
Cox M, Gilks B (2001) Healed or quiescent temporal arteritis versus senescent changes in temporal artery biopsy specimens. Pathology 33:163–166
Schmidt D, Hetzel A, Reinhard M, Auw-Haedrich C (2003). Comparison between color duplex ultrasonography and histology of the temporal artery in cranial arteritis (giant cell arteritis). Eur J Med Res 8:1–7
Ashton-Key MR, Gallagher PJ (1992) False-negative temporal artery biopsy. Am J Surg Pathol 16:634–635
Hall S, Persellin S, Lie JT, O’Brien PC, Kurland LT, Hunder GG (1983) The therapeutic impact of temporal artery biopsy. Lancet 2:1217–1220
Dixon AS, Beardwell C, Kay A, Wanka J, Wong YT (1966) Polymyalgia rheumatica and temporal arteritis. Ann Rheum Dis 25:203–208
Puechal X, Chauveau M, Menkes CJ (1995) Temporal Doppler-flow studies for suspected giant-cell arteritis. Lancet 345:1437–1438
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reinhard, M., Schmidt, D. & Hetzel, A. Color-coded sonography in suspected temporal arteritis—experiences after 83 cases. Rheumatol Int 24, 340–346 (2004). https://doi.org/10.1007/s00296-003-0372-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-003-0372-6