Abstract
In Saccharomyces cerevisiae the Ras/cAMP/PKA signalling pathway controls multiple metabolic pathways, and alterations in the intracellular concentrations of cAMP through modification of signalling pathway factors can be lethal or result in severe growth defects. In this work, the important role of Ras2p in metabolic regulation during growth on the non-fermentable carbon source glycerol is further investigated. The data show that the overexpression of RAS2 suppresses the growth defect of the glyoxylate cycle citrate synthase mutant, cit2Δ. The overexpression results in enhanced proliferation and biomass yield when cells are grown on glycerol as sole carbon source, and increases citrate synthase activity and intracellular citrate concentration. Interestingly, the suppression of cit2Δ and the enhanced proliferation and biomass yield are only observed when RAS2 is overexpressed and not in strains containing the constitutively active allele RAS2 val19. However, both RAS2 and RAS2 val19upregulated citrate synthase activity. We propose that the RAS2 overexpression results in a combination of general upregulation of respiratory growth capacity and an increase in mitochondrial citrate/citrate synthases, which together, complement the metabolic requirements of the cit2Δ mutant. The data therefore provide new evidence for the role of Ras2p as a powerful modulator of metabolism during growth on a non-fermentable carbon source.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Saccharomyces cerevisiae, Ras signals via the cAMP/protein kinase A (PKA) pathway to regulate cellular metabolism in response to the type of carbon source available for utilisation (Broach and Deschenes 1990; Thevelein 1994). On non-fermentable carbon sources, a lower basal level of intracellular cAMP is observed compared to glucose, and alterations in the intracellular concentrations of cAMP through modification of the signalling pathway factors can result in severe growth defects (e.g. ras2Δ, bcy1Δ, ira1Δ ira2Δ and RAS2 val19). Therefore, the Ras/cAMP/PKA pathway is an important regulator of cellular machinery during growth on non-fermentable carbon sources and identifying the downstream metabolic targets is essential in understanding its role.
Two Ras proteins are present in yeast and are encoded by RAS1 and RAS2 (DeFeo-Jones et al. 1983; Powers et al. 1984; Dhar et al. 1984; Toda et al. 1985). Regulatory signals are transmitted by Ras by shuttling between the inactive GDP-bound form and the active GTP-bound form through the activity of the guanine nucleotide exchange factor, Cdc25p, and the GTPase activating proteins, Ira1p and Ira2p (Tanaka et al. 1989, 1990a, b; Jones et al. 1991). The GTP-bound Ras protein stimulates adenylyl cyclase, Cyr1p, which synthesises cyclic AMP, thereby increasing intracellular concentrations of cAMP (Toda et al. 1985; De Vendittis et al. 1986; Field et al. 1988). High cAMP concentrations stimulates the binding of cAMP to the PKA regulatory subunit, Bcy1p, thereby releasing the catalytic subunits, Tpk1p, Tpk2p and Tpk3p, which subsequently phosphorylate a variety of proteins involved in cellular metabolism and regulation (Matsumoto et al. 1982; Toda et al. 1987b; Broach and Deschenes 1990; Thevelein 1994; Robertson et al. 2000). Intracellular cAMP is degraded by cyclic nucleotide phosphodiesterases, Pde1p and Pde2p (Sass et al. 1986; Nikawa et al. 1987).
The effect of Ras activation on levels of cAMP appears most apparent when strains growing on non-fermentable carbon sources are switched to glucose-containing media (Mbonyi et al. 1990). In these conditions a rapid and significant increase in the cAMP levels are observed, as much as a 50-fold increase after 1–2 min. However, the cAMP levels rapidly decline and, after a relatively short time, near basal levels are reached (Nikawa et al. 1987). After the cAMP spike, the basal levels of cAMP required for continuous growth on glucose is slightly higher than the basal level of cAMP required for growth on non-fermentable carbon sources. Indeed, the level of cAMP must drop in order for the cell to adapt its metabolism from fermentative growth to respiratory growth (Russell et al. 1993).
The absence of Ras2p downregulates the Ras/cAMP/PKA pathway (decrease of intracellular cAMP concentrations) and this results in a severe growth defect on non-fermentable carbon sources (Fraenkel 1985; Tatchell et al. 1985; Toda et al. 1985). On fermentable carbon sources, e.g. glucose, the absence of Ras2p or Ras1p has no effect on growth; however, when they are both absent, the strain is not viable (Kataoka et al. 1984; Tatchell et al. 1984). Hyperactivation of the Ras/cAMP/PKA pathway (increase of intracellular cAMP concentrations), through the presence of the constitutively active RAS2 val19allele, also results in a severe growth defect on non-fermentable carbon sources (Kataoka et al. 1984; Toda et al. 1985; Nikawa et al. 1987). In addition, hyperactivated Ras strains show distinctive phenotypes that include reduction in glycogen and trehalose levels, heatshock sensitivity, nutrient-starvation sensitivity, pronounced pseudohyphal differentiation and invasive growth (Toda et al. 1987a; Engelberg 1994; Pan and Heitman 1999; Stanhill et al. 1999). The same phenotypes, including a growth defect on glycerol, are observed in strains without the PKA regulatory unit, Bcy1p, where the Tpk’s are permanently liberated and therefore constitutively active (Toda et al. 1985, 1987a). Thus, it is clear that Ras2p/cAMP/PKA pathway is an important regulator of respiratory growth on non-fermentable carbon sources. It has also been shown on the non-fermentable carbon source lactate, that hyperactivation of the cAMP/PKA pathway (e.g. RAS2 val19) or constitutive activation of PKA (e.g. bcy1Δ) results in an increase in mitochondrial enzyme content, while a loss of Ras activity (e.g. ras2Δ), results in a decrease in mitochondrial enzyme content (Dejean et al. 2002). Recently, the Tpk3p was shown to be specifically involved in the regulation of mitochondrial content for growth on the non-fermentable carbon source lactate (Chevtzoff et al. 2005).
The glyoxylate cycle is an essential metabolic process required for growth on non-fermentable carbon sources. The cycle is a modified version of the TCA cycle, incorporating two acetyl-CoA units per cycle and releasing succinate (Kornberg 1966). The succinate produced by the glyoxylate cycle is transferred to the mitochondria in order to supply the TCA cycle with C4 intermediates (van Roermund et al. 1995). The carnitine shuttle also supplies the TCA cycle with carbon units. In this shuttle, acetyl-CoA combines with carnitine to form acetylcarnitine, which is transported into the mitochondria where the acetylgroup is released to form acetyl-CoA, which can then enter the TCA cycle (Bremer 1983). Because of the impermeability of the mitochondrial membrane to acetyl-CoA, the glyoxylate cycle and the carnitine shuttle are the only two pathways through which the carbon groups of cytosolic and peroxisomal acetyl-CoA can be transferred to the mitochondria (van Roermund et al. 1995; Swiegers et al. 2001). In the absence of carnitine (and therefore the carnitine shuttle), the glyoxylate cycle citrate synthase mutant, cit2Δ, has a severe growth defect on glycerol (Swiegers et al. 2001). In this mutant, the inflow of acetyl-CoA into the glyoxylate cycle is blocked. However, because the glyoxylate cycle is required for gluconeogenesis and anaplerotic reactions, growth of the cit2Δ mutant on non-fermentable carbon sources when supplemented with carnitine implies that the glyoxylate cycle is still functional. Therefore, it has been proposed that in this mutant, citrate is recruited from the mitochondria in order to keep the glyoxylate cycle functioning (van Roermund et al. 1995).
Here, we present data showing that both N. crassa ras-1 and S. cerevisiae RAS2 could suppress the cit2Δ strains when grown on synthetic media with glycerol as the carbon source. We show that overexpression of RAS2 and ras-1 enhances the ability of yeast to proliferate on glycerol-containing media in general. Interestingly, the RAS2 proliferation effect is more pronounced in the cit2Δ strain, suggesting communication between the mitochondria and the glyoxylate cycle. We confirm the role of the PKA pathway in this process by showing that in the absence of Tpk1p, the growth enhancement caused by RAS2 overexpression is blocked. Differences in glycogen levels and flocculation phenotypes suggest that the overexpression of RAS2 activates the cAMP/PKA pathway but less severely than in the case of the RAS2 val19 allele. Furthermore, our data show that the overexpression of RAS2 increases and ras2Δ decreases mitochondrial citrate synthase activity of cells grown on glycerol. We propose that a combination of the upregulation of respiratory growth capacity and increase in mitochondrial citrate/citrate synthases complements the metabolic needs of the cit2Δ mutant. Therefore, the suppression does not act directly on the glyoxylate cycle but reflects the indirect effects resulting from general mitochondrial upregulation.
Materials and methods
Yeast strains and plasmids
FY23 (MATa leu2 trp1 ura3) was used as a wild-type strain, while the FY23cit2Δ (MATa leu2 ura3 cit2::TRP1) was used as the glyoxylate citrate synthase deficient strain (Winston et al. 1995; Swiegers et al. 2001). The RAS2 gene was cloned by PCR from plasmid YCP50-RAS2 using the primers RAS2F(EcoRI) 5′-GATCGAATTC ATG CCT TTG AAC AAG TCG AAC A-3′ and RAS2R(XhoI) 5′-GATCCTCGAG TTA ACT TAT AAT ACA ACA GCC AC-3′ with introduced restriction sites (underlined). The gene was subcloned into pGEM-T-easy (Promega) and cloned into the EcoRI/XhoI sites of expression vector pHVXII between the PGK1 promoter and terminator (Volschenk et al. 1997). Transformation of yeast was done using the lithium acetate procedure (Becker and Guarente 1991). The RAS2 val19 allele was supplied by David Engelberg (plasmid B2562). The pYPGE15 ras-1 plasmid was isolated from PG15 cDNA library (Fungal Genetic Stock Center, Kansas City, KS, USA; Brunelli and Pall 1993). This library is based on the pYPGE15 plasmid; 2 μm, URA3 and the cDNA’s were cloned under regulation of the constitutive PGK1 yeast promoter. The FY23ras2Δ strains were prepared by transforming the PCR product of the disruption cassette of strain BY4742ras2Δ (Euroscarf) using the primers RAS2-Fp AGT GGG TGG TGT GGC TAA TC and RAS2-Rp CAT CGT CGT CTT CCT CG. Other strains used were BY4742wt, BY4742tpk1Δ, BY4742tpk2Δ and BY4742tpk3Δ (Euroscarf), and disruptions were verified using PCR.
Media and growth conditions
Yeast were grown in 2% rich glucose medium (YPD), synthetic glucose medium (SCD); 6.7 g/l yeast nitrogen base without amino acids (Difco) and 2% glucose. Synthetic glycerol medium (SCG) contained 6.7 g/l yeast nitrogen base without amino acids (Difco) and 3% glycerol. Amino acids were supplied according to the requirement of each strain.
cDNA library screen
A N. crassa cDNA yeast expression library (Fungal Genetic Stock Center) was used to transform the FY23cit2Δ strain using the lithium acetate method (Becker and Guarente 1991). About 40,000 transformants were replica-plated on YNG medium. Plates were incubated for 2 weeks at 30°C, clones that grew were selected and the plamids isolated from the individual colonies. Isolated plasmids were retransformed into the FY23cit2Δ strain to confirm the phenotype. Sequencing was done using the ABI-Prism automated sequencer.
Citrate synthase and citrate assay
The strains were grown in selective medium (SCD and SCG) and 4 ml of the culture was harvested and the supernatant decanted. The culture was centrifuged and the remaining supernatant removed by pipetting. The cells were resuspended in 200 μl ice cold Triton-X-100 (0.05%); Tris–HCl 0.1 M solution and 150 μl glass beads were added. The suspension was vortexed rigorously at 8°C for 15 min and 800 μl ice cold water was added. A volume of 25–50 μl was used for enzyme analysis. The citrate synthase (EC 4.1.3.7) activity was determined by monitoring at 412 nm the oxidation of coenzyme A (produced by citrate synthase activity) by 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) as a function of time using a photometer (Srere 1969). The enzyme activity was calculated using an extinction coefficient of 13,600 M−1 cm−1 at 412 nm. One citrate synthase unit was equal to 1 μmole of DTNB reduced per minute per milligram wet weight.
For intracellular citrate determination strains were grown on the selective SCD medium for 48 h and 40 ml harvested through centrifugation for 5 min at 5,000 rpm. Cells were washed with 10 ml distilled water, harvested and resuspended in 1 ml distilled water to be transferred to a microcentrifuge tube. After centrifuging for 1 min at 12,000 rpm and removal of the supernatant, 0.5 g of glass beads were added and 0.3 ml Triton-X-100. Rigorous vortexing was applied for 30 min at 8°C. The microcentrifuge tubes with cell suspension were centrifuged 10 min, 12,000 rpm at 4°C to remove the debris. The supernatant was used to assess the citrate content using the Citric Acid Enzymatic UV test kit (Roche).
cAMP assay
The BiotrakTM cAMP competitive enzyme-immunoassay system was used to determine intracellular levels of the cAMP (Amersham Pharmacia Biotech). Strains were grown in selective media, and a total of 10–40 ml of cells were harvested depending on the growth stage (Fig. 3c). Cells were resuspended in 1 ml water and transferred to a microcentrifuge tube. The cells were harvested and the wet weight determined. The cells were resuspended in 300 μl lysis buffer 1B (Amersham) and 150 μl glass beads added. The suspension was vortexed for 30 min at 8°C and then spun down 10 min at 4°C. The supernatant (100 μl per sample) was used for analysis.
Results
Cloning of heterologous suppressors of cit2Δ
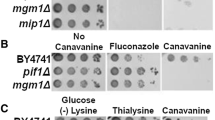
We used the N. crassa cDNA yeast expression library to screen for suppressors of the cit2Δ mutation in S. cerevisiae (Brunelli and Pall 1993). The library was transformed into the cit2Δ mutant and transformants selected on SCD media. Approximately 40,000 transformants were replica-plated onto SCG plates without carnitine. After 2 weeks, four growing colonies were isolated and the plasmids were retrieved and sequenced. Two plasmids contained cDNA that was identified as the ras-1 gene, the N. crassa homologue to yeast RAS1 and RAS2 (NCBI accession no. X53533 protein id. CAA37612.1; Altschuler et al. 1990). The other two suppressors were identified as coding for an ATPase but were not investigated further. No citrate synthase homologues were isolated indicating that the library was not saturated. The ras-1 gene codes for a protein of 213 aa. The translated protein has 59 and 57% identity to yeast RAS1 and RAS2, respectively. Retransformation of the ras-1 clone confirmed the suppression of the cit2Δ (Fig. 1). Transformation of the plasmid into S. cerevisiae ras2Δ strains suppressed the growth defect of this mutant on non-fermentable carbon sources indicating the functionality of the N. crassa ras-1 gene in S. cerevisiae (data not shown). The interchangeability of Ras proteins between organisms is well known and this is the first time it has been shown for N. crassa (Kataoka et al. 1985; Parrini et al. 1996).
Suppression of cit2Δ by Ras on glycerol-containing media (SCG). a Suppression of the cit2Δ phenotype by Neurospora crassa ras-1. Strains were grown on SCD to avoid carnitine carry-over from rich media and then streaked on SCG and grown for 14 days at 30°C. b Suppression of the cit2Δ phenotype by RAS2. Strains were grown on SCD solid media and then for 2 days on SCD liquid media to stationary phase. Strains were serially diluted to equal cell counts and then spotted on SCG media and grown for 8 days at 30°C
Yeast RAS2 suppresses the cit2Δ growth defect
The identification of a Ras gene as a suppressor of cit2Δ was surprising but an interesting find. In order to assess if this suppression was exclusively linked to the N. crassa Ras, or if the S. cerevisiae native Ras genes could also suppress the cit2Δ mutant, the RAS2 gene was cloned into the multiple-copy expression vector pHVXII under the regulation of the PGK1 promoter. The FY23cit2Δ strain was transformed with pHVXII-RAS2, grown under selective conditions in SCD medium, serially diluted and spotted on 3% SCG agar plates. After 8 days a clear suppression of the growth defect of the cit2Δ mutant was observed in pHVXII-RAS2 transformed strains, similar to the suppression of cit2Δ by N. crassa ras-1 overexpression (Fig. 1). However, the constitutively active RAS2 val19 could not suppress the cit2Δ mutant.
The suppression of the cit2Δ phenotype by overexpression of both ras-1 and RAS2 takes a few days to present itself clearly. The reason for this could be that transformants of the cit2Δ need to be streaked on the SCD twice and then on the SCG, which results in a very slow growth on the glycerol media of wild-type strains in general. This step has to be taken because of the sensitivity of the cit2Δ mutant to complementation by trace quantities of carnitine. For example, glycerol media containing small quantities of yeast extract or peptone complement the cit2Δ phenotype fully due to the large quantities of carnitine present in these extracts. The suppression was also more prominent when transformants were spotted on amino acid selective glycerol (SCG) plates. Furthermore, the suppression of the cit2Δ phenotype by RAS2 overexpression could also be observed on ethanol containing medium, but not as clearly as on glycerol containing medium (data not shown).
RAS2 and ras-1 overexpression improves proliferation and biomass yield on glycerol
Ras is involved in a complex array of cellular processes and control mechanisms for metabolic regulation. Therefore, because of the far-reaching influence of Ras on metabolism, we wanted to determine if the suppression was specific to the cit2Δ mutant, or if the suppression could be due to a general enhancement of proliferation on glycerol. The Ras genes were overexpressed in FY23 wild-type and the growth monitored on glucose and glycerol (Fig. 2). Indeed, the overexpression of Ras in wild-type cells improved the proliferation on glycerol media (SCG). The wild-type strains with overexpressed Ras reached a much higher optical density on glycerol at stationary phase compared to the wild-type with plasmid alone. However, on glucose-containing media (SCD), the opposite was observed. Strains with overexpressed Ras had a lower optical density at stationary phase compared to wild-type. This would indicate that the high biomass yield reached at stationary phase is not a general effect present on all carbon sources.
Proliferation effects of RAS2 overexpression. FY23 strains were grown in 100 ml synthetic medium with 2% glucose (SCD) (a) and synthetic medium with 3% glycerol (SCG) (b). Precultures were grown in SCD media for 1 day and inoculated at OD600 of 0.05 for glucose growth curves and OD600 of 0.2 for glycerol growth curves. Symbols are as follows: Wild-type strain (filled triangle); Wild-type strain with RAS2 overexpressed (filled square); Wild-type strain with Nc-ras1 overexpressed (open square); Wild-type strain with RAS2 val19 (open triangle); cit2Δ strain (open circle); cit2Δ strain with RAS2 overexpressed (filled circle). Strains were transformed with vector pHVXII or pHVXII-RAS2 and growth curves were done in triplicate
The enhancement of proliferation by RAS2 overexpression is in contrast to the well-known growth defect caused by the RAS2 val19allele on glycerol. Indeed, the FY23 wild-type strains harbouring the RAS2 val19allele showed a growth defect on glycerol media and reduced biomass yield at stationary phase (Fig. 2). Interestingly, the highest optical density (OD600) reached at stationary phase for strains growing on glycerol was observed in the FY23cit2Δ strains with RAS2 overexpressed. In this case, carnitine was added to complement the cit2Δ mutant’s growth on glycerol. However, adding carnitine to the FY23 wild-type and the wild-type overexpressing RAS2, did not change the optical density reached at stationary phase. Furthermore, the cit2Δ mutant grew similar to wild-type when carnitine was added. Therefore, the effect is not related to carnitine but to RAS2 overexpression in the cit2Δ background. For the cit2Δ strain grown on glycerol medium containing carnitine (SCG + carnitine), a twofold increase in optical density at stationary phase was shown when RAS2 was overexpressed. In the same conditions and in wild-type, only a 1.3-fold increase in optical density was observed when RAS2 was overexpressed. This would indicate regulatory effects between Ras2p and Cit2p. Indeed, it has been shown previously that the RAS2 regulates the retrograde response, which is the communication between the mitochondria and the nucleus in response to mitochondrial dysfunction or damage (Kirchman et al. 1999). The CIT2 gene is known to be upregulated in response to mitochondrial dysfunction or damage (Liao et al. 1991). In our case, the absence of CIT2 allowed RAS2 to enhance proliferation on glycerol significantly better than when the CIT2 was present.
RAS2 overexpression results in increased cAMP/PKA activity
The RAS2 val19 allele translates a Ras protein that is locked in the GTP bound form and therefore constitutively activates the Ras/cAMP/PKA pathway through elevated intracellular cAMP concentrations (Toda et al. 1985; Nikawa et al. 1987). A large quantity of Ras2p in the cell would not necessarily mean higher activity as would be the case for most enzymes. The level of activation of the cAMP/PKA pathway would depend on the relative quantity of GTP bound Ras2p and not on the total quantity of Ras2p present. In addition, various forms of regulation might influence this Ras2p-GTP/Ras2p-GDP ratio when these proteins are in abundance. Previous reports have indicated that overexpression of RAS2 does not result in significant elevation of intracellular cAMP, thereby implying limited activation of the PKA pathway (Sun et al. 1994; Colombo et al. 1998).
In this work we show that the overexpression of RAS2 results in a decrease in glycogen content. However, the reduction in glycogen levels was less than in the case of RAS2 val19 (Fig. 3a). Furthermore, the RAS2 overexpressed strains were sensitive to nutrient starvation (data not shown).
Activation of the cAMP/PKA pathway by RAS2 val19 and overexpression of RAS2. a Iodine/iodide staining of transformants. Transformants were grown on SCD medium for 2 days and then spotted on SCD medium and grown for 5 days at 30°C. A solution of 0.2% iodine/0.4% potassium iodide was gently poured over the colonies and photographs taken 3 minutes later. The darker the colour, the more glycogen is present and lighter the colour, the less glycogen is present. b Flocculation phenotypes. Strains were grown in SCD media for 1 day at 30°C on a rotating wheel and photographs taken. Optical densities did not vary more than 10%. c Intracellular cAMP measurements. Strains were grown on SCD and SCG media and harvested in log phase (L) or late stationary phases (S). Intracellular cAMP was measured as described in ‘Materials and methods’
We also observed a flocculation phenotype in glucose-containing media (SCD) when RAS2 was overexpressed (Fig. 3b). The Ras pathway is known to act on related phenotypes, such as pseudohyphal differentiation and invasive growth (Mosch et al. 1999; Pan and Heitman 1999). For instance, FLO11, which has been implicated in flocculation and invasive growth, is known to be upregulated when the Ras/cAMP/PKA pathway is activated (Pan and Heitman 1999). We further showed that the tpk2Δ strain blocked the flocculation phenotype in RAS2 overexpressed strains (data not shown). The blocking of invasive growth in Ras-activated strains by the tpk2Δ deletion has previously been shown, confirming the correlation (Pan and Heitman 1999). As expected, the RAS2 val19 also caused flocculation on synthetic glucose media. However, the flocculation was much more intense for strains with RAS2 val19.
To finally implicate the RAS2 overexpression in the activation of the cAMP/PKA pathway, we measured intracellular cAMP in S288c isogenic strains grown on glucose and glycerol media. Cells harvested in late stationary phase growing on glycerol showed a clear increase in intracellular cAMP levels when RAS2 was overexpressed (Fig. 3c). In addition, we also confirmed the hypothesis that a lower basal level of cAMP is required for growth on glycerol compared to glucose. The drop in cAMP in cells moving from exponential to stationary phase in glucose was also confirmed (Russell et al. 1993) Interestingly, the cAMP was much higher in late stationary phase of glycerol grown cells compared to exponentially grown cells (Fig. 4c). Collectively, all these data would therefore indicate that overexpressing RAS2 does result in the activation of the cAMP/PKA pathway.
Proliferation effect of RAS2 overexpression in tpkΔ strains. a Growth of BY4742 strains on SCG. Precultures were grown in SCD media for 1 day and inoculated at an OD600 of 0.2 to start the measurement of the growth curves. Symbols are as follows: Wild-type strain (filled circle); wild-type strain with RAS2 overexpressed (open circle); tpk1Δ strain (open triangle); tpk1Δ strain with RAS2 overexpressed (filled triangle); tpk2Δ strain (filled square); tpk2Δ strain with RAS2 overexpressed (open square); tpk3Δ strain (filled diamond) tpk3Δ strain with RAS2 overexpressed (open diamond). Strains were transformed with vector pHVXII or pHVXII-RAS2 and growth curves done at least in triplicate. b The average stationary phase OD600 reached for SCD grown BY4742 strains
The RAS2 proliferation and biomass yield effect is blocked in the tpk1Δ mutant
Ras2p can signal, independently of cAMP/PKA, through the MAPK cascade to promote filamentous growth and cell integrity (Lee and Elion 1999; Mosch et al. 1999; Pan et al. 2000). Here, we show that the proliferation effect of RAS2 overexpression does act through the cAMP/PKA pathway. We overexpressed the RAS2 in BY4742 strains (Euroscarf), which carry deletions in the catalytic subunits encoded by TPK1, TPK2 and TPK3. The growth of these strains was then monitored in glycerol-containing media (SCG) (Fig. 4a).
In these conditions, the tpk3Δ strains showed a significant reduction in biomass yield at stationary phase compared to wild-type. However, the RAS2 overexpression in tpk3Δ resulted in growth similar to wild-type overexpressing RAS2. Therefore, the RAS2 also suppresses the growth defect on glycerol of the tpk3Δ strain. The tpk2Δ strain grew the same as the wild-type strain. The tpk2Δ strain also grew identical to wild-type when the RAS2 was overexpressed in both strains. The tpk1Δ strain had a higher biomass yield at stationary phase compared to the wild-type strain. However, the RAS2 overexpression did not result in the high biomass yield observed for the wild-type, tpk2Δ and tpk3Δ strains when RAS2 was overexpressed. However, on glucose-containing media (SCD) the characteristic reduction in biomass yield at stationary phase was observed for tpk1Δ when RAS2 was overexpressed (as for all the other strains used; Fig. 4b). This would indicate that the RAS2 overexpression confers its proliferation effect of cells grown on glycerol through Tpk1p.
Overexpression of RAS2 increases mitochondrial citrate synthase activity and intracellular citrate content
The cit2Δ mutant may be suppressed on non-fermentable carbon due to the leakage of mitochondrial citrate synthase and/or citrate to the cytosol. The S. cerevisiae genome encodes three citrate synthases, the cytosolic Cit2p and the mitochondrial Cit1p and Cit3p (Kim et al. 1986, Rosenkrantz et al. 1986; Jia et al. 1997).
In order to determine if Ras increases citrate synthase activity, enzyme assays were done on glucose and glycerol grown cells. On glycerol grown cells, RAS2 overexpression increased citrate synthase activity and in the cit2Δ mutant citrate sythase activity almost doubled, indicating that the mitochondrial citrate synthase is upregulated (Fig. 5). In contrast, the opposite effect was seen on glucose where citrate synthase activity decreased when RAS2 was overexpressed. On glucose grown cells, no significant change in citrate synthase activity was monitored for the ras2Δ strain, but a significant drop in activity was seen when the strain was grown on glycerol-containing medium (SCG). Interestingly, the RAS2 val19 strain showed increased citrate synthase activity in wild-type cells grown on glycerol and decreased activity in wild-type cells grown on glucose, similar to the RAS2 overexpression but in both cases more pronounced. However, the RAS2 val19could not suppress the cit2Δ mutant and caused a growth defect in wild-type cells grown on glycerol (Figs. 1, 2).
Citrate synthase activity of Ras affected strains. FY23 strains were grown in SCD (white bars) and SCG (black bars) media. For the cit2Δ stains, carnitine was added to complement growth on glycerol medium. The ras2Δ and RAS2 val19 strains were inoculated at OD600 of 0.5 in SCG to allow growth to OD600 of 1. The SCD strains were grown for 24 h, and the SCG strains were harvested at OD600 of 1–1.5. Citrate synthase activity was measured as described in ‘Materials and methods’
Intracellular citrate levels are an indication of respiratory activity in yeast. Citrate concentrations were therefore determined in the RAS2 overexpressed strains. The level of citrate in a wild-type strain was 93 μg/gWW and in cit2Δ strains 46 μg/gWW. When RAS2 was overexpressed citrate levels increased in wild-type twofold to 201 μg/gWW and 2.5-fold more in the cit2Δ strain at 117 μg/gWW. Therefore, levels of citrate in the cit2Δ strains with overexpressed RAS2 were even higher than in wild-type strains.
From these data, it is clear that the RAS2 tightly regulates citrate synthase activity in the cell. This seems to be part of a regulatory circuit involved in upregulation of mitochondrial biogenesis and mitochondrial enzyme content. Therefore, possible leakage of mitochondrial citrate synthases and citrate from the mitochondria to the cytosol are probably part of the general increase in respiratory and proliferation capacity of RAS2 overexpressing cells grown on glycerol.
Discussion
In this work, we have identified the N. crassa ras-1 gene as a suppressor of the S. cerevisiae cit2Δ mutant when grown on glycerol medium. We subsequently showed that the native RAS2 gene could also suppress the cit2Δ mutant when overexpressed. Further investigation indicated that the RAS2 overexpression in cells grown on glycerol: (1) enhances biomass yield of wild-type; (2) activates the cAMP/PKA pathway; and (3) upregulates mitochondrial citrate synthase activity. We further showed that the increase in biomass yield in wild-type cells grown on glycerol and overexpressing RAS2 is blocked in the tpk1Δ mutant. Tpk1p has been implicated in respiratory growth through its apparent regulation of iron content in the mitochondria (Robertson et al. 2000). In the same work, Tpk1p has also been shown to regulate BAT1 expression, which is involved in the regulation of stationary phase. Together, these data indicate the important role of Tpk1p in growth on non-fermentable carbon sources.
We also compared the effect of RAS2 val19 with that of the RAS2 overexpression and found that both result in: (1) a decrease in glycogen content; (2) nutrient starvation sensitivity; (3) the flocculation of cells grown on glucose; (4) a decrease in biomass yield and citrate synthase activity for wild-type cells grown on glucose-containing medium (SCD); and (5) an increase in citrate synthase activity of wild-type cells grown on glycerol (SCG). In support of the last point, it has previously been shown that the cAMP addition increases CIT1 expression and citrate synthase activity in cells grown on the non-fermentable carbon source lactate (Dejean et al. 2002). In this work we did not investigate the expression of CIT1, CIT2 or CIT3, and we can therefore not conclude how each of them contributes to the increased citrate synthase activity. However, in the cit2Δ mutant there was a large increase in the citrate synthase activity and it would therefore implicate Cit1p or Cit3p or both but whether the Cit1p or Cit3p is more expressed does not affect our hypothesis and we therefore deemed it unnecessary to conduct expression analysis. Furthermore, most of our observations were performed in the cit2Δ background, therefore, analysing the expression of CIT2 would fall outside the scope of this study. At the same time, determining whether the overexpression of CIT1 or CIT3 would lead to suppression of the cit2Δ phenotype would not be conclusive as the physiological condition of the cell would be different in the case where the RAS2 is overexpressed. Furthermore, other factors could also come into play such as the leakage of mitochondrial tri-carboxylic acid cycle intermediates, such as fumarate, malate and citrate. Or, the overexpression of RAS2 could actually result in the mislocalisation of Cit1p and/or Cit3p to the cytosol. At the same time, it was not feasible to investigate if the RAS2 overexpression could suppress the cit1Δ cit2Δ strain as cit1Δ has a growth defect on non-fermentable carbon sources, including glycerol (Kim et al. 1986; Steinmetz et al. 2002). Furthermore, a cit1Δ cit2Δ double deletion causes, in addition to the growth defect on non-fermentable carbon sources, a glutamate auxotrophy (Kim et al. 1983). It is important also to take note that both RAS2 overexpression and RAS2 val19 resulted in an increase in citrate synthase activity but that RAS2 val19could not suppress the cit2Δ growth defect. Therefore, the suppression of cit2Δ cannot be looked at in isolation but rather as a combination of multiple effects. However, we show that all these phenotypes were more pronounced in the case of strains with the RAS2 val19 allele. Surprisingly, in reciprocal effects, the RAS2 overexpression enhanced proliferation and biomass yield of cells growing on glycerol compared to the decreased proliferation and biomass yield of cells with RAS2 val19 in these conditions.
The importance of the Ras/cAMP/PKA regulatory pathway in respiratory growth on non-fermentable carbon sources is well known. Most of the deletions in this pathway result in growth defects on glycerol (e.g. ras2Δ, bcy1Δ, ira1Δira2Δ), as does constitutive activation of the RAS2 val19 allele. In this work we also investigated the BY4742 strains with a deletion in the cAMP phosphodiesterase genes PDE1 and PDE2. We found that the deletion of PDE2 (but not the PDE1) displays a growth defect when grown on glycerol medium (data not shown). Therefore, over- and underactivation of the Ras/cAMP/PKA pathway results in growth defects on non-fermentable carbon sources. Indeed, it appears that the Ras/cAMP/PKA pathway plays a much more important role on non-fermentable carbon sources than on fermentable carbon sources (Robertson et al. 2000). In addition, it has been shown that activation of the cAMP pathway increases mitochondrial enzyme content when the cells are grown on the non-fermentable carbon source lactate (Dejean et al. 2002). This increase in mitochondrial enzyme content appears to be dependent on Tpk3p when cells are grown on lactate. Indeed, the tpk3Δ strain had reduced citrate synthase activity on lactate compared to wild-type (Chevtzoff et al. 2005). Interestingly, we observed a growth defect in the tpk3Δ strain on glycerol-containing media (probably due to the low mitochondrial enzyme content) and this growth defect could be suppressed by the RAS2 overexpression, indicating that the Tpk1p and Tpk2p could probably increase mitochondrial enzyme expression when RAS2 is overexpressed.
In our work, and in contrast to the growth defects exhibited by the ras2Δ mutant, we show that the RAS2 overexpression enhances proliferation on glycerol-containing media. Other examples of these reciprocal effects are the effect of RAS2 overexpression on life span extension vs the effect of ras2Δ on life span curtailing (Sun et al. 1994). On the other hand, it has previously been shown that the RAS2 val19overexpression and bcy1Δ curtails life span (Sun et al. 1994; Pichova et al. 1997). These findings lead researchers in the field to propose that the RAS2 overexpression acts independently of the cAMP/PKA pathway (Sun et al. 1994). However, later, the same workers indicated that overexpression of the RAS2 can reverse the effect of chronic stress on life span strictly through the cAMP/PKA pathway (Shama et al. 1998). Our data on proliferation of cells on glycerol as a sole carbon source correlate with these observations. We show that the RAS2 overexpression enhances proliferation of cells growing on glycerol in contrast to the growth defect of ras2Δ in these conditions. We show that the RAS2 overexpression proliferation effect is mediated by the cAMP/PKA pathway. It has been shown that the RAS2 val19 can act independently of the cAMP/PKA pathway to generate reactive oxygen species and lock respiration in a non-phosphorylating state (Hlavata et al. 2003). These cAMP/PKA independent effects of RAS2 val19 may contribute to the growth defect of cells grown on glycerol. This is supported by the fact that, on non-fermentable carbon sources, both RAS2 val19 and RAS2 overexpression result in an increase in mitochondrial enzyme content (including citrate synthase activity and ATPase activity; Dejean et al. 2002; Mabuchi et al. 2000). However, we show that the RAS2 overexpression and not the RAS2 val19, improves proliferation of cells grown on glycerol and suppresses cit2Δ phenotype. The more pronounced activation of the cAMP/PKA pathway by RAS2 val19 may also be responsible for the adverse effect regarding growth on glycerol. This is supported by the phenotype of the bcy1Δ mutant, showing that constitutively active PKAs result in a growth defect on glycerol. Therefore, the RAS2 overexpression might activate the cAMP/PKA at an optimal level in order to alter the mitochondrial machinery in a positive way to promote growth and proliferation on glycerol.
In support of our data, it has been shown that the ATPase mutant (atp1–2), which has a growth defect on glycerol, could be suppressed by overexpression of RAS2 on a multi-copy plasmid under its native promotor (Mabuchi et al. 2000). It was also shown that the RAS2 overexpression increases ATPase activity and deletion of RAS2 decreases ATPase activity of cells grown on glycerol medium. This correlates with our data regarding citrate synthase activity. In our case, the ATPase activity should also increase when the RAS2 is overexpressed in cells grown on glycerol medium. The high biomass yield achieved in these conditions would support this notion, as more ATP would be required for this process. In conclusion, it is clear that the RAS2 overexpression in S. cerevisiae alters the mitochondrial metabolism and regulation in a way that supports growth on glycerol.
We propose that the suppression of the cit2Δ mutant on glycerol by RAS2 overexpression is a combination of indirect effects related to mitochondrial capacity. The overexpression of RAS2 in cells grown on glycerol results in: (1) enhanced proliferation of wild-type strain and cit2Δ strain; and (2) increase in citrate synthase activity in wild-type strain and cit2Δ strain. However, in both cases the effects were much more pronounced in the cit2Δ strain. It would therefore seem that there is some type of communication between the glyoxylate cycle and Ras2p with regard to mitochondrial regulation, which allows the cell to upregulate its mitochondrial machinery, even better than is the case for wild-type. This could result in citrate synthase/citrate leakage to the cytosol, alteration in metabolic pools of the mitochondria, and superior respiratory growth machinery, which together, would suppress the growth defect of the cit2Δ strain when grown on glycerol medium.
Finally, in this work we expanded on our knowledge of the role of the Ras/cAMP/PKA pathway and found that depending on the type of modification of the Ras2p, different effects are seen. The knowledge generated in this work can be useful to researchers in this field, in particular those working on non-fermentable carbon sources wanting a ‘gentle’ induction of the cAMP/PKA pathway without the apparent negative side effects of ‘over activation’ as in the case of RAS2 val19. Furthermore, the investigation of how the Ras/cAMP/PKA pathway affect mitochondrial enzyme content, especially citrate synthases, might lead to the identification of novel mitochondrial targets of this pathway. A global analysis in conditions of the RAS2 overexpression and RAS2 val19 activation would be an incredible insightful resource and should be a focus of future research in this field.
References
Altschuler DL, Muro A, Schijman A, Almonacid FB, Torres HN (1990) Neurospora crassa cDNA clones coding for a new member of the ras protein family. FEBS Lett 273:103–106
Becker DM, Guarente L (1991) High efficiency transformation of yeast by electroporation. In: Guthrie C, Fink GR (eds) Guide to yeast genetics and molecular biology. Academic, San Diego, pp 182–187
Bremer J (1983) Carnitine-metabolism and functions. Physiol Rev 63:1420–1480
Broach JR, Deschenes RJ (1990) The function of ras genes in Saccharomyces cerevisiae. Adv Cancer Res 54:79–139
Brunelli JP, Pall ML (1993) A series of yeast/Escherichia coli lambda expression vectors designed for directional cloning of cDNAs and cre/lox-mediated plasmid excision. Yeast 9:1309–1318
Chevtzoff C, Vallortigara J, Averet N, Rigoulet M, Devin A (2005) The yeast cAMP protein kinase Tpk3p is involved in the regulation of mitochondrial enzymatic content during growth. Biochim Biophys Acta 1706:117–125
Colombo S, Ma P, Cauwenberg L, Winderickx J, Crauwels M, Teunissen A, Nauwelaers D, De Winde JH, Gorwa MF, Colavizza D, Thevelein JM (1998) Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J 17:3326–3341
De Vendittis E, Vitelli A, Zahn R, Fasano O (1986) Suppression of defective RAS1 and RAS2 functions in yeast by an adenylate cyclase activated by a single amino acid change. EMBO J 5:3657–3663
DeFeo-Jones D, Scolnick EM, Koller R, Dhar R (1983) ras-Related gene sequences identified and isolated from Saccharomyces cerevisiae. Nature 306:707–709
Dejean L, Beauvoit B, Bunoust O, Guerin B, Rigoulet M (2002) Activation of Ras cascade increases the mitochondrial enzyme content of respiratory competent yeast. Biochem Biophys Res Commun 293:1383–1388
Dhar R, Nieto A, Koller R, DeFeo-Jones D, Scolnick EM (1984) Nucleotide sequence of two rasH related-genes isolated from the yeast Saccharomyces cerevisiae. Nucleic Acids Res 12:3611–3618
Engelberg D, Zandi E, Parker CS, Karin M (1994) The yeast and mammalian Ras pathways control transcription of heat shock genes independently of heat shock transcription factor. Mol Cell Biol 14:4929–4937
Field J, Nikawa J, Broek D, MacDonald B, Rodgers L, Wilson IA, Lerner RA, Wigler M (1988) Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol 8:2159–2165
Fraenkel DG (1985) On ras gene function in yeast. Proc Natl Acad Sci USA 82:4740–4744
Hlavata L, Aguilaniu H, Pichova A, Nystrom T (2003) The oncogenic RAS2 val19 mutation locks respiration, independently of PKA, in a mode prone to generate ROS. EMBO J 22:3337–3345
Jia YK, Becam AM, Herbert CJ (1997) The CIT3 gene of Saccharomyces cerevisiae encodes a second mitochondrial isoform of citrate synthase. Mol Microbiol 24:53–59
Jones S, Vignais ML, Broach JR (1991) The CDC25 protein of Saccharomyces cerevisiae promotes exchange of guanine nucleotides bound to ras. Mol Cell Biol 11:2641–2646
Kataoka T, Powers S, McGill C, Fasano O, Strathern J, Broach J, Wigler M (1984) Genetic analysis of yeast RAS1 and RAS2 genes. Cell 37:437–445
Kataoka T, Powers S, Cameron S, Fasano O, Goldfarb M, Broach J, Wigler M (1985) Functional homology of mammalian and yeast RAS genes. Cell 40:19–26
Kim KS, Rosenkrantz MS, Guarente L (1986) Saccharomyces cerevisiae contains two functional citrate synthase genes. Mol Cell Biol 6:1936–1942
Kirchman PA, Kim S, Lai CY, Jazwinski SM (1999) Interorganelle signaling is a determinant of longevity in Saccharomyces cerevisiae. Genetics 152:179–190
Kornberg HL (1966) The role and control of the glyoxylate cycle in Escherichia coli. Biochem J 99:1–11
Lee BN, Elion EA (1999) The MAPKKK Ste11 regulates vegetative growth through a kinase cascade of shared signaling components. Proc Natl Acad Sci USA 96:12679–12684
Liao XS, Small WC, Srere PA, Butow RA (1991) Intramitochondrial functions regulate nonmitochondrial citrate synthase (CIT2) expression in Saccharomyces cerevisiae. Mol Cell Biol 11:38–46
Mabuchi T, Ichimura Y, Takeda M, Douglas MG (2000) ASC1/RAS2 suppresses the growth defect on glycerol caused by the atp1–2 mutation in the yeast Saccharomyces cerevisiae. J Biol Chem 275:10492–10497
Matsumoto K, Uno I, Oshima Y, Ishikawa T (1982) Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc Natl Acad Sci USA 79:2355–2359
Mbonyi K, Van Aelst L, Arguelles JC, Jans AW, Thevelein JM (1990) Glucose-induced hyperaccumulation of cyclic AMP and defective glucose repression in yeast strains with reduced activity of cyclic AMP-dependent protein kinase. Mol Cell Biol 10:4518–4523
Mosch HU, Kubler E, Krappmann S, Fink GR, Braus GH (1999) Crosstalk between the Ras2p-controlled mitogen-activated protein kinase and cAMP pathways during invasive growth of Saccharomyces cerevisiae. Mol Biol Cell 10:1325–1335
Nikawa J, Cameron S, Toda T, Ferguson KM, Wigler M (1987) Rigorous feedback control of cAMP levels in Saccharomyces cerevisiae. Genes Dev 1:931–937
Pan X, Heitman J (1999) Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol Cell Biol 19:4874–4887
Pan X, Harashima T, Heitman J (2000) Signal transduction cascades regulating pseudohyphal differentiation of Saccharomyces cerevisiae. Curr Opin Microbiol 3:567–572
Parrini MC, Bernardi A, Parmeggiani A (1996) Determinants of Ras proteins specifying the sensitivity to yeast Ira2p and human p120-GAP. EMBO J 15:1107–1111
Pichova A, Vondrakova D, Breitenbach M (1997) Mutants in the Saccharomyces cerevisiae RAS2 gene influence life span, cytoskeleton, and regulation of mitosis. Can J Microbiol 43:774–781
Powers S, Kataoka T, Fasano O, Goldfarb M, Strathern J, Broach J, Wigler M (1984) Genes in S. cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell 36:607–612
Robertson LS, Causton HC, Young RA, Fink GR (2000) The yeast A kinases differentially regulate iron uptake and respiratory function. Proc Natl Acad Sci USA 97:5984–5988
Rosenkrantz M, Alam T, Kim KS, Clark BJ, Srere PA, Guarente LP (1986) Mitochondrial and nonmitochondrial citrate synthases in Saccharomyces cerevisiae are encoded by distinct homologous genes. Mol Cell Biol 6:4509–4515
Russell M, Bradshaw-Rouse J, Markwardt D, Heideman W (1993) Changes in gene expression in the Ras/adenylate cyclase system of Saccharomyces cerevisiae: correlation with cAMP levels and growth arrest. Mol Biol Cell 4:757–765
Sass P, Field J, Nikawa J, Toda T, Wigler M (1986) Cloning and characterization of the high-affinity cAMP phosphodiesterase of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 83:9303–9307
Shama S, Kirchman PA, Jiang JC, Jazwinski SM (1998) Role of RAS2 in recovery from chronic stress: effect on yeast life span. Exp Cell Res 245:368–378
Srere PA (1969) Citrate synthase. Meth Enzymol 13:3–11
Stanhill A, Schick N, Engelberg D (1999) The yeast ras/cyclic AMP pathway induces invasive growth by suppressing the cellular stress response. Mol Cell Biol 19:7529–7538
Steinmetz LM, Scharfe C, Deutschbauer AM, Mokranjac D, Herman ZS, Jones T, Chu AM, Giaever G, Prokisch H, Oefner PJ, Davis RW (2002) Systematic screen for human disease genes in yeast. Nat Genet 31:400–404
Sun J, Kale SP, Childress AM, Pinswasdi C, Jazwinski SM (1994) Divergent roles of RAS1 and RAS2 in yeast longevity. J Biol Chem 269:18638–18645
Swiegers JH, Dippenaar N, Pretorius IS, Bauer FF (2001) Carnitine-dependent metabolic activities in Saccharomyces cerevisiae: three carnitine acetyltransferases are essential in a carnitine-dependent strain. Yeast 18:585–595
Tanaka K, Matsumoto K, Toh-E A (1989) IRA1, an inhibitory regulator of the RAS-cyclic AMP pathway in Saccharomyces cerevisiae. Mol Cell Biol 9:757–768
Tanaka K, Nakafuku M, Satoh T, Marshall MS, Gibbs JB, Matsumoto K, Kaziro Y, Toh-e A (1990a) S. cerevisiae genes IRA1 and IRA2 encode proteins that may be functionally equivalent to mammalian ras GTPase activating protein. Cell 60:803–807
Tanaka K, Nakafuku M, Tamanoi F, Kaziro Y, Matsumoto K, Toh-e A (1990b) IRA2, a second gene of Saccharomyces cerevisiae that encodes a protein with a domain homologous to mammalian ras GTPase-activating protein. Mol Cell Biol 10:4303–4313
Tatchell K, Chaleff DT, DeFeo-Jones D, Scolnick EM (1984) Requirement of either of a pair of ras-related genes of Saccharomyces cerevisiae for spore viability. Nature 309:523–527
Tatchell K, Robinson LC, Breitenbach M (1985) RAS2 of Saccharomyces cerevisiae is required for gluconeogenic growth and proper response to nutrient limitation. Proc Natl Acad Sci USA 82:3785–3789
Thevelein JM (1994) Signal transduction in yeast. Yeast 10:1753–1790
Toda T, Uno I, Ishikawa T, Powers S, Kataoka T, Broek D, Cameron S, Broach J, Matsumoto K, Wigler M (1985) In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell 40:27–36
Toda T, Cameron S, Sass P, Zoller M, Scott JD, McMullen B, Hurwitz M, Krebs EG, Wigler M (1987a) Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol Cell Biol 7:1371–1377
Toda T, Cameron S, Sass P, Zoller M, Wigler M (1987b) Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell 50:277–287
Van Roermund CW, Elgersma Y, Singh N, Wanders RJ, Tabak HF (1995) The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO J 14:3480–3486
Volschenk H, Viljoen M, Grobler J, Petzold B, Bauer F, Subden RE, Young RA, Lonvaud A, Denayrolles M, Van Vuuren HJ (1997) Engineering pathways for malate degradation in Saccharomyces cerevisiae. Nat Biotechnol 15:253–257
Winston F, Dollard C, Ricupero-Hovasse SL (1995) Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11:53–55
Acknowledgements
We would like to thank the South African National Research Foundation and Winetech for financial support. We are grateful to David Engelberg for supplying the B2562 plasmid.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann
Rights and permissions
About this article
Cite this article
Swiegers, J.H., Pretorius, I.S. & Bauer, F.F. Regulation of respiratory growth by Ras: the glyoxylate cycle mutant, cit2Δ, is suppressed by RAS2 . Curr Genet 50, 161–171 (2006). https://doi.org/10.1007/s00294-006-0084-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-006-0084-z