Abstract
When glucose is available, Saccharomyces cerevisiae prefers fermentation to respiration. In fact, it can live without respiration at all. Here, we study the role of respiration in stress tolerance in yeast. We found that colony growth of respiratory-deficient yeast (petite) is greatly inhibited by canavanine, the toxic analog of arginine that causes proteotoxic stress. We found lower amounts of the amino acids involved in arginine biosynthesis in petites compared with WT. This finding may be explained by the fact that petite cells exposed to canavanine show reduction in the efficiency of targeting of proteins required for arginine biosynthesis. The retrograde (RTG) pathway signals mitochondrial stress. It positively controls production of arginine precursors. We show that canavanine abrogates RTG signaling especially in petite cells, and mutants in the RTG pathway are extremely sensitive to canavanine. We suggest that petite cells are naturally ineffective in production of some amino acids; combination of this fact with the effect of canavanine on the RTG pathway is the simplest explanation why petite cells are inhibited by canavanine. Surprisingly, we found that canavanine greatly inhibits colony formation when WT cells are forced to respire. Our research proposes a novel connection between respiration and proteotoxic stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In eukaryotic cells, the citric acid cycle generates NADH and FADH2, electron carriers used in oxidative phosphorylation of the electron transport chain (ETC) to create ATP, a process collectively known as respiration (Woelders 1989). This occurs in the mitochondrial matrix and inner mitochondrial membrane (Larosa and Remacle 2018). While mitochondria are well known for their role in ATP production and regeneration of reducing power via respiration, this work aims to contribute to the growing body of research on the importance of respiration outside of energy production (Jambhekar and Amon 2008; Knupp et al. 2019; Peng et al. 2012; Vlahakis et al. 2017).
Saccharomyces cerevisiae, the common baker’s yeast, is a valuable tool for exploring the alternate roles of respiration due to its ease of genetic manipulation and versatile metabolism. S. cerevisiae is able to respire yet prefers to create ATP through glycolysis even under aerobic conditions (de Alteriis et al. 2018; de Deken 1966). In fact, in the presence of sufficient glucose, transcription of genes involved in respiration and gluconeogenesis are repressed (Carlson 1999; Gancedo 1998). Furthermore, yeasts with mutations in proteins of the ETC are able to survive despite their inability to respire, a class of mutants known as petites due to their small colony size (a result of reduced ATP production) (Chen and Clark-Walker 2000; Day 2013), yet yeasts cannot survive without mitochondria (Contamine and Picard 2000; Evans and Neuman 2016).
Besides respiration, mitochondria carry out a variety of essential cellular tasks, including cell signaling, amino acid biosynthesis (Jauniaux et al. 1978; Zelenaya-Troitskaya et al. 1995), and nitrogen metabolism (Evans and Neuman 2016; Magasanik and Kaiser 2002). The amino acids partially or completely synthesized in the mitochondria are arginine, ornithine, proline, citrulline, and glutamate, which can be easily converted to glutamine. Glutamate and glutamine are the sole nitrogen source for all biosynthetic reactions (Guillamon et al. 2001; Magasanik and Kaiser 2002). Generation of these two amino acids is proposed to be one of the primary functions of the retrograde pathway (Liu and Butow 2006).
The retrograde (RTG) pathway is a feedback mechanism wherein mitochondria communicate with the nucleus under both normal and stressful conditions (Chelstowska and Butow 1995; Liao and Butow 1993; Liu and Butow 1999). Although mitochondria have their own DNA (mtDNA), mtDNA encodes for a small number of proteins (compared to nuclear DNA), all of which encode for translational machinery and components of the ETC (Anderson et al. 1981). However, because the mitochondria are responsible for a variety of cellular tasks that depend on input from the nuclear genome, they must be able to alert the nucleus when a metabolic shift or protein under nuclear control is needed. Genome-wide transcriptional profiling of yeast cells undergoing mitochondrial dysfunction has identified key players involved in the RTG response (Wodicka et al. 1997). DLD3 and CIT2 are two genes under the regulation of the RTG pathway. In petites, these genes have elevated expression levels (Burns et al. 1994; Chelstowska et al. 1999; Liao et al. 1991). DLD3 converts d-2-hydroxyglutarate (D-2HG) to α-ketoglutarate, a citric acid cycle intermediate, with simultaneous reduction of pyruvate to d-lactate (Becker-Kettern et al. 2016). CIT2 encodes for citrate synthase, a peroxisomal isozyme that catalyzes the production of citrate, another citric acid cycle intermediate occurring upstream of α-ketoglutarate, from acetyl-coA and oxaloacetate (Lewin et al. 1990). α-Ketoglutarate combines with ammonia to form glutamate. Glutamate can then form glutamine from glutamine synthetase and ammonia.
We use canavanine, a toxic amino acid analog of arginine, to examine mechanisms underlying proteotoxic stress in yeast. Canavanine can be mistakenly incorporated into proteins during translation, causing protein misfolding and aggregation (Rosenthal 1977). However, the cellular processes governing canavanine tolerance once it enters the cell are unknown. Through serendipity we found that cells lacking respiration capacity (petites) are highly susceptible to canavanine. By working with sublethal doses of canavanine we can elucidate the role of respiration in the tolerance of proteotoxic stress, as well as identify the genetic networks involved in tolerance to canavanine-induced toxicity. This work will demonstrate two novel effects of canavanine: (1) canavanine affects the RTG pathway, and (2) canavanine causes a reduction in the efficiency of mitochondrially targeted arginine biosynthesis proteins.
Materials and methods
Yeast strains
BY4741 was used as our WT laboratory strain. pif1∆, mgm1∆, mip1∆, mks1∆, rtg2∆, and rtg3∆ of the BY4741 background were of the library constructed by Giaever et al. (Giaever et al. 2002). CS47, a complete arginine auxotroph, is a derivative of S288C strains with an arg4 mutation. C-terminally tagged-GFP strains were from the library constructed at Professor Erin O’Shea’s lab at UCSF (Huh et al. 2003). Arg5,6 C-terminally tagged-GFP was transformed with a plasmid expressing N-terminally tagged cherry-Tom22 under TEF2 promoter from URA Cen/Ars plasmid using the delitto perfetto approach (Storici and Resnick 2006). Petite strains used for microscopy were generated from fluorescently labeled parent strains exposed to ethidium bromide for 1 h in liquid YPD at 250 RPM (Goldring et al. 1970).
Media
Unless otherwise stated, all experiments with canavanine were carried out in complete synthetic media (CSM) without arginine [(−) arginine]. Similarly, experiments using thialysine were carried out on media lacking lysine. YPD solid media contains 2% Bacto Agar, 2% Bacto Peptone, 1% Bacto Yeast Extract (Difco, Sparks, MD, USA), and 2% anhydrous dextrose (Avantor Performance Materials, Center Valley, PA, USA) dissolved in DDW. All other media (complete, YPG, or media lacking a specific amino acid) contain 2% Bacto Agar, 0.67% Yeast Nitrogen Base without Amino Acids (Difco Laboratories), 0.082% Complete Supplement Mixture (no amino acid drop-out) or 0.074% Complete Supplement Mixture Drop-out: ARG or LYS (Formedium), respectively. All media are brought to pH 5.8 using NaOH pearls (Bio-Lab LTD, Jerusalem, IL, USA), autoclaved, and the desired carbon source (2% for glucose and 3% for non-fermentable carbon sources such as lactic acid, ethanol, and glycerol), is added after autoclaving. For nitrogen-starvation experiments, 0.17% Yeast Nitrogen Base lacking nitrogen and amino acids was used, with 0.067% ammonium sulfate and 0.074% Complete Supplement Mixture Drop-out: ARG (Formedium) added. For glutamate addition experiments, l-glutamic acid (Formedium) was added to autoclaved media at a final concentration of 38 mg/l and filter-sterilized. For α-ketoglutarate addition, α-ketoglutaric acid disodium salt dihydrate was added to autoclaved media at a final concentration of 10 mM and filter-sterilized. Liquid media were prepared in the same fashion as solid media with the exclusion of Bacto Agar.
Growth assays on solid media
Using a 96-well tissue culture plate (Jet Biofil), single colony undergoes a tenfold serial dilution. If there were great discrepancies between colony sizes of various isolates, multiple colonies were used to obtain comparable cell numbers per well. Using a 6-by-8-column metal prong, approximately 1 μl from each well is pronged onto desired media. After allowing suspended cells to fully absorb into the plate, the plate is put into 30 °C and growth followed for 1–2 weeks.
Growth assays in liquid media
A single colony was put into 10 ml of media of choice in a breathable Erlenmeyer flask and grown overnight in 30 °C at 250 RPM. The following day, the culture was diluted 50-fold and OD600 was read at various time points. For colony-forming unit (CFU) counts, 100 ul was removed, diluted as needed, and 100 μl spread onto YPD medium. After 2 days, CFUs were counted.
Real-time PCR
A single colony was patched onto CSM (−) arginine or CSM (−) arginine 1 μg/ml canavanine solid media and grown for 4 days in 30 °C. Cells were removed from solid media, resuspended in DDW, and total RNA was isolated using the RNeasy kit (Qiagen) and the MasterPure™ Yeast RNA Purification Kit (Epicentre). cDNAs were synthesized with the FastQuant RT Kit (with gDNase) according to the manufacturer’s protocol. Real-time PCRs were done in a 10 μl volume using the Absolute quantitative PCR SYBR Green mix (Sigma) in a 96-well plate. Triplicates for each sample were included for each reaction. The real-time PCR primers used are forward 5′–3′ primer for ACT1: TCCAAGCCGTTTTGTCCTTG; reverse 5′–3′ primer for ACT1: AAGATTGAGCAGCGGTTTGC; forward 5′–3′ primer for CIT2: CGGAACTACCTAGTCATGTCGTTCA; reverse 5′–3′ primer for CIT2: CATCCTTAGAACCAATCAAGTTGACCAG; forward 5′–3′ primer for DLD3: ACGTCAGGGTCCAATAAGAGACAC; reverse 5′–3′ primer for DLD3: CAAACCGGCTGCGTTTAATCTCTC; forward 5′–3′ primer for ACO1: AGTAACTGCGTTCGCCATTG; reverse 5′–3′ primer for ACO1: AGGCAAACCATCACCATGTG; forward 5′–3′ primer for IDH1: TTGTCGACAATGCCTCCATG; reverse 5′–3′ primer for IDH1: TCAAAGCAGCGCCAATGTTG; forward 5′–3′ primer for IDH2: TTGCCGGTCAAGATAAAGCG; reverse 5′–3′ primer for IDH2: TGTTTTCTGGACCTGATGCG; forward 5′–3′ primer for CIT1: TGGTCGTGCCAATCAAGAAG; reverse 5′–3′ primer for CIT1: AAAACCGCATGGCCATAACC. ACT1 was used as the internal control, with RNA levels of the genes of interest normalized to ACT1 levels. PCRs were initiated at 95 °C for 20 s in the holding stage. The cycling stage consisted of 3 s at 95 °C, 30 s at 60 °C, and 15 s at 95 °C, repeated for 40 cycles. Melt curve stage consisted of 15 s at 95 °C followed by 1 min at 60 °C and finally 15 s at 95 °C.
Microscopy
For each strain, a single colony was put into glucose (−) arginine media for 3 h, then divided evenly into two separate cultures with one culture inoculated with 1 μg/ml canavanine. Cultures grew overnight in 30 °C at 250 RPM. OD600 was read the following morning and adjusted as needed to a final OD600 of 0.6 for all samples. 50 μl of sample was placed in a glass-bottom microplate (MGB101-1-2-LG-L, Brooks). Cells were allowed to settle for 20 min, followed by three washes with 50 μl DDW. A fourth and final addition of 50 μl DDW was added for imaging of the wells in the microscope. Analysis of GFP fluorescence was done using ImageJ. Fluorescence strength and area were calculated by converting GFP images to a “Thresholded” image (thresholding method: default; threshold color: B&W; color space: HSB), followed by “Analyze Particles” [Size (pixel2): 30-infinity; Circularity: 0.00–1.00; excluded on edges and not including holes] (https://imagej.nih.gov/ij/docs/menus/analyze.html). Reasoning that strong localization to the mitochondria results in a smaller area and poor localization results in a larger area, density was calculated as fluorescence/area.
Membrane potential
Arg5,6 C-terminally tagged-GFP and its petite derivative were grown overnight as described above in Microscopy methods with OD600 adjusted to 0.6 the following day. 100 μl of cell suspension was inoculated with 1 μM TMRE (ab113852, Abcam) for a final concentration of 100 nM TMRE. Each sample was incubated for 20 min at 37 °C followed by two washes with 100 μl PBS; cells were then resuspended in 100 μl PBS. 50 μl of sample were placed in a glass-bottom microplate (MGB101-1-2-LG-L, Brooks). Cells were allowed to settle for 20 min, followed by a wash with 50 μl DDW. A final addition of 50 μl DDW was added for imaging of the wells in the microscope.
Hot methanol extraction of amino acids
A single colony of yeast was grown overnight in 5 ml of liquid media at 30 °C shaking in a 50-ml test tube. After overnight growth, 100 ml of sample is diluted and spread onto YPD to count cells. The remaining suspended cells are centrifuged at 3000 RPM for 3 min (Eppendorf Centrifuge 5810 R). The supernatant is disposed of and the remaining pellet washed three times with 3 ml of DDW via vortex, followed by centrifugation at 3000 RPM for 3 min, followed by removal of the supernatant. The remaining pellet is suspended in 1 ml DDW in a 1.5-ml Eppendorf tube and spun down at 14,000 RPM (Eppendorf Centrifuge 5418) for 2 min, followed by removal of supernatant with a pipette. 1 ml of 66% methanol was added to each sample and put into a hot water bath of 70 °C for 30 min with occasional vortexing. Sample is then spun down for 10 min at 14,000 RPM and the supernatant saved and stored at − 20 °C until analysis was done at the Interdepartmental Equipment Unit of The Robert H. Smith Faculty of Agriculture, Food and Environment.
Amino acid measurement
Free amino acid concentrations were determined using LC–MS/MS method. Samples were analyzed on LC–MS system which consisted of Dionex Ultimate 3000 RS HPLC coupled to Q Exactive Plus hybrid FT mass spectrometer equipped with heated electrospray ionization source (Thermo Fisher Scientific Inc.). The HPLC separation of compounds was carried out on Acclaim C18 column (2.1 × 150 mm, particle size 2.2 µm, Dionex) employing linear gradient of acetonitrile/water (with 1.5% acetic acid) binary solvent composition. The mass spectrometer was operated in positive ionization mode, ion source parameters were as follows: spray voltage 3 kV, capillary temperature 300 °C, ESI capillary temp. 300 °C, sheath gas rate (arb) 35, and auxiliary gas rate (arb) 10. Mass spectra were acquired in “full MS”—“all ions fragmentation” at resolving power 70,000. The LC–MS system was controlled and data were analyzed using Xcalibur and TraceFinder software, respectively (Thermo Fisher Scientific Inc.).
Sample preparation An aliquot of unknown sample methanolic solution (10 or 50 µl depending on concentration) was spiked with 10 µl of labeled amino acid internal standard mix (0.2 µg of all labeled amino acids in 10 µl) following addition of 600 µl borate buffer (0.05 M, pH 11). Ethyl carbamates of amino acids were prepared by addition of 30 µl ethylchloroformate solution in acetonitrile (1:9) to the buffer. We allowed 10–15 min for carbamates to form. The buffer is acidified with 30% HCl to pH 1–2 following extraction of amino acid derivatives with 800 µl ethyl acetate. Aqueous and organic phases are separated by centrifugation and the upper, organic phase is accurately transferred to HPLC vial. Ethyl acetate is gently evaporated upon a stream of nitrogen, residue is dissolved in 1 ml of acetonitrile/water/acetic acid (20:79:1) and filtered through 0.2-µm RC membrane filter (regenerated cellulose) prior to LC–MS analysis.
Internal standards (IS) The solution of IS was prepared in 0.1 N HCl from “cell free” labeled amino acid mix (20 AA, 13C, 15N) purchased from Cambridge Isotope (P/N CNLM-6696-1) at final concentration 0.2 mg (of all amino acids)/ml. There was separately added labeled methionine (methyl-D3, Cambridge Isotope, P/N DLM-431-1) to the IS mixture because it was not detected in the “cell free” mix.
Calibration Standards of 24 amino acids were purchased from Sigma-Aldrich. Calibration solutions of amino acids were prepared in 0.1 N HCl at concentration ranges from 1 to 2000 ng/ml. Preparation and derivatization of calibration samples were carried out as described above with a small modification; water was evaporated from 1000 to 150–50 µl volume prior to derivatization. All raw results of amino acid measurements are found in Table S1.
Results
Canavanine-induced growth inhibition of petites on solid media
By serendipity we found that respiration-deficient colonies (petites) undergo extreme growth inhibition in the presence of sublethal doses of canavanine when grown on solid media. This observation was first identified in spontaneous petite colonies; nevertheless, the work presented here was done using lab strains that have permanently lost mitochondrial DNA due to lack of enzymes that are needed for mitochondrial DNA maintenance, such as DNA helicase (pif1Δ), GTPase (mgm1Δ), and DNA polymerase gamma (mip1Δ). The severe growth inhibition of petite cells by canavanine is not due to a general weakness of petites, as they are able to grow to wild-type (WT) level when exposed to other stressors, such as heat shock (not shown), fluconazole, and thialysine (Fig. 1). However, we do not see the same canavanine-induced growth inhibition when petites are grown in liquid phase (Fig. 2). Petite cells were able to grow to stationary stage in liquid media containing canavanine as determined both by OD measurements (data not shown) and colony formation (Fig. 2). We hypothesized that petite cells lack a specific metabolite required for canavanine tolerance; in liquid phase, cells have many opportunities to come into contact with the resources needed to produce or utilize this metabolite, but in solid phase, acquiring necessary resources from the environment is difficult due to low diffusion rate.
Respiration-deficient yeasts are specifically sensitive to canavanine. a Cells were pronged onto glucose (−) arginine with no canavanine, 2 µg/ml canavanine YNB, or YPG (carbon source glycerol). Cells were grown for 1 week at 30 °C. b WT and petites were pronged onto glucose (−) arginine 2 µg/ml canavanine, complete media and complete media containing fluconazole. Cells were grown for 1 week at 30 °C. c WT and petites cells were pronged onto glucose (−) arginine 2 µg/ml canavanine, glucose (−) lysine, and glucose (−) lysine with thialysine. There was no observable difference in toxicity between WT and petites when increasing doses of fluconazole or thialysine
Respiration-deficient yeasts can tolerate canavanine in liquid phase. A single colony of each genotype was grown overnight in 1 ml glucose (−) arginine 2 µg/ml canavanine in 30 °C, 250 RPM shaking. The following day, cultures were diluted 50-fold and continued to grow for a total of 96 h. At seven time points (marked with error bars), 100 microliter of culture was diluted and spread onto YPD plates and CFUs counted after 1-week growth in 30 °C
Arginine addition somewhat rescues the petite phenotype
Arginine, which is synthesized in the mitochondria, competes with canavanine for cellular uptake and incorporation into proteins (Ahmad and Bussey 1986). We asked if addition of arginine to the media could rescue the petite phenotype from canavanine-induced growth inhibition. A small amount of arginine was added to the media and gradually increased. Respiratory-competent cells that are completely auxotrophic for arginine (strain CS47) were unable to form colonies under these conditions, indicating that we did not saturate the system with arginine (Fig. 3). In contrast, the viability of petites grown in the presence of canavanine was improved, but did not reach WT levels, an indication that petites do not produce enough arginine and that arginine availability alone cannot explain petites’ sensitivity to canavanine.
Arginine addition somewhat rescues petites. Strains were grown on glucose (−) arginine and glucose with increasing amounts of arginine, either with 2 µg/ml canavanine (top) or with no canavanine (bottom). The amount of added arginine was based on the amount of arginine in complete media and the amount shown is proportional to that in complete media (from left to right: 0%, 0.5%, 1%, 5%, and 10%). CS47 is an arginine auxotroph and serves as a control to show the presence or absence of arginine in the media. Growth after 10 days. Experiment was also done in mgm1Δ and mip1Δ of the BY4741 background (Fig. S3)
Amino acid profile of petites is different than WT cells
The above results suggest that petite cells do not generate enough arginine. To further examine petites’ availability of arginine, we grew WT and petite cells in media lacking arginine and then extracted amino acids using hot methanol. Through GC–MS analysis we were able to measure the relative amounts of free amino acids in petites versus the WT. Both WT and petites were tested under three conditions: growth in liquid media without arginine, growth on solid media without arginine, and growth on solid media with 1 μg/ml canavanine without arginine. Exclusion of arginine in the media allows us to roughly characterize the state of arginine biosynthesis in each condition.
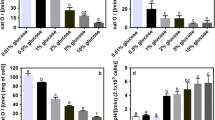
Petite cells are not arginine auxotrophs and can in fact produce arginine, though in liquid (−) arginine media, petite cells have relatively less free arginine than WT, with pif1Δ cells having an average of 6% arginine versus WT 12% arginine (Fig. 4). These differences were not observed in solid media—both WT and petite cells had around 12% free arginine. The relative arginine amounts in WT were similar irrespective of whether canavanine was added to solid media or not. However, the amount of arginine was slightly lower in pif1Δ cells grown in canavanine solid media compared with media without canavanine (10% versus 13%, respectively, Fig. 4). Moreover, differences between WT and pif1Δ cells were observed for other amino acids in the arginine biosynthesis pathway, specifically glutamine, glutamate and ornithine (Fig. S1, Fig. 4). In liquid, WT has a higher amount of glutamine (26% versus 8%) and ornithine (8% versus 2%) than pif1Δ; in solid media, 8% versus 2% glutamine and 11% versus 4% ornithine in WT versus pif1Δ, respectively; in solid media with canavanine, 6% versus 4% glutamine and 13% versus 3% ornithine in WT versus pif1Δ, respectively. In liquid media, WT and pif1Δ had an equal amount of glutamic acid (19%), but in solid media, glutamic acid was depleted both with canavanine (15% in WT versus 8% in pif1Δ) and without canavanine (15% in WT versus 6% in pif1Δ) in the petite background. Citrulline, the most immediate biosynthetic precursor to arginine, comprised less than 1% under all conditions in both genotypes. While it is known that growth in media lacking a specific amino acid will increase production of that amino acid, our data suggest that petites are unable to replenish glutamine/glutamate stores during this shift to arginine production, resulting in depleted quantities of these precursor amino acids.
Amino acid analysis. Only amino acids comprising greater than 2% have been included (unless they are produced in the mitochondria). Relative amounts of free amino acids were determined by nanograms of free amino acids divided by total amount (in nanograms) of free amino acids per sample. a BY4741 grown overnight in liquid glucose (−) arginine. bpif1Δ grown overnight in liquid glucose (−) arginine. c BY4741 grown for 2 days on solid glucose (−) arginine. dpif1Δ grown for 2 days on solid glucose (−) arginine. e BY4741 grown for 2 days on solid glucose (−) arginine with 1 μg/ml canavanine. fpif1Δ grown for 2 days on solid glucose (−) arginine with 1 μg/ml canavanine. Liquid analysis was also done in mgm1Δ and mip1Δ of the BY4741 background, as well as mgm1Δ from the HAY75 background (56) with similar results (not included for brevity)
Interestingly, compared to growth in liquid, alanine is overrepresented in both WT and pif1Δ grown on solid media with and without canavanine, with the petite having double than that of WT. The increase in alanine on solid media is intriguing given the fact that pyruvate, the product of glycolysis that can enter the citric acid cycle, can be converted into alanine in a reversible reaction via alanine aminotransferase encoded by ALT1 (α-ketoglutarate + l-alanine ⟺ l-glutamate + pyruvate).
Glutamate and alpha-ketoglutarate support canavanine tolerance
Based on the amino acid profile of petite cells we reasoned that α-ketoglutarate and glutamate availability should decrease canavanine-induced growth inhibition in petites. We added glutamate and α-ketoglutarate to (−) arginine media containing canavanine and followed growth for 9 days. Both glutamate and α-ketoglutarate had a transient effect on canavanine-induced growth inhibition. After 4 days of growth, petite colonies grown on glutamate and 1 μg/ml canavanine were clearly visible and larger in size when compared to those grown on canavanine only. Petite and WT cells grown on canavanine 2 μg/ml also showed improved growth on the glutamate background (Fig. 5a). A similar but less pronounced effect was observed with α-ketoglutarate addition (Fig. 5b). mgm1∆ showed the greatest improvement in growth on canavanine under both glutamate and α-ketoglutarate addition.
Glutamate and α-ketoglutarate effect on canavanine-induced growth inhibition. a Glutamic acid addition. A single colony was placed in 200 µl DDW, serial dilutions performed, and pronged onto glucose (−) arginine media with 0, 1, or 2 μg/ml canavanine, with or without addition of l-glutamic acid. b α-Ketoglutarate addition. A single colony was placed in 200 µl DDW, serial dilutions were performed, and pronged onto glucose (−) arginine media with 0, 1, or 2 μg/ml canavanine, with or without addition of α-ketoglutarate. Images show growth after 4 and 9 days
Canavanine affects localization of arginine biosynthesis enzymes
Our data indicate that petite cells have reduced capacity to generate intermediates in the arginine pathway. Arginine biosynthesis enzymes are encoded in nuclear DNA, yet four of these ultimately localize to the mitochondria (Arg2, Arg5,6, Arg7, and Arg8). We asked if petites’ ability to transport arginine biosynthesis proteins was compromised, possibly due to decreased mitochondrial membrane potential resulting from a lack of ETC and oxidative phosphorylation. When grown in glucose (−) arginine liquid media, we did not observe a major difference between WT and petite mitochondrial localization of these enzymes. When grown in glucose (−) arginine 1 μg/ml canavanine, delocalization in both WT and petites was observed, with petites displaying a more extreme phenotype (Fig. 6a). We quantified the strength and localization of GFP in Arg5,6 and Arg8, represented as density (fluorescence/area) in Fig. 6b. Next, we studied if the delocalization was due to mistargeting or due to disruption of mitochondrial structure. On the basis of the Arg5,6-GFP strains described above, we constructed Tom22 RFP strains.
Canavanine affects localization of arginine biosynthesis genes. A single colony was grown in glucose (−) arginine for 3 h and then divided into two separate cultures with one culture inoculated with 1 μg/ml canavanine. Cultures grew overnight in 30 °C at 250 RPM and pictures were taken with a fluorescent microscope. a Microscope images showing localization of GFP-labeled enzymes. A representative 704 × 704-pixel selection is shown for each of the total 2048 × 2048 pixel images. Arg2 not included due to poor imaging. b Bar graph showing the density of two GFP-labeled enzymes. Quantification is of the total 2048 × 2048 pixel images
Tom22 is a central component of the TOM (translocase of outer membrane) receptor complex (Honlinger et al. 1995; Lithgow et al. 1994). It does not require the mitochondrial membrane potential to be inserted into the mitochondrial membrane. In respiration-proficient strains with or without canavanine addition, the GFP (Arg5,6) and the RFP (Tom22) signals overlapped (Fig. 7). The same was true for respiration-deficient strains without canavanine exposure. However, respiration-deficient strains that were exposed to canavanine showed only partial overlap between the Arg5,6 and Tom22 (Fig. 7). To test if the delocalization of enzymes within the arginine biosynthesis pathway was due to reduction in the mitochondrial membrane potential, we measured mitochondrial membrane potential using TMRE (tetramethylrhodamine, ethyl ester) staining. TMRE is a red fluorescent dye that is accumulated in active mitochondria—loss of mitochondrial membrane potential is reflected in loss of the TMRE signal. Adding canavanine to respiration-proficient strains caused reduction in the relative amounts of cells with positive TMRE signal (Fig. 8). Respiration-deficient strains showed reduction in the relative amounts of cells with positive TMRE signal compared with respiration-proficient ones, a trend that was further decreased when canavanine was added (Fig. 8). The proportion of TMRE-stained mitochondria in petite cells treated with canavanine was minimal, indicating a combined effect of canavanine and loss of respiration on the mitochondrial membrane potential (Fig. 8).
Canavanine-affected cells have functioning mitochondria. Arg5,6-GFP Tom22-RFP respiration-proficient and -deficient strains were grown in glucose (−) arginine for 3 h, then divided into two separate cultures with one culture inoculated with 1 μg/ml canavanine. Cultures grew overnight in 30 °C at 250 RPM and pictures taken with a fluorescent microscope, green and red signals were overlaid. White arrows in the bottom-right image point to examples of mislocalization of Arg5,6
Canavanine reduces mitochondria membrane potential. Respiration-proficient and -deficient strains were grown in glucose (−) arginine for 3 h, then divided into two separate cultures with one culture inoculated with 1 μg/ml canavanine. Cultures grew overnight in 30 °C at 250 RPM and pictures taken with a fluorescent microscope after 20 min incubation with 100 nM TMRE as described in the manufacturer protocol
Nitrogen starvation increases canavanine-induced growth inhibition more than glucose starvation
We have shown above that petite cells produce relatively less amino acids in the pathway leading to arginine. Next, we asked what resources are limiting in the production of amino acids. One such resource required for petite growth is glucose (or any other fermentable carbon source). We grew petites in increased concentrations of glucose but found no effect on canavanine tolerance (data not shown). Conversely, we grew petites on reduced concentrations of glucose in the presence of canavanine and found petites could tolerate these reduced glucose concentrations, though not as well as WT (Fig. 9a). This could be due to the relief of glucose repression—that is, under sufficient glucose levels, the transcription of genes involved in respiration and gluconeogenesis is inhibited, but under glucose-scarce conditions (like those used in this experiment), transcription of such genes can proceed.
Nitrogen starvation increases canavanine sensitivity more than glucose starvation. a Strains were grown on media containing 2% glucose and either 1/10th the normal amount of ammonium sulfate (top; 0.5 mg/ml) or normal nitrogen (bottom; 5 mg/ml), without arginine, and in the presence of either no canavanine, 0.5 μg/ml, 1 μg/ml, or 2 μg/ml. b Strains were grown on (−) arginine media with 0.2%, 1%, or 2% glucose containing 1 μg/ml canavanine. Decreased glucose concentration had little effect on canavanine-induced growth inhibition. Growth after 1 week
Arginine synthesis ultimately begins with the availability of nitrogen in the form of ammonia or the amino acid glutamate; glutamate and glutamine are not supplied in the growing media and, therefore, are synthesized from simple compounds. Addition of ammonium sulfate to the media had no effect on colony formation in petites, likely due to saturation of nitrogen transporters (data not shown). However, reduction of ammonium sulfate in the media caused an increase in petite cells’ sensitivity to canavanine, and even the WT showed an increase in canavanine-induced growth inhibition, although to a lesser extent (Fig. 9b). This shows the importance of nitrogen availability in the presence of canavanine, especially in respiration-deficient cells.
Canavanine interferes with the RTG pathway
We have observed amino acid production defects of petite cells; some of the defects were exacerbated in the presence of canavanine, such as reduction in arginine amounts in solid media. We were able to link these differences to reduction in the mitochondrial membrane potential. The retrograde (RTG) pathway connects between mitochondrial function and regulation of nuclear gene expression (Liu and Butow 2006). Petite cells of various backgrounds have the RTG pathway turned on even under normal conditions (Wodicka et al. 1997). We asked if addition of canavanine would cause an increase in mitochondrial stress, measured by the abundance of the two prototypical RTG pathway genes in petites, CIT2 and DLD3; surprisingly, we found that canavanine caused a decrease in mRNA expression levels (Fig. 10a–c). We used the Δ/ΔCt method to quantify the relative abundance of these two genes, with comparisons between four different pairs: pif1Δ glucose/BY4741 glucose, pif1Δ canavanine/BY4741 canavanine, pif1Δ canavanine/pif1Δ glucose, and BY4741 canavanine/BY4741 glucose. Under normal conditions, pif1Δ expresses more CIT2 (2.45-fold expression) and DLD3 (1.49-fold expression) than WT (Fig. 10a, blue bars), as expected. However, we see an overall trend of decreased expression upon exposure to canavanine, with pif1Δ expression of CIT2 reducing from 2.45-fold to 1.15-fold, and there is a significant reduction in DLD3 mRNA expression in pif1Δ compared to the WT, reducing from 1.49-fold to 0.47-fold in pif1Δ versus WT upon exposure to canavanine (Fig. 10a, red bars). When we compare mRNA levels of exposure to canavanine versus non-exposure within the same genotype, we continue to see this downward trend in which canavanine-exposed cells, both WT and petite, express less mRNA of both CIT2 and DLD3 (Fig. 10b, c). pif1Δ exposed to canavanine expresses CIT2 at a level of just 0.02 when compared to mRNA expression of CIT2 in pif1Δ without canavanine, and similarly, DLD3 drops to 0.18-fold expression in the presence of canavanine (Fig. 10b). WT expression of CIT2 mRNA drops to just 0.07-fold and DLD3 drops to 0.59-fold expression when exposed to canavanine and compared to WT growth without canavanine (Fig. 10c).
Canavanine affects the RTG pathway. Fold change was calculated using the Δ/ΔCt method with ACT1 as a reference gene. a Blue columns represent mRNA expression of pif1Δ compared to WT when grown on (−) arginine media. Red columns represent mRNA expression of pif1Δ compared to WT when grown on (−) arginine media 1 μg/ml canavanine. b Bar chart represents mRNA expression of pif1Δ grown on (−) arginine media with 1 μg/ml canavanine versus pif1Δ grown on (−) arginine media. c Bar chart represents mRNA expression of WT grown on (−) arginine media with 1 μg/ml canavanine versus WT grown on (−) arginine media
Canavanine alters expression of genes that are under RTG control in rho0 cells
The Hap2,3,4,5p transcription complex is needed for expression of many mitochondrial proteins involved in electron transport and the TCA cycle. Liu and Butow (1999) showed that a decrease or loss of respiration caused expression of CIT1, ACO1, IDH1, and IDH2 to switch from HAP control to RTG control. These four TCA genes encode for enzymes that synthesize the immediate precursors of α-ketoglutarate. Since expression of these four TCA genes is dependent on the RTG genes in rho0 cells, induction of their expression in petites is a retrograde response (Traven et al. 2001). We again used the Δ/ΔCt method to measure the relative abundance of these genes in petites versus WT. Under normal conditions, pif1Δ expresses more CIT1 mRNA (4.93-fold), ACO1 (14.62-fold), and IDH1 (2.08-fold) than WT (Fig. 10a, blue bars). Taking into account the standard deviation, IDH2 mRNA expression in pif1Δ was roughly equal to WT under these conditions (1.05-fold). When grown on canavanine, expression of all four genes decreases in pif1Δ compared to WT: CIT1 expression drops from 4.93-fold to 0.91-fold, ACO1 expression drops from 14.62-fold to 0.21-fold, IDH1 expression drops from 2.08-fold to 0.36-fold, and IDH2 expression drops from 1.05-fold to 0.02-fold (Fig. 10a, red bars). Comparing mRNA levels of exposure to canavanine versus non-exposure within the same genotype, mRNA levels in pif1Δ and WT had opposite responses to canavanine (Fig. 10b, c). In pif1Δ, canavanine caused a decrease in mRNA expression (CIT1 = 0.64-fold, ACO1 = 0.03-fold, IDH1 = 0.48-fold, IDH2 = 0.37-fold) (Fig. 10b). In WT, canavanine caused an increase in mRNA expression (CIT1 = 3.47-fold, ACO1 = 2.05-fold, IDH1 = 2.79-fold, IDH2 = 15.59-fold) (Fig. 10c).
RTG2, the central regulator of the RTG pathway, is required for canavanine tolerance
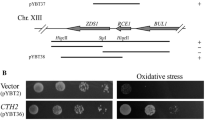
We found that canavanine exposure correlates with decreased expression of the prototypical RTG genes mRNA in both petites and WT, with petites displaying a more severe phenotype. Thus, we hypothesized that a switching off of the RTG pathway should induce petite-like canavanine sensitivity in a respiration-proficient cell. To test this, we used rtg2Δ; Rtg2p is a sensor of mitochondrial dysfunction and a central regulator of the RTG pathway (Liu et al. 2003). In agreement with our RT-qPCR findings, rtg2Δ is unable to grow on canavanine (Fig. 11). rtg3Δ and mks1Δ were also unable to grow on canavanine (Fig. 11). All three knockout strains grew to WT level on YPD media, but grew slowly on (−) arginine media, suggesting they are deficient in arginine production. Rtg3p is a transcription factor that forms a complex with Rtg1p to activate the RTG response. Mks1p is a negative transcriptional regulator involved in RTG signaling.
The Goldilocks effect: enforcing respiration causes severe inhibition by canavanine
Based on our work with petites, we see respiration is a requirement for growth in the presence of canavanine, so we examined how respiration as the sole means of ATP production would affect canavanine tolerance. Unlike petites (Fig. 2), canavanine has a fungistatic effect on WT cells grown in a liquid non-fermentable carbon source (Fig. 12a). When grown on a solid non-fermentable carbon source, WT cells experience canavanine-induced growth inhibition at a level similar to that of petites (Fig. 12b). This leads us to hypothesize that different mechanisms govern canavanine-induced growth inhibition among respiratory-proficient cells grown on a non-fermentable carbon source and respiratory-deficient cells grown on a fermentable carbon source, especially considering that a can1∆ mutant is also sensitive to the combination of a non-fermentable carbon source and canavanine (Fig. S2).
Canavanine is fungistatic with a non-fermentable carbon source. a Growth of BY4741 in glucose (blue) versus lactic acid (red). A single colony of WT BY4741 was grown overnight in 1 ml lactic acid (−) arginine with 1 µg/ml canavanine in 30 °C, 250 RPM shaking. The following day, cultures were diluted 50-fold and continued to grow for a total of 96 h. At seven time points (marked with error bars), 100 microliter of culture was diluted and spread onto YPD plates. b BY4741 growth on non-fermentable carbon sources versus growth on glucose with or without canavanine. WT was grown on a non-fermentable carbon source (−) arginine with or without canavanine. Growth after 1 week
In conclusion, our data show an important role of respiration and fermentation in facilitating growth in the presence of canavanine. The system presented here can model the effect of varying respiration capacities on stress tolerance, a topic at the heart of cancer biology research.
Discussion
Saccharomyces cerevisiae is an elegant model to study how different respiration capacities affect the cell’s ability to deal with stress, because, unlike many eukaryotic organisms, S. cerevisiae is petite positive and exhibits the Crabtree effect—fermentation in the presence of oxygen (Merico, et al. 2007). Despite their lack of respiration, and thus decreased ATP production (Rodrigues et al. 2006; Williamson et al. 1971), petite cells are not generally weak. This is supported by our work (Fig. 1), as well as prior work showing petite cells are more resistant to heat shock and certain drugs, as they exhibit pleiotropic drug resistance via upregulation of PDR5 (Moye-Rowley 2005; Traven et al. 2001). In the past, pif1Δ was identified as a mutant conferring increased resistance to canavanine in a high-throughput screen of a BY4741 knockout library (Shi et al. 2011); however, our data show that petite cells, and specifically pif1Δ, are sensitive to canavanine. This false positive is likely due to the gain of a can1 mutation since absence of Pif1 increases mutation rates, especially in telomere proximal genes such as CAN1 (Myung et al. 2001; Zhang and Singh 2014).
We hypothesize that even low rates of respiration contribute to overall mitochondrial integrity and, therefore, is necessary for tolerance of canavanine. Our investigation points to two factors: first, petite cells are deficient in producing amino acids within the arginine biosynthesis pathway (Figs. 4, 13), and canavanine reduces the efficiency of targeting to the mitochondria of enzymes in the arginine biosynthesis pathway, probably through reducing mitochondrial membrane potential (Figs. 6, 7, 8). Second, we show for the first time that canavanine interferes with the RTG pathway in yeast cells. This interference can be tolerated in respiration-proficient yeast, but respiration-deficient ones rely more on the retrograde pathway (Liao et al. 1991; Liu and Butow 1999) and are thus more susceptible to canavanine interference. Moreover, the expression of CIT1, ACO1, IDH1 and IDH2, all part of the citric acid cycle, is induced in WT cells following canavanine exposure (Fig. 10c). In contrast, the expression of these genes is suppressed in petite cells following canavanine exposure (Fig. 10b). This difference can be partly explained by these four genes being under different regulation in WT and petite (HAP in WT (Forsburg and Guarente 1989), RTG in petite (Liu and Butow (1999); discussed further below). We think that the differential effect of canavanine on petite cells from an arginine biosynthesis standpoint and activation of the RTG pathway are major determinants in the sensitivity of petite cells to canavanine. However, other explanations are valid and may contribute significantly to this phenomenon. For example, enhanced permeability of petite cells to canavanine or more efficient uptake of the toxic analog into proteins in petite is possible.
To the best of our knowledge, the amount of information regarding the effect of respiration capacity on amino acid biosynthesis is limited (Martiez-Force and Benitez 1992). Martinez-Force and Benitez measured amino acid pools in fermentative versus respiratory and mixed fermentative–respiratory media. Their data show increases in arginine, lysine, and glutamate as metabolism shifts towards respiratory only. Our findings were somewhat different; while we did see a significant increase in arginine pools in WT, there was no difference in glutamate concentrations between WT and petite, and free lysine was almost 20% greater under fermentative conditions (Fig. 4a, b). Importantly, our results were obtained under conditions of arginine starvation, not like the data obtained by Martinez-Force and Benitez. Therefore, from an arginine biosynthesis standpoint, our results are more relevant because there is no influence of arginine uptake from the media. In addition, we describe here for the first time the differences in the free amino acid profile between respiration-proficient and respiration-deficient yeast grown on solid media where diffusion is limited. Our results show that compared to liquid phase, solid phase growth has a major effect on the status of relative free amino acids in both WT and petite, with petites undergoing the most drastic changes (Figs. 4, 13). pif1Δ grown in solid media produced less glutamine, glutamate and ornithine, all of which are amino acids that are part of the arginine biosynthesis pathway (Jauniaux et al. 1978). We show that addition of arginine and glutamate somewhat relieves the growth inhibition imposed by canavanine on solid media (Figs. 3, 5, Fig. S3). The simplest explanation is that under canavanine stress there is a need to produce more arginine to compete with canavanine for uptake into proteins; since petite cells have less glutamate and glutamine to start with (Fig. 4d, f), the reservoir for arginine production is lower than WT cells. Alternatively, the low pools of glutamate and glutamine may itself cause stress on the proteome that is then exacerbated by the stress of canavanine. Another level of complexity is the observation that canavanine reduces the efficiency of targeting of arginine biosynthetic enzymes to the mitochondria, especially in petite cells (Fig. 6). We found that canavanine reduces the ability to generate mitochondrial membrane potential in both WT and petites. This, combined with petites’ inherently reduced mitochondrial membrane potential, could explain why canavanine exposure causes such a drastic reduction in the ability of some proteins to be targeted to the mitochondria in respiration-deficient cells (Figs. 6, 7, 8).
We also show that addition of α-ketoglutarate has a small but reliable effect on canavanine tolerance in petite cells, though the effect is not equal between all petite mutants; a stronger effect was observed in mgm1Δ cells. The difference between the mutants could be because Mgm1 unlike Mip1 and Pif1 also functions in mitochondria morphology and fusion that may affect permeability of α-ketoglutarate or retention of the relevant enzymes in the mitochondria (Sesaki et al. 2003). Ammonium and α-ketoglutarate are the simplest compounds required for glutamate biosynthesis (Roon et al. 1974). Enzymes of the citric acid cycle are functional in petite cells, but those downstream of α-ketoglutarate (KGD1, SDH1, FUM1, and MDH1) are all downregulated in rho0 petites, and petite cells lacking any of the RTG genes show severe reduction in citric acid cycle enzymes upstream of α-ketoglutarate (CIT1, ACO1, IDH1, IDH2) (Liu and Butow 1999). It is unknown how respiration capacity affects the cellular concentrations of α-ketoglutarate in yeast. One of the roles of the RTG pathway is to increase the amounts of α-ketoglutarate in mitochondrially stressed cells; this is largely modeled after phenotypes of respiratory-deficient cells (Epstein et al. 2001; Liu and Butow 1999; Traven et al. 2001), but it is unknown if the RTG pathway can elevate α-ketoglutarate levels in petites to a comparable α-ketoglutarate level in WT. Our data indicate that there is not enough α-ketoglutarate in petite cells to produce glutamate and glutamine at a WT level (Figs. 4, 5, 13). Furthermore, our finding that canavanine hampers expression of CIT1, ACO1, IDH1, and IDH2 in petites yet increases expression of these four genes in WT may explain why petites are so much more sensitive to canavanine that WT: inhibition of the RTG response, whether by canavanine or otherwise, inherently decreases petites’ ability to generate α-ketoglutarate; however, CIT1, ACO1, IDH1, and IDH2 are under HAP control in respiration-competent cells, thus they are still able to produce α-ketoglutarate. The modest effect of α-ketoglutarate addition on canavanine tolerance could be due to poor uptake or rapid metabolism that is different from glutamate biosynthesis (Contreras-Shannon et al. 2005; Quezada et al. 2013; Smith et al. 2007).
Reduction in the amount of the nitrogen source sensitizes petite cells more than WT to canavanine (Fig. 9). This finding may suggest that the poor production of glutamate is not only a result of poor flow of citric acid cycle metabolites but due to either poor uptake of nitrogen or inefficient activity of the enzymes required for glutamine or glutamate biosynthesis. Gdh1 is a key enzyme in glutamate production that is expressed in fermentative growth and uses α-ketoglutarate as a substrate (DeLuna et al. 2001); we hypothesize that it is either downregulated or inhibited in petite cells. Alternatively, Gdh1 could be directly or indirectly inactivated by canavanine.
The RTG pathway has been extensively studied in petites (Epstein et al. 2001; Liu and Butow 1999, Traven et al. 2001); however, its relation to canavanine has not been reported. This pathway regulates genes involved in glutamate and α-ketoglutarate homeostasis, which leads to the conclusion that the RTG pathway plays a role in nitrogen homeostasis (Magasanik and Kaiser 2002). Our results show that nitrogen starvation increases canavanine sensitivity, and loss of RTG2, RTG3 or MKS1 eliminates a colony’s ability to tolerate canavanine under arginine-limiting conditions (Figs. 9b, 11). It has been reported that nitrogen starvation induces mitophagy (Camougrand et al. 2008; Kanki et al. 2009), yet rtg∆ mutants undergo less mitophagy (Torelli et al. 2015). All rtg mutants (rtg1∆, rtg2∆, and rtg3∆) have a great reduction in CIT2 expression, both in respiration-proficient and -deficient strains (Guaragnella and Butow 2003; Liao and Butow 1993). In fact, petite cells with mutations in any of the rtg genes show a reduction in most citric acid enzymes (the exceptions being SDH1 and FUM1) (Liu and Butow 1999). We suggest that the inhibition of the RTG pathway by canavanine is the one explanation for the various petite-related phenotypes we observe here. This is because petites rely more than WT on the RTG pathway for nitrogen homeostasis and amino acid production, two processes closely tied to the mitochondria (Jauniaux et al. 1978; Komeili et al. 2000; Magasanik and Kaiser 2002). At this point we do not know how canavanine interferes with the RTG pathway. The response to canavanine is mediated by Msn2/4 transcription factors that govern cytosolic stress (Shor et al. 2013). Could it be that the cytosolic stress response overrides the mitochondrial one?
Our observations may have an applied side; several fungicides target succinate dehydrogenase and, therefore, respiration (Scalliet et al. 2012). In light of our results, synthetic lethality of the succinate dehydrogenase inhibitor boscalid and canavanine (or similar compounds) is possible.
Finally, we observe that growth in a non-fermentable carbon source greatly sensitizes yeast cells to canavanine. At this point, we do not understand the mechanism of this synthetic interaction. It is possible that canavanine directly interferes with the assembly of respiratory complexes even in very low concentrations—much lower than needed to inhibit the rest of the proteome. However, other more complicated scenarios are possible. Interestingly, a connection between the Snf1 kinase, which is activated in cells growing on a non-fermentable carbon source, and the response to misfolded proteins were previously reported (Simpson-Lavy et al. 2017). It was previously reported that growth on a carbon source that is not glucose changes the morphology of mitochondria and activates the RTG pathway (Epstein et al. 2001; Guaragnella and Butow 2003; Prevost et al. 2018; Soontorngun 2017). It is, therefore, possible that the toxic effect of canavanine in the presence of a non-fermentable carbon source occurs through inhibition of the RTG pathway or disruption of mitochondria morphology. Zhang and Cao provide a more general view on respiration capacity and response to stress. They suggest that regulation of mitochondrial respiration can modulate metabolic reprogramming and stress response, thus restriction of the ability to regulate respiration either by growth on a non-fermentable carbon source or using respiration-deficient cells may restrict the ability of the cell to deal with canavanine stress (Zhang and Cao 2017).
We think that the mode of inhibition under forced respiratory conditions is different than the mode of inhibition of canavanine imposed on petite cells grown on glucose. This is due to the fungistatic effect of canavanine and a non-fermentable carbon source in liquid culture (compare Fig. 2 with Fig. 12). If so, from an evolutionary standpoint, what is the meaning of such confined respiratory capacity, i.e., the Goldilocks effect?
References
Ahmad M, Bussey H (1986) Yeast arginine permease: nucleotide sequence of the CAN1 gene. Curr Genet 10(8):587–592
Anderson Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PHJ, Smith AJ, Staden R, Young IG (1981) Sequence and organization of the human mitochondrial genome. Nature 290(5806):457–465
Becker-Kettern J, Paczia N, Conrotte J-F, Kay DP, Guignard C, Jung PP, Linster CL (2016) Saccharomyces cerevisiae forms d-2-hydroxyglutarate and couples its degradation to d-lactate formation via a cytosolic transhydrogenase. J Biol Chem 291(12):6036–6058. https://doi.org/10.1074/jbc.m115.704494 (Epub 2016 Jan 16)
Burns N, Grimwade B, Ross-Macdonald PB, Choi EY, Finberg K, Roeder GS, Snyder M (1994) Large-scale analysis of gene expression, protein localization, and gene disruption in Saccharomyces cerevisiae. Genes Dev 8(9):1087–1105
Camougrand N, Kissová I, Salin B, Devenish RJ (2008) Chapter 8 monitoring mitophagy in yeast. Methods in enzymology. Academic, New York, pp 89–107
Carlson M (1999) Glucose repression in yeast. Curr Opin Microbiol 2(2):202–207
Chelstowska A, Butow RA (1995) RTG genes in yeast that function in communication between mitochondria and the nucleus are also required for expression of genes encoding peroxisomal proteins. J Biol Chem 270(30):18141–18146
Chelstowska A, Liu Z, Jia Y, Amberg D, Butow RA (1999) Signalling between mitochondria and the nucleus regulates the expression of a new d-lactate dehydrogenase activity in yeast. Yeast 15(13):1377–1391
Chen XJ, Clark-Walker GD (2000) The petite mutation in yeasts: 50 years on. Int Rev Cytol 194:197–238
Contamine V, Picard M (2000) Maintenance and integrity of the mitochondrial genome: a plethora of nuclear genes in the budding yeast. Microbiol Mol Biol Rev 64(2):281–315
Contreras-Shannon V, Lin AP, McCammon MT, McAlister-Henn L (2005) Kinetic properties and metabolic contributions of yeast mitochondrial and cytosolic NADP + -specific isocitrate dehydrogenases. J Biol Chem 280(6):4469–4475
Day M (2013) Yeast petites and small colony variants: for everything there is a season. Adv Appl Microbiol 85:1–41
de Alteriis E, Carteni F, Parascandola P, Serpa J, Mazzoleni S (2018) Revisiting the Crabtree/Warburg effect in a dynamic perspective: a fitness advantage against sugar-induced cell death. Cell Cycle 17(6):688–701. https://doi.org/10.1080/15384101.2018.1442622
de Deken RH (1966) The Crabtree effect: a regulatory system in yeast. J Gen Microbiol 44(2):149–156. https://doi.org/10.1099/00221287-44-2-149
DeLuna A, Avendano A, Riego L, Gonzalez A (2001) A NADP-glutamate dehydrogenase isoenzymes of Saccharomyces cerevisiae. Purification, kinetic properties, and physiological roles. J Biol Chem 276(47):43775–43783
Epstein CB, Waddle JA, Hale W, Davé V, Thornton J, Macatee TL, Garner HR, Butow RA (2001) Genome-wide responses to mitochondrial dysfunction. Mol Biol Cell 12(2):297–308
Evans A, Neuman N (2016) The mighty mitochondria. Mol Cell 61(5):641. https://doi.org/10.1016/j.molcel.2016.02.002
Forsburg SL, Guarente L (1989) Communication between mitochondria and the nucleus in regulation of cytochrome genes in the yeast Saccharomyces cerevisiae. Annu Rev Cell Biol 5:153–180
Gancedo JM (1998) Yeast carbon catabolite repression. Microbiol Mol Biol Rev 62(2):334–361
Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B, Arkin AP, Astromoff A, El-Bakkoury M, Bangham R, Benito R, Brachat S, Campanaro S, Curtiss M, Davis K, Deutschbauer A, Entian KD, Flaherty P, Foury F, Garfinkel DJ, Gerstein M, Gotte D, Güldener U, Hegemann JH, Hempel S, Herman Z et al (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418(6896):387–391
Goldring ES, Grossman LI, Krupnick D, Cryer DR, Marmur J (1970) The petite mutation in Yeast: loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J Mol Biol 52:323–335. https://doi.org/10.1016/0022-2836(70)90033-1
Guaragnella N, Butow RA (2003) ATO3 encoding a putative outward ammonium transporter is an RTG-independent retrograde responsive gene regulated by GCN4 and the Ssy1-Ptr3-Ssy5 amino acid sensor system. J Biol Chem 278(46):45882–45887
Guillamon JM, van Riel NA, Giuseppin ML, Verrips CT (2001) The glutamate synthase (GOGAT) of Saccharomyces cerevisiae plays an important role in central nitrogen metabolism. FEMS Yeast Res 1(3):169–175
Honlinger A, Kubrich M, Moczko M, Gartner F, Mallet L, Bussereau F, Eckerskorn C, Lottspeich F, Dietmeier K, Jacquet M et al (1995) The mitochondrial receptor complex: Mom22 is essential for cell viability and directly interacts with preproteins. Mol Cell Biol 15(6):3382–3389
Huh WK, Falvo JV, Gerke LC, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK (2003) Global analysis of protein localization in budding yeast. Nature 425(6959):686–691
Jambhekar A, Amon A (2008) Control of meiosis by respiration. Curr Biol 18(13):969–975. https://doi.org/10.1016/j.cub.2008.05.047
Jauniaux JC, Urrestarazu LA, Wiame JM (1978) Arginine metabolism in Saccharomyces cerevisiae: subcellular localization of the enzymes. J Bacteriol 133(3):1096–1107
Kanki T, Kang D, Klionsky DJ (2009) Monitoring mitophagy in yeast: the Om45-GFP processing assay. Autophagy 5(8):1186–1189
Knupp J, Arvan P, Chang A (2019) Increased mitochondrial respiration promotes survival from endoplasmic reticulum stress. Cell Death Differ 26(3):487–501. https://doi.org/10.1038/s41418-018-0133-4 (Epub 2018 May 23)
Komeili A, Wedaman KP, O’Shea EK, Powers T (2000) Mechanism of metabolic control. Target of rapamycin signaling links nitrogen quality to the activity of the Rtg1 and Rtg3 transcription factors. J Cell Biol 151(4):863–878
Larosa V, Remacle C (2018) Insights into the respiratory chain and oxidative stress. Biosci Rep 38(5):BSR20171492
Lewin AS, Hines V, Small GM (1990) Citrate synthase encoded by the CIT2 gene of Saccharomyces cerevisiae is peroxisomal. Mol Cell Biol 10(4):1399–1405
Liao X, Butow RA (1993) RTG1 and RTG2: Two yeast genes required for a novel path of communication from mitochondria to the nucleus. Cell 72(1):61–71
Liao XS, Small WC, Srere PA, Butow RA (1991) Intramitochondrial functions regulate nonmitochondrial citrate synthase (CIT2) expression in Saccharomyces cerevisiae. Mol Cell Biol 11(1):38–46
Lithgow T, Junne T, Suda K, Gratzer S, Schatz G (1994) The mitochondrial outer membrane protein Mas22p is essential for protein import and viability of yeast. Proc Natl Acad Sci USA 91(25):11973–11977
Liu Z, Butow RA (1999) A transcriptional switch in the expression of yeast tricarboxylic acid cycle genes in response to a reduction or loss of respiratory function. Mol Cell Biol 19(10):6720–6728
Liu Z, Butow RA (2006) Mitochondrial retrograde signaling. Annu Rev Genet 40:159–185
Liu Z, Sekito T, Spirek M, Thornton J, Butow RA (2003) Retrograde signaling is regulated by the dynamic interaction between Rtg2p and Mks1p. Mol Cell 12(2):401–411
Magasanik B, Kaiser CA (2002) Nitrogen regulation in Saccharomyces cerevisiae. Gene 290(1–2):1–18
Martiez-Force E, Benitez T (1992) Changes in yeast amino acid pool with respiratory versus fermentative metabolism. Biotechnol Bioeng 40(6):643–649
Merico A, Sulo P, Piškur J, Compagno C (2007) Fermentative lifestyle in yeasts belonging to the Saccharomyces complex. FEBS J 274(4):976–989
Moye-Rowley WS (2005) Retrograde regulation of multidrug resistance in Saccharomyces cerevisiae. Gene 354:15–21
Myung K, Chen C, Kolodner RD (2001) Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature 411(6841):1073–1076
Peng Y, Dong D, Jiang C, Yu B, Wang X, Ji Y (2012) Relationship between respiration deficiency and azole resistance in clinical Candida glabrata. FEMS Yeast Res 12(6):719–727. https://doi.org/10.1111/j.1567-1364.2012.00821.x (Epub 2012 Jul 11)
Prevost CT, Peris N, Seger C, Pedeville DR, Wershing K, Sia EA, Sia RAL (2018) The influence of mitochondrial dynamics on mitochondrial genome stability. Curr Genet 64(1):199–214. https://doi.org/10.1007/s00294-017-0717-4 (Epub 2017 Jun 1)
Quezada H, Marin-Hernandez A, Arreguin-Espinosa R, Rumjanek FD, Moreno-Sanchez R, Saavedra E (2013) The 2-oxoglutarate supply exerts significant control on the lysine synthesis flux in Saccharomyces cerevisiae. FEBS J 280(22):5737–5749
Rodrigues F, Ludovico P, Leão C (2006) Sugar metabolism in yeasts: an overview of aerobic and anaerobic glucose catabolism. Biodiversity and ecophysiology of yeasts. Springer, Berlin, pp 101–121
Roon RJ, Even HL, Larimore F (1974) Glutamate synthase: properties of the reduced nicotinamide adenine dinucleotide-dependent enzyme from Saccharomyces cerevisiae. J Bacteriol 118(1):89–95
Rosenthal GA (1977) The biological effects and mode of action of L-canavanine, a structural analogue of l-arginine. Q Rev Biol 52(2):155–178
Scalliet G, Bowler J, Luksch T, Kirchhofer-Allan L, Steinhauer D, Ward K, Niklaus M, Verras A, Csukai M, Daina A, Fonné-Pfister R (2012) Mutagenesis and functional studies with succinate dehydrogenase inhibitors in the wheat pathogen Mycosphaerella graminicola. PLoS One 7(4):e35429
Sesaki H, Southard SM, Yaffe MP, Jensen RE (2003) Mgm1p, a dynamin-related GTPase, is essential for fusion of the mitochondrial outer membrane. Mol Biol Cell 14(6):2342–2356 (Epub 2003 Feb 6)
Shi Y, Stefan CJ, Rue SM, Teis D, Emr SD (2011) Two novel WD40 domain-containing proteins, Ere1 and Ere2, function in the retromer-mediated endosomal recycling pathway. Mol Biol Cell 22(21):4093–4107
Shor E, Fox CA, Broach JR (2013) The yeast environmental stress response regulates mutagenesis induced by proteotoxic stress. PLoS Genet 9:e1003680. https://doi.org/10.1371/journal.pgen.1003680
Simpson-Lavy K, Xu T, Johnston M, Kupiec M (2017) The Std1 activator of the Snf1/AMPK kinase controls glucose response in yeast by a regulated protein aggregation. Mol Cell 68:1120–1133. https://doi.org/10.1016/j.molcel.2017.11.016
Smith EH, Janknecht R, Maher LJ III (2007) Succinate inhibition of α-ketoglutarate-dependent enzymes in a yeast model of paraganglioma. Hum Mol Genet 16(24):3136–3148
Soontorngun N (2017) Reprogramming of nonfermentative metabolism by stress-responsive transcription factors in the yeast Saccharomyces cerevisiae. Curr Genet 63(1):1–7. https://doi.org/10.1007/s00294-016-0609-z (Epub 2016 May 14)
Storici F, Resnick MA (2006) The delitto perfetto approach to in vivo site-directed mutagenesis and chromosome rearrangements with synthetic oligonucleotides in yeast. Methods Enzymol 409:329–345
Torelli NQ, Ferreira-Júnior JR, Kowaltowski AJ, da Cunha FM (2015) RTG1- and RTG2-dependent retrograde signaling controls mitochondrial activity and stress resistance in Saccharomyces cerevisiae. Free Radic Biol Med 81:30–37
Traven A, Wong JM, Xu D, Sopta M, Ingles CJ (2001) Interorganellar communication Altered nuclear gene expression profiles in a yeast mitochondrial DNA mutant. J Biol Chem 276(6):4020–4027
Vlahakis A, Lopez Muniozguren N, Powers T (2017) Mitochondrial respiration links TOR complex 2 signaling to calcium regulation and autophagy. Autophagy 13(7):1256–1257. https://doi.org/10.1080/15548627.2017.1299314 (Epub 2017 Mar 21)
Williamson DH, Maroudas NG, Wilkie D (1971) Induction of the cytoplasmic petite mutation in Saccharomyces cerevisiae by the antibacterial antibiotics erythromycin and chloramphenicol. Mol Gen Genet 111(3):209–223
Wodicka L, Dong H, Mittmann M, Ho M-H, Lockhart DJ (1997) Genome-wide expression monitoring in Saccharomyces cerevisiae. Nat Biotechnol 15(13):1359–1367. https://doi.org/10.1038/nbt1297-1359
Woelders H (1989) Mitochondrial oxidative phosphorylation: studies on the chemiosmotic coupling between respiration and ATP synthesis. Dissertation, University of Amsterdam
Zelenaya-Troitskaya O, Perlman PS, Butow RA (1995) An enzyme in yeast mitochondria that catalyzes a step in branched-chain amino acid biosynthesis also functions in mitochondrial DNA stability. EMBO J 14(13):3268–3276
Zhang N, Cao L (2017) Starvation signals in yeast are integrated to coordinate metabolic reprogramming and stress response to ensure longevity. Curr Genet 63(5):839–843. https://doi.org/10.1007/s00294-017-0697-4 (Epub 2017 Apr 25)
Zhang H, Singh KK (2014) Global genetic determinants of mitochondrial DNA copy number. PLoS One 9(8):e105242
Acknowledgements
We thank the Schuldiner lab for technical support and most valuable input. This work was supported by RCDA Grant of ICRF to SC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Arginine biosynthesis pathway.
Original representation based on arginine biosynthesis pathway from SGD database (yeastgenome.org). Red denotes location in mitochondria; blue denotes location in cytosol (TIFF 1540 kb)
294_2019_974_MOESM2_ESM.tiff
can1Δis sensitive to growth on a non-fermentable carbon source with canavanine. A can1Δ mutant was pronged onto glucose (-) arginine with 20 μg/ml canavanine and lactic acid (-) arginine with 20 μg/ml canavanine. Growth after one week (TIFF 104 kb)
Arginine addition somewhat rescues petites.
Strains were grown on 2 µg/ml canavanine glucose (-) arginine and glucose with increasing amounts of arginine. Growth after 10 days (TIFF 739 kb)
Rights and permissions
About this article
Cite this article
Druseikis, M., Ben-Ari, J. & Covo, S. The Goldilocks effect of respiration on canavanine tolerance in Saccharomyces cerevisiae. Curr Genet 65, 1199–1215 (2019). https://doi.org/10.1007/s00294-019-00974-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-019-00974-y