Abstract
Large-vessel vasculitis (LVV) is a group of diseases mainly comprised of giant-cell arteritis (GCA), Takayasu arteritis, and a series of rare diseases like Behçet’s disease, IgG4-related disease, infectious aortitis, and other unfrequent entities. Besides clinical and laboratory features, Doppler sonography (DS) can assist in establishing the diagnosis. Its diagnostic sensitivity has been evaluated in various studies, most of them, however, in temporal arteritis (TA) respectively in LVV with involvement of the temporal artery. Little is known in extracranial LVV. We retrospectively evaluated the diagnostic accuracy of DS in 30 patients with extracranial, non-temporal LVV using the highly sensitive PET/CT as method of reference in comparison to 20 controls who were found to have no LVV. We investigated ten arterial sites and documented the presence of the sonographic halo sign. Sensitivities of DS for LVV were highest in the subclavian and axillary arteries (71.4%/72.2%) and low in the abdominal aorta (26.1%) and the common femoral artery (16.7%). DS detected 24 out of 30 cases of LVV (overall sensitivity 80.0%). The LVV cases where DS was completely negative did not significantly differ in leukocyte count, C-reactive protein, or erythrocyte sedimentation rate from LVV cases with positive DS. DS is a potent method in diagnosing extracranial LVV especially in the axillary and the subclavian arteries. Aortic, intraabdominal, and lower extremity artery manifestations, however, are often missed by DS. A second imaging modality (e.g., PET/CT) is therefore required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The diagnosis of large-vessel vasculitis (LVV) can be challenging since clinical signs and symptoms are heterogeneous and might be misleading. Giant-cell arteritis (GCA) with or without temporal arteritis (TA) is by far the most prominent representative of LVV [1]. Takayasu arteritis and Behçet’s disease are less common [2], and entities such as IgG4-related aortitis [3], infectious aortitis (e.g., in syphilis), and Cogan’s syndrome [4] are considered to be rare causes of LVV. The leading clinical features are myalgiform limb pain, fever, weight loss, and night sweats. Laboratory studies are characterized by increased C-reactive protein (CRP) levels and an elevated erythrocyte sedimentation rate (ESR). Color Doppler sonography (DS) is capable of detecting vascular inflammation displayed as hypoechoic wall thickening reflecting vessel wall edema (also known as halo sign [5]), stenosis, or complete vessel occlusion. Its sensitivity in TA ranges between 10 and 100% [6,7,8,9], and in extracranial LVV, it is reported to be between 55 and 100% [10,11,12]. The standard of reference in most of these studies was either the American College of Rheumatology (ACR) criteria from 1990 and/or a temporal artery biopsy (TAB).
18-Fluoro-deoxyglucose positron emission tomography in hybrid with computed tomography (FDG-PET/CT) is an emerging diagnostic technique in the field of LVV [13,14,15]. Its strength lies in its capability to perform a whole body scan in a single session and to diagnose LVV in an early state of the disease, i.e., in a phase of T cell and macrophage recruitment and vessel wall infiltration before the formation of edema [16,17,18,19]. Evidence is mounting that FDG-PET/CT might be more sensitive than MRI [16]. However, in contrast to ultrasound, PET/CT is costly, is not widely available, and comes with a significant radiation exposure. We therefore evaluated DS at various arterial sites in 30 patients with LVV diagnosed by clinical, laboratory, and PET/CT findings and compared our findings with 20 controls.
Patients and methods
Patients
We retrospectively assessed 50 consecutive patients with suspected LVV who presented themselves to our departments between March 2012 and June 2016. We obtained a thorough medical history and a complete physical examination from all patients. In each patient, we documented leukocyte count (normal range 4.5–10/nl), C-reactive protein levels (normal range < 5 mg/l), and the erythrocyte sedimentation rate (normal range < 15 mm after 1 h), as well as anti-neutrophile antibodies (ANCA, immunofluorescence) and anti-nuclear antibodies (ANA, immunofluorescence) where available. All subjects received a Doppler sonography (DS) of the temporal and the extracranial arteries. Patients with suspected or proven TA were excluded from the study. PET/CT was performed in all patients. Patients in whom LVV was suspected on the basis of their symptoms, examination, and/or laboratory workup, but in whom PET/CT found no evidence of LVV, were included as control cases. In some of the control patients, PET/CT revealed other diagnoses such as malignancy, infection, or other autoimmune diseases. DS always preceded PET/CT in that the sonographers were blinded to the results of the PET/CT. The radiologist performing PET/CT had no knowledge of the ultrasound findings. Approval of the local ethics committee was obtained for analysis of this retrospective cohort (Medizinische Ethikkommission II, Universitätsmedizin Mannheim, approval no. 2016-843R-MA).
Doppler sonography (DS)
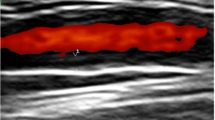
Both LVV and control patients underwent Doppler sonography of the common carotid, subclavian, axillary, and femoral arteries bilaterally as well as of the abdominal aorta and the proximal parts of its visceral branches, i.e., the truncus coeliacus and the superior mesenteric artery. A circumferential hypoechoic wall thickening greater than 1.5 mm not showing typical signs of atherosclerosis such as hyperechoic depositions with dorsal sound cancelation was classified as halo sign suggestive for vasculitis (Fig. 1). Spectral Doppler analysis was performed to investigate a hemodynamically relevant stenosis. We used an Aplio 400 (Toshiba, Minato, Japan) with a linear transducer head (7.5–14 MHz) for extra-abdominal arteries and a curved array transducer head (3–7 MHz) for the abdominal and retroperitoneal vessels. A total of three experienced sonographers performed the examinations in our study. Uncertain cases were debated, and consensus was reached. There was no blinded second investigation. Two of the three sonographers were certified level 2 and 3, respectively (3 being the highest level of expertise, i.e., ultrasound trainer), by the German Society of Ultrasound in Medicine (DEGUM).
PET/CT
After an overnight fasting, blood glucose level was measured and patients received 250 MBq of 18F-fluorodeoxyglucose (FDG) intravenously. One hour after injection, images were acquired from vertex to knees in three-dimensional mode with 1.5 min per bed position using dedicated PET/CT scanners (Philips Gemini TF 64 and Siemens Biograph mCT). Images were reconstructed using the CT data for attenuation correction.
The images were analyzed primarily by visual interpretation of transverse, coronal, and sagittal slices. Visual results were substantiated by placing regions of interest over the wall of affected vessels and over the liver which served as reference organ [20]. Vessels with a circumferential and linear uptake in their wall equal to or higher than the uptake of the liver were considered positive for vasculitis [20, 21]. Strong atherosclerosis-causing tracer enrichments potentially leading to falsely positive diagnosis of vasculitis were excluded by evaluating the according CT images. None of the patients were under immunosuppressive therapy at the time of the PET. Figure 2 shows a typical PET-positive LVV.
Diagnosis of LVV
There are no classification or diagnosis criteria for general LVV. The 1990 ACR criteria for GCA mainly apply for TA and are not suited for extracranial large-vessel GCA [22]. In our cohort, we diagnosed active LVV when appropriate clinical symptoms, elevated CRP and/or ESR, tracer uptake in FDG-PET/CT in at least one large artery consistent with vasculitis, and a response to immunosuppressive treatment were present. Patients > 50 years of age were classified as GCA in accordance with the revised 2012 Chapel Hill consensus criteria (CHCC) [23]. Takayasu arteritis was diagnosed according to the ACR criteria from 1990. Patients with isolated LVV of the aorta were also included in our study.
Statistical analyses
All statistical analyses were performed using the SPSS v11.5. We calculated means with standard deviations and t tests for parametric and Mann-Whitney U tests for non-parametric comparisons. To calculate sensitivities, specificities, and positive and negative predictive values (PPV, NPV), we applied crosstables using χ 2 test. Significance was defined as a two-tailed p < 0.05.
Results
We analyzed data from 50 patients. In 30 (60.0%), we diagnosed LVV. The remaining 20 (40.0%) were included as control patients. The mean age was 63.3 ± 12.6 years (range 26–85). The male to female ratio in the entire cohort was 1:3.2. In the control group, the female contingent was 60.0% compared to 86.7% in the LVV group reflecting the fact that LVV, especially GCA, is more common in women. That difference was statistically significant (p < 0.05). A complete list of patient characteristics is presented in Table 1.
Unlike in gender distribution, the two groups were not different regarding age, leukocyte count, CRP levels, and ESR (age p = 0.293, 95% CI −3.387–10.954; leukocytes p = 0.394, 95% CI −2468–0.990; CRP p = 0.877, 95% CI −44.804–38.369; ESR p = 0.322, 95% CI −28.781–9.663).
In patients diagnosed with LVV, 26/30 (86.7%) were classified as GCA, 3/30 (10.0%) had isolated aortitis, and 1/30 (3.3%) was diagnosed with Takayasu arteritis.
Clinical and laboratory findings
Night sweats were more common in patients diagnosed with LVV (LVV 14/30, 46.7% vs. control 2/20, 10.0%, p < 0.01), whereas fever (LVV 8/30, 26.7%, control 5/20, 25.0%) and weight loss (LVV 16/30, 53.3%, control: 8/20, 40.0%) were not statistically different between the two groups.
Mean leukocyte count was 10.0 ± 2.8/nl in the LVV group compared to 9.2 ± 3.2/nl in the control group, and CRP and ESR tended to be higher in patients with LVV (CRP 99.7 ± 59.6 vs. 96.5 ± 85.3 mg/l; ESR 87.8 ± 33.9 vs. 78.2 ± 31.3); however, for all three evaluated laboratory values, differences were not statistically significant.
In 25/30 (83.3%) patients, we diagnosed aortic involvement. In cases of PET positivity in the aorta fever, night sweats and weight loss did not differ significantly from those who had no aortic involvement (fever p = 0.104, night sweats p = 0.105, weight loss p = 0.280). Leukocytes, CRP, and ESR tended to be higher in patients with PET-positive aorta; however, a modest statistically significant difference was only achieved for leukocytes (p < 0.05, 95% CI −5.658 to −0.462). In patients with PET-proven LVV, clinical and laboratory parameters were not statistically different between patients who were completely unremarkable in DS compared with those who had at least one positive DS sign at any arterial site.
Diagnosing LVV by Doppler sonography and PET/CT
In all patients, we investigated ten arterial sites by DS as mentioned above, and all patients underwent PET/CT. None of our patients had clinical or sonographic signs of TA involvement. All 20 control patients were PET negative regarding vasculitis. In 24/30 cases of PET-proven LVV, DS detected a halo sign in at least one arterial vessel leading to an overall sensitivity of 80.0% with a specificity of 70.0% (PPV 80.0%, NPV 70.0%). Analyzing each artery separately, we found the highest diagnostic yield in the axillary artery (sensitivity 72.2%, specificity 87.5%) in contrast to the abdominal aorta and its visceral branches where DS detected a manifestation of LVV in 6/23 respectively 1/9 cases (sensitivity aorta 26.1, visceral arteries 11.1%). Table 2 informs about all results from DS and PET/CT in detail.
Furthermore, PET/CT detected involvement of the thoracic aorta in 25/30 cases (83.3%) most parts of which are not accessible by abdominal ultrasound. For a detailed list of all calculations, please refer to Table 3.
Discussion
In our study, we retrospectively evaluated the diagnostic yield of DS in extracranial, non-temporal LVV at different arterial sites compared to the findings from FDG-PET/CT. PET/CT can be considered a highly sensitive method in the detection of LVV [14]. One of the challenges in evaluating a diagnostic method in LVV lies in the choice of the method of reference. In our study, we used PET/CT as gold standard for two reasons: Firstly, the ACR criteria from 1990 cannot be applied to extracranial LVV. At that time, GCA was considered to be a disease mainly of the temporal artery [22]. More recent criteria are not available. Secondly, it is mostly feasible to perform a TAB to prove vasculitis histologically; however, in extracranial LVV, obtaining histology generally is not an option except within in the context of vascular surgery. TAB is false negative in more than 40% of cases of extracranial LVV and even in about 15% of cases with clinically suspected involvement of the temporal artery [24].
Our data suggest that DS shows the highest diagnostic sensitivity in the axillary and subclavian arteries (72.2 and 71.4%, respectively). In our cohort, a negative result in axillary artery DS ruled out LVV in about 88% of cases. These findings are in line with previous investigations [25]. It could be speculated that the apparent preference of GCA to upper extremity arteries that is suggested by some authors [26, 27] causes a more advanced vessel wall inflammation in these areas leading to an increased sonographic detectability. In our study, in 25/30 cases of LVV (83.3%), PET/CT diagnosed an involvement of the aorta, 24 of which (80.0%) had positivity in the entire aorta (including the abdominal segment which is accessible to ultrasound). DS detected only 26% of abdominal aortic manifestations. Aortic manifestations of LVV require special attention during follow-up in order not to miss the formation of potentially life-threatening aneurysms. According to our data, aortic manifestations are missed in nearly three-quarters of cases and thoracic aortic involvement is mostly undetectable for abdominal ultrasound. These facts pose a strong call for a diagnostic method that sensitively detects aortic inflammation. Clinical and laboratory features were not able to predict an aortic involvement in our study.
The overall sensitivity of DS in detecting LVV (meaning positivity of ultrasound at any arterial site) was 80%. Conversely, it can be concluded that 1 out of 5 cases of LVV in our investigation would have been missed if the diagnosis had been based on ultrasound alone. In literature, the sensitivity of DS is debated controversially and shows extreme variability: Some authors describe sensitivity rates of up to 100% for DS [12, 13], and others such as Aschwanden and coworkers report detection rates of 55% [11]. Maldini et al. found a sensitivity of 10 to 17% in biopsy-proven TA [8]. It appears as if the results were strongly influenced by the preselection of patients and by the method of reference applied.
The question remains why in some cases of LVV ultrasound completely fails to detect pathology despite strong tracer uptake in arterial vessel walls revealed by PET. One possible explanation could be the various histological entities in GCA. Beside the classic histological finding of a transmural inflammation, there are patterns of periadventitial small-vessel vasculitis and of vasa vasorum vasculitis [28]. Muratore and colleagues found a highly significant difference of the appearance of the sonographic halo sign when accounting for these different histological subtypes. According to their investigation, the sensitivity of DS in classic GCA was 80%, whereas in periadventitial or vasa vasorum vasculitis, it only reached 20% [29].
One can also speculate that time might be of the essence. There seems to be a link not only between the halo sign and the type of histological inflammation but also to its extent. The more inflammation within a vessel wall, the greater the wall edema, the more likely is the appearance of a halo sign in DS [30]. Hence, the time between onset of symptoms and the establishment of the diagnosis could be critical because it can be assumed that in early stages of the disease the extent of the vascular inflammation is less than in more advanced stages. In contrast to that assumption, we did not find any difference in serological markers of inflammation between LVV patients who were completely DS negative versus those who were DS positive.
In summary, according to the data presented here, we found that DS is a potent method in diagnosing extracranial LVV. It detected 4 out 5 cases of LVV in our cohort given PET/CT as gold standard. The axillary and the subclavian arteries are the sites where DS is most likely capable of establishing respectively ruling out the diagnosis. Its weaknesses lie in aortic, intraabdominal, and lower extremity artery manifestations. We therefore must conclude, especially in light of the potentially dramatic consequences of aortic involvement, that an additional imaging modality (e.g., PET/CT or another imaging method equally sensitive) is necessary to complement the diagnosis in LVV patients.
We need to address some limitations of our work: Firstly, the retrospective nature of this study. Although the sonographers were unaware of the PET results, the analysis was not performed prospectively. As a consequence, our control group was not standardized and is therefore very heterogeneous comprised of patients with either autoimmune, malignant, or unknown diseases. Secondly, we cannot provide for interrater/interobserver reliability since there was no programmed second look investigation between the sonographers. This might have an influence on the results because different levels of sonographic expertise will affect the sonographic findings. Thirdly, validated classification criteria for extracranial LVV are still missing. We used PET/CT as the method of reference to evaluate the diagnostic accuracy of DS. However, PET/CT is not the official gold standard to diagnose extracranial LVV. It is our opinion that classification criteria for non-temporal LVV are necessary and the ACR criteria for GCA from 1990 need to be updated. Most likely, these criteria would have to consider clinical, laboratory, and imaging findings.
References
Weyand CM, Goronzy JJ (2003) Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med 139:505–515
Calamia KT, Schirmer M, Melikoglu M (2011) Major vessel involvement in Behçet’s disease: an update. Curr Opin Rheumatol 23:24–31. doi:10.1097/BOR.0b013e3283410088
Löffler C, Hoffend J, Rebel M et al (2016) A rare cause for lower back pain: a case of an IgG4-related periaortitis. Clin Rheumatol 35:265–270. doi:10.1007/s10067-014-2535-0
Haynes BF, Kaiser-Kupfer MI, Mason P, Fauci AS (1980) Cogan syndrome: studies in thirteen patients, long-term follow-up, and a review of the literature. Medicine (Baltimore) 59:426–441
Schmidt WA, Kraft HE, Vorpahl K et al (1997) Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 337:1336–1342
Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JPA (2005) Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med 142:359–369
Arida A, Kyprianou M, Kanakis M, Sfikakis PP (2010) The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Disord 11:44. doi:10.1186/1471-2474-11-44
Maldini C, Dépinay-Dhellemmes C, Tra TTS et al (2010) Limited value of temporal artery ultrasonography examinations for diagnosis of giant cell arteritis: analysis of 77 subjects. J Rheumatol 37:2326–2330. doi:10.3899/jrheum.100353
Salvarani C, Silingardi M, Ghirarduzzi A et al (2002) Is duplex ultrasonography useful for the diagnosis of giant-cell arteritis? Ann Intern Med 137:232–238
Förster S, Tato F, Weiss M et al (2011) Patterns of extracranial involvement in newly diagnosed giant cell arteritis assessed by physical examination, colour coded duplex sonography and FDG-PET. Vasa 40:219–227. doi:10.1024/0301-1526/a000096
Aschwanden M, Kesten F, Stern M et al (2010) Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2 × 11 arterial regions. Ann Rheum Dis 69:1356–1359. doi:10.1136/ard.2009.122135
Diamantopoulos AP, Haugeberg G, Hetland H et al (2014) Diagnostic value of color Doppler ultrasonography of temporal arteries and large vessels in giant cell arteritis: a consecutive case series. Arthritis Care Res 66:113–119. doi:10.1002/acr.22178
Brodmann M, Lipp RW, Passath A et al (2004) The role of 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology 43:241–242. doi:10.1093/rheumatology/keh025
Henes JC, Müller M, Krieger J et al (2008) [18F] FDG-PET/CT as a new and sensitive imaging method for the diagnosis of large vessel vasculitis. Clin Exp Rheumatol 26:47–52
Prieto-González S, Depetris M, García-Martínez A et al (2014) Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 73:1388–1392. doi:10.1136/annrheumdis-2013-204572
Meller J, Strutz F, Siefker U et al (2003) Early diagnosis and follow-up of aortitis with [(18)F]FDG PET and MRI. Eur J Nucl Med Mol Imaging 30:730–736. doi:10.1007/s00259-003-1144-y
Belhocine T, Blockmans D, Hustinx R et al (2003) Imaging of large vessel vasculitis with 18FDG PET: illusion or reality? A critical review of the literature data. Eur J Nucl Med Mol Imaging 30:1305–1313. doi:10.1007/s00259-003-1209-y
Hara M, Goodman PC, Leder RA (1999) FDG-PET finding in early-phase Takayasu arteritis. J Comput Assist Tomogr 23:16–18
Meller J, Grabbe E, Becker W, Vosshenrich R (2003) Value of F-18 FDG hybrid camera PET and MRI in early Takayasu aortitis. Eur Radiol 13:400–405. doi:10.1007/s00330-002-1518-8
Hautzel H, Sander O, Heinzel A et al (2008) Assessment of large-vessel involvement in giant cell arteritis with 18F-FDG PET: introducing an ROC-analysis-based cutoff ratio. J Nucl Med 49:1107–1113. doi:10.2967/jnumed.108.051920
Puppo C, Massollo M, Paparo F et al (2014) Giant cell arteritis: a systematic review of the qualitative and semiquantitative methods to assess vasculitis with 18F-fluorodeoxyglucose positron emission tomography. Biomed Res Int 2014:574248. doi:10.1155/2014/574248
Hunder GG (1990) Giant cell (temporal) arteritis. Rheum Dis Clin N Am 16:399–409
Jennette JC, Falk RJ, Bacon PA et al (2012) 2012 Revised international Chapel Hill consensus conference nomenclature of vasculitides. Theol Rev 20:5–15. doi:10.1002/art.37715
Gonzalez-Gay MA, Garcia-Porrua C, Llorca J et al (2001) Biopsy-negative giant cell arteritis: clinical spectrum and predictive factors for positive temporal artery biopsy. Semin Arthritis Rheum 30:249–256. doi:10.1053/sarh.2001.16650
Czihal M, Zanker S, Rademacher A et al (2012) Sonographic and clinical pattern of extracranial and cranial giant cell arteritis. Scand J Rheumatol 41:231–236. doi:10.3109/03009742.2011.641581
Lie JT (1995) Aortic and extracranial large vessel giant cell arteritis: a review of 72 cases with histopathologic documentation. Semin Arthritis Rheum 24:422–431
Klein RG, Hunder GG, Stanson AW, Sheps SG (1975) Large artery involvement in giant cell (temporal) arteritis. Ann Intern Med 83:806–812
Cavazza A, Muratore F, Boiardi L et al (2014) Inflamed temporal artery: histologic findings in 354 biopsies, with clinical correlations. Am J Surg Pathol 38:1360–1370
Muratore F, Boiardi L, Restuccia G et al (2013) Comparison between colour duplex sonography findings and different histological patterns of temporal artery. Rheumatology (Oxford) 52:2268–2274
Schmidt D, Hetzel A, Reinhard M, Auw-Haedrich C (2003) Comparison between color duplex ultrasonography and histology of the temporal artery in cranial arteritis (giant cell arteritis). Eur J Med Res 8:1–7
Acknowledgements
We thank Dr. Uta Löffler for her assistance and aid in the statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Funding
No funding was involved in this work.
Rights and permissions
About this article
Cite this article
Löffler, C., Hoffend, J., Benck, U. et al. The value of ultrasound in diagnosing extracranial large-vessel vasculitis compared to FDG-PET/CT: A retrospective study. Clin Rheumatol 36, 2079–2086 (2017). https://doi.org/10.1007/s10067-017-3669-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3669-7