Abstract
Protein–polysaccharide Maillard conjugate has recently got much attention for improving protein functionality. In this study, soy protein isolate (SPI) was conjugated to maltodextrin (MD) at the protein-to-polysaccharide ratio of 1:2, 1:1 and 2:1 (w/w) via Maillard reaction by dry heating (60 °C, 0–9 days) or wet heating (90 °C, 0–120 min). The influences on the degree of graft (DG), functionalities and structure of conjugates were evaluated. The mass ratio of reactants and reaction time greatly impacted the DG of conjugates. The conjugates with protein-to-polysaccharide ratio of 1:2 (w/w) prepared via dry- and wet-heating methods exhibited the highest DG. The DG of dry heat-induced conjugates raised significantly as incubation for 3 days and prolonged heating has no effect of DG, whereas the DG of wet-heat conjugates increased as extended heating period. The emulsifying properties were sharply improved in the conjugates by dry heating as prolonging the incubation time. Protein solubility and foaming properties of the conjugates by dry heating incubated for a day were enhanced. Extending the reaction period negatively impacted those functionalities. On the other hand, wet heating profoundly enhanced the protein solubility and foaming properties of the conjugates. The structural characteristics by Fourier transform infrared spectrum (FTIR) and circular dichroism (CD) confirmed the conformational changes in the secondary structure of the conjugates prepared by dry- and wet-heating methods. The appropriate Maillard reaction conditions could formulate the conjugate with better functionalities that will be useful for food applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant-derived proteins are recently obtained much attention in food applications due to their low cost, sustainability and high nutritious. Soy protein isolate (SPI) consists of more than 90% protein which is extracted from defatted soybean meal through a conventional alkaline solubilization-acid precipitation. Upon sedimentation coefficient after centrifugation, SPI consists of two major globular protein subunits, conglycinin (7S) and glycinin (11S). Due to its amphiphilic nature, SPI acts as a good emulsifier at oil–water interface and is extensive utilized in food industry [1]. Nonetheless, the functional properties of native SPI have been well recognized, and its application is still not sufficient to meet the various needs in food industry owing to its structural limitations and variability. Thus, the appropriate modification methods were required to improve functionality of protein.
Glycosylation is a chemical modification by a Maillard reaction involving the amino-carbonyl interactions. Since Maillard reaction is a spontaneous and taken place by heating without addition of any chemical reagent, it is an ideal method that can potentially improve protein functionalities, such as solubility, emulsifying and foaming properties for food purpose [2, 3]. The Maillard reactivity and the properties of products by conjugates are greatly influenced by the types of polysaccharides. Maltodextrin (MD), a hydrolysis product of starch, consists of D-glucose as a monomer which links together by α-(1,4)-glycosidic bonds. MD is commonly utilized in food emulsion as a stabilizer and possesses excellent water solubility and oxidative stability [4]. The structural characteristics of protein and polysaccharide greatly impact the Maillard reactivity and the functional properties of conjugates [5]. Additionally, glycation method is another vigorous parameter influencing the rate and degree of glycation, as well as functionalities of conjugates [6].
The conjugation via Maillard reaction is known to be induced by dry or wet heating. Conjugation of protein with carbohydrate is usually conducted through dry heating; however, the reaction generally takes several days or weeks. Maillard conjugate via wet heating shortens the reaction time and more flexible for industrial applications [7]. Maillard reaction leads to structural modification of proteins, and the changes of structure varies in dry and wet heating conditions [8]. Xue et al. [9] investigated the functional properties and structural characterization of the SPI-MD and SPI-gum Arabic conjugates via dry-heated Maillard reaction at 60 °C, 79% relative humidity for three and seven days, respectively. Boostani et al. reported that the best Maillard reaction condition based on grafting degree to formulate the SPI-dextran conjugate is operated through dry heating at pH 8.5, 60 °C for eight days and exhibited the enhancement of techno-functional properties of SPI [10]. Zhuo et al. carried out that the optimum condition to prepare the SPI-dextran conjugates is at pH 6.5, 60 °C for 30 h under macromolecular crowding conditions and reported that Maillard-induced structural flexibility further contributes to better functional characteristics [11]. Very limited reports have so far been published and revealed on the influence of Maillard reaction conditions of the SPI-MD conjugates prepared via dry- and wet-heating method on their reactivity and functionalities.
The present work thus aimed (1) to study the influence of Maillard reaction conditions (glycation method, reaction time and mass ratio of reactants) on the Maillard reactivity and techno-functionalities (protein solubility, emulsifying properties, foam properties and water and oil holding capacity) of the SPI-MD conjugates and (2) to evaluate the conformational changes of the conjugate between SPI and MD prepared via dry- and wet-heating method.
Materials and methods
Materials and reagents
SPI (protein content 91%, w/w) was purchased from Club Protein Co. Ltd., Thailand. MD with the dextrose equivalent 10–12 was purchased from CTi & Science Co., Ltd. (Bangkok, Thailand). Corn oil was purchased from local supermarket. O-phthaldialdehyde, sodium tetraborate, sodium dodecyl sulfate, β-mercaptoethanol, bovine serum albumin, acrylamide/bis-acrylamide solution and glacial acetic acid were purchased from Sigma-Aldrich (St Louis, MO, USA). All other reagents used were of analytical grade.
Preparation of SPI-MD conjugates
For dry heat treatment, SPI and MD were mixed at three different weight ratios (1:2, 1:1 and 2:1, w/w). The mixtures were dispersed in distilled water to obtain an aqueous suspension of 10% (w/w total solid) and stirred with magnetic stirrer at 500 rpm (Stuart, model UC152D, Staffordshire, UK) for 4 h at 25 °C. The mixtures were further hydrated overnight at 4 °C, followed by lyophilized at − 55 °C for 24 h in a freeze-dryer (Scanvac Coolsafe 55-4, Labogene, Denmark). The lyophilized mixtures were further ground to produce fine powder using a grinder (Le caisson blender, model SX-886, China) and sieved through an 80-mesh sieve (Tyler, Ohio, USA). The dried mixtures were further incubated in the controlled humidity desiccator (75% relative humidity) at different interval periods (0, 1, 3, 5, 7 and 9 days).
For wet heat treatment, the three different ratios of SPI to MD (1:2, 1:1 and 2:1, w/w) were developed following the method described by Nasrin and Anal with some modifications [12]. The mixture of SPI and MD was suspended in warm water (60 °C) to obtain aqueous suspension of 10% (w/w). The pH was adjusted to 7.5. The mixtures were further heated at 90 °C at different interval periods (0, 30, 60, 90 and 120 min) with continuous stirring at 500 rpm using a hotplate stirrer (Stuart, model UC152D, Staffordshire, UK). The mixtures were cooled in an ice-bath and lyophilized at − 55 °C for 24 h in a freeze-dryer. Fine powder was obtained after sieving through an 80-mesh sieve.
The conjugated samples were further dissolved in 10 mM phosphate buffer solution (pH 7) to obtain a concentration of 1 mg/mL to determine the Maillard reaction products. The absorbance of the solutions was measured at 294 and 420 nm wavelength as indicators of intermediates and final-stage products of the reaction, respectively, using a UV–visible spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) [13].
Degree of graft (DG)
The o-phthaldialdehyde (OPA) assay was used to evaluate the number of MD molecules attached to SPI by measuring a reduction of amine group as degree of grafting (DG) following the method described by Wen et al. [14]. The OPA reagent was prepared freshly by mixing 40 mg OPA (dissolved in 1 mL of methanol), 25 mL of 0.1 M sodium tetraborate (pH 9.85), 2.5 mL of sodium dodecyl sulfate (20%, w/v) and 0.1 mL of β-mercaptoethanol. The OPA reagent (4 mL) was added to 0.2 mL of SPI-MD conjugate solution (2 mg/mL) and incubated at 35 °C for 2 min. The absorbance at 340 nm was then measured using a UV–visible spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). The calibration curve was obtained from 0.2 to 2 mM L-leucine. DG was calculated on the basis of following Eq. (1);
where A0 represents the free amino group content of unheated SPI and MD mixture and At represents the free amino group content of SPI-MD conjugates.
Functional properties of SPI-MD conjugates
Protein Solubility
Protein solubility of conjugates was determined following the method described by Saatchi et al. with slight modifications [2]. The conjugated samples (1 mg/mL) were dissolved in 10 mM phosphate buffer solution (pH 7) and centrifuged (CN-2060, Hsiang-Tai, Taipei, Taiwan) at 1456 g for 10 min at 25 °C. The supernatants were collected and determined by Bradford assay using BSA as the standard. The absorbance was recorded at 595 nm by a UV–visible spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). The protein solubility was calculated as a percentage of soluble proteins to the total protein content.
Emulsifying properties of the conjugates
Emulsion stability index (ESI) and emulsifying activity index (EAI) were analyzed by the turbidimetric method as described by Ma et al. [15] with some modifications. For emulsion formation, 90 mL of SPI-MD conjugates (2 mg/ mL) in 10 mM phosphate buffer solution pH 7 and 10 ml of corn oil were pre-homogenized using a high-speed blender (HR-2118, Philips, Indonesia) for 1 min and then homogenized by a high-pressure homogenizer (IKA Labor-pilot, 2000/4, Staufen, Germany) for 10 min. After homogenization for 10 min, 50 µL of the emulsion was immediately taken from the bottom of the beaker and diluted as 1:100 with 0.1% (w/v) SDS solution. The absorbance of diluted emulsion was recorded at 500 nm by UV–Vis Spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). ESI and EAI were calculated by using Eqs. (2) and (3), respectively:
where A0 and A10 are the absorbance of the emulsion at 0 and 10 min, respectively. Dilution factor is 100, C is the protein concentration (g/ mL), \(\varphi \) is the oil volume fraction (v/ v) of the emulsion, and \(\theta \) is the optical path (1 cm).
Foaming properties
The foaming properties of SPI-MD conjugates were evaluated following the method of Jain and Anal with slightly modifications [16]. SPI-MD conjugate solution (2 mg/mL) was placed in a beaker, and the volume of the sample was recorded as A1. The solution was further homogenized by using a homogenizer (Servodyne Model 50000-25, Cole-Parmer Instrument Co., Vernon Hills, IL, USA) at 900 rpm for 5 min, and the sample volume was recorded as A2. After 30 min, the sample volume was recorded as A3. The calculation formula of the foaming capacity and foaming stability of the sample was represented in Eqs. (4) and (5).
Water and oil holding capacity
Water holding capacity (WHC) and oil holding capacity (OHC) were analyzed following the method of Jain and Anal with slight modifications [16]. For WHC test, conjugated samples were accurately weighted (W0, approximately 0.01 g) and thoroughly mixed with 1 mL of distilled water in an Eppendorf (W2), followed by centrifugation (Centurion K2R series, Chichester, UK) at 726 g for 30 min. The supernatant was discarded and the Eppendorf was kept up-side-down for 5 min. The Eppendorf containing the sample was then weighted (W1). The WHC was calculated using Eq. (6). For the OHC, the procedure was done similar to WHC but using corn oil instead of distilled water. The OHC was calculated using Eq. (7). The WHC and OHC were carried out as g water/g sample and g oil/g sample, respectively.
Characterization of SPI-MD conjugates
Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR) was performed using a Thermo Scientific Nicolet iS50 FT-IR (Fisher Scientific, Chino, CA, USA). Prior to the measurement, a background of air absorbance spectrum was recorded as a blank. The fine powder samples were measured in the wavenumber range of 400–4000 cm−1 at resolution of 4 cm−1 with 32 scans. The data were collected using OMNIC 9 software (Thermo Fisher Scientific, Madison, WI, USA).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis
SDS-PAGE was operated on a Mini-PROTEAN Tetra cell system (Bio-rad, Hercules, USA) using 12.5% (w/v) acrylamide as separating gel and 4% (w/v) acrylamide as stacking gel. Samples (5 mg/mL in protein) were mixed with loading buffer in a ratio of 1:3 of buffer to sample. The resulted samples were further heated at 95 °C for 5 min. Each sample (20 µL) was loaded onto the gel and set voltage at 150 V. Gel was further stained with Coomassie brilliant blue R-250 and de-stained by the solution consisting of ethanol and glacial acetic acid.
Circular dichroism (CD) spectroscopy
The secondary structure of SPI-MD conjugates was investigated by a CD spectrometer (ORDM-401, Jasco, Japan). The samples (1 mg/ mL) were prepared in 10 mM phosphate buffer solution (pH 7). The CD spectra were observed at the wavelengths of 190–250 nm with scanning rate of 100 nm/min under nitrogen purge. The secondary structures of samples were analyzed using the DICHROWEB software.
X-ray diffraction (XRD)
The X-ray diffractograms were obtained on the X-ray Diffractometer model 10190376 (Bruker, Siemens, Germany) equipped with a Cu tube (λ = 1.5418 Å) to determine the crystalline structure present in SPI-MD conjugates. The XRD patterns were recorded over a diffraction angle (2θ) range from 5 to 60°, with a step size of 0.05° and a scan speed of 1° min−1. The diffractometer was operated at 40 kV and 40 mA. The crystallinity index of conjugate samples was determined following the method described by Pirestani et al. [17] using Origin software. The crystallinity index refers to the ratio of the crystalline peak to the total area of peak as represented in Eq. (8).
Statistical analysis
Total experimental design was performed by a completely randomized design with two factors of reaction time and protein-to-polysaccharide ratio. The statistical analysis was interpreted using SPSS software (SPSS, 25.0). Tukey’s honest significance test was used to determine the significant differences (p < 0.05) among the means.
Results and discussion
Effects of reaction conditions on SPI-MD Maillard conjugates via dry and wet heating
Measurement of Maillard reaction progress
Maillard reaction products were monitored at the UV absorbances of 294 and 420 nm, which correspond to the development of the intermediate Amadori compounds in an early stage and melanoidins in the final stage of Maillard reaction, respectively [18]. A continue increase in the absorbance at 294 nm during the first 3 days of the reaction, followed by nearly constant and a decline at 9 days of dry reaction, was observed (Fig. 1A). This indicates the formation of Amadori compound occurred within 3 days. A constant in absorbance at 294 nm after 3 days of the dry reaction is due to irreversible degradation and polymerization of the intermediate Amadori compounds to form further high molecular weight melanoidins [19]. Likewise, there was a gradual increase in absorbance value at 420 nm of the conjugates up to 7 days and a slight decline at 9 days of incubation (Fig. 1A). This happens due to the formation of high molecular weight melanoidins over the reaction time.
For wet heat method, there was a sharp increase (p < 0.05) in absorbance at 294 nm after heating the SPI-MD conjugates for 30–60 min followed by constant as the heating time extended up to 120 min (Fig. 1B). This indicates that the formation of intermediate substances occurred during the first 30 min of wet heating. Pirestani et al. reported that the intermediate products formed within 15 min of heating for the canola protein-gum Arabic conjugates [3]. A gradually increase in absorbance at 420 nm was also observed during wet heat treatment (Fig. 1B). Moreover, it was observed that the SPI:MD ratio of 1:2 exhibited a higher absorbance at 294 and 420 nm than the others. This could be possibly because the SPI:MD ratio of 1:2 contains a higher level of carbonyl groups from MD, thus promoting the formation of intermediate compounds. Nooshkam and Varidi also exhibited a significant increment (p < 0.05) in the absorbance at 294 nm of the whey protein isolate-low acyl gellan gum conjugates as increasing gellan gum concentration [20]. Similarly, Benjakul et al. [21] showed that the browning intensity (absorbance at 420 nm) of porcine plasma protein–sugar conjugates raised up with the increased sugar concentration.
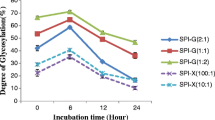
Degree of graft
The glycation of SPI and MD through Maillard reaction occurs between free amino groups of protein and carbonyl groups of polysaccharide molecule. A reduction of free amino group content in SPI is attributed to the level of grafting reaction. The DG of SPI-MD conjugates by dry heating was significantly increased (p < 0.05) during first 3 days of reaction and was constant for further reaction (Fig. 2A). The intermolecular reaction between protein–protein takes place with the prolonged incubation period, resulting in protein aggregation. These aggregated proteins possibly compete with the conjugation of polysaccharide later [22].
For wet heating, a dramatic increased (p < 0.05) in the DG of the SPI-MD conjugates with increasing the reaction time was observed (Fig. 2B). A continue rise in the DG upon the heating time is likely due to heat that unfolds the protein chain, resulting in more availability of the ε-amino groups on SPI chain that can graft with MD molecules. Moreover, it was observed that at the same reaction time the DG increased with the MD content in the mixture. The DG of the conjugates at the SPI:MD ratio of 1:2 was profoundly higher than those of the 1:1 and 2:1 conjugates for both dry and wet treatments (Fig. 2A and B). In general, the development of Maillard reaction is promoted as an availability of excess carbonyl groups in the molar ratio which facilitates the conjugation with amino groups. The result was well consistent to the study of Mao et al. which demonstrated the higher DG was obtained for the conjugate of SPI-carrageenan at 1:3 (62%) in comparison with the conjugate of 3:1 (23%) [23].
Functional properties of SPI-MD conjugates
Protein solubility
Maillard conjugation induced by dry or wet heat treatments exhibited protein solubility differently. Protein solubility significantly increased for the dry heat-induced conjugates incubated 1 day, followed by a gradually decreased as extended the reaction time (Fig. 3A). This result was similar to the previous study reported by de Oliveira et al. [23]. The overreaction of conjugation occurred during thermal process with the extended incubation period resulted in reduce solubility [24]. The accumulation of insoluble melanoidins and other advanced glycation end products (AGEs), generally generated by further protein cross-linking and intermediate degradation at the later stage of Maillard reaction, was observed [25]. Likewise, the increased accumulation of final stage products (melanoidins) was observed with the extended incubation periods as indicated from the UV absorbance readings at 420 nm (Fig. 1A). Moreover, the reduction in conjugate solubility is explained by the structural changes and the enhancement of surface hydrophobicity of protein. This leads to the increment of hydrophobic–hydrophobic interaction and facilitated the aggregation of protein [20].
In contrast, there was increment in protein solubility of all conjugates via wet heating method (Fig. 3B). The solubility increased from 7–10% to 19–40% after 120 min of heating. Qu et al. [26] reported the improvement in solubility of the rapeseed protein isolate-dextran conjugates by wet heating. The grafting treatment leads to an increased number of hydrophilic groups (–OH) on the conjugated protein, resulting in a decreased surface hydrophobicity, thus likely enhancing solubility. Du et al. [27] confirmed that the conjugation of protein with hydrophilic polysaccharides during the early stage of the Maillard reaction promoted protein hydration. The improvement in protein solubility by wet heating at 90 °C is possibly due to the unfolding of proteins, breaking peptide bonds and exposing hydrophilic amino acid residues [28]. As a consequent, Maillard conjugation improves the protein solubility by controlling the Maillard reaction stage.
Emulsifying properties
Emulsifying properties are usually determined by two indices known as emulsion stability index (ESI) and emulsifying activity index (EAI). The ESI and EAI of the SPI-MD conjugates were significantly (p < 0.05) enhanced along with the progression of dry heating reaction up to 7 days and a slightly decline at 9 days of reaction (Fig. 4A and B). A similar result has been reported by Chen et al. which the EAI and ESI of the whey protein isolate-gum acacia conjugates were gradually increased as extending the dry heating period up to 5 days followed by slightly decline for further reaction [22]. A significant improvement in the emulsifying properties after covalent bonding is ascribed to the formation of macromolecule as a result of protein–polysaccharide conjugation, which is large enough to cover and provide steric stabilization at the surface of emulsion droplets [22]. On the other hand, loss of the emulsifying index after certain period of the reaction can be attributed to the formation of the polymerization products which reduce the molecule mobility, slowing the absorption of the conjugates at oil–water interface [29]. Likewise, the improvement in emulsifying properties of conjugates is highly correlated with the Maillard-induced structural conformation. This facilitated protein to be exposed to the hydrophobic groups, increasing surface hydrophobicity and enhancing absorption rate of conjugate to the oil–water interface [30].
A similar trend of the improvement in the emulsifying index of the SPI-MD conjugates prepared via wet heating method was observed. The ESI and EAI were enhanced for a certain heating period (30–60 min) and slightly decreased as extending the reaction (Fig. 4C and D). This result was in accordance with Li et al. in which the emulsion stability was gradually enhanced during the first 5 h of wet heating reaction and significantly decreased as prolonging the reaction period [1]. The better emulsion stability after wet heat-induced Maillard conjugate is due to the higher flexibility of protein molecules [26]. However, longer reaction causes proteins to reaggregate, thus deceasing emulsifying properties [1]. There was no significant improvement of the EAI of the SPI-MD conjugates by wet heating at the SPI:MD ratio of 1:1 and 2:1 (Fig. 4D). Ma et al. revealed that there were no significant differences between the EAI and ESI of the SPI-pectin conjugates prepared by wet heating in comparison with the mixture of unheated sample due to their low DG [15].
The SPI-MD conjugates with exhibited highest ESI from each dry and wet heating condition were selected for further characterization as reported in Sect. 3.3.
Foaming properties
The FC and FS of the SPI-MD conjugates by dry heating were obviously improved at 1 day of reaction and gradually dropped as further extending the incubation period (Fig. 5A and B), whereas the FC and FS of the SPI-MD conjugates via wet heating were noticeably (p < 0.05) raised during heating for 30–60 min and constant as expending the reaction period (Fig. 5C and D). The increment of foam properties of the SPI-MD conjugates is probably related to solubility. Wen et al. [14] also reported that the higher FC and FS is likely due to more solubility of the SPI-lentinan conjugates and the conformational changes by increasing random structure of proteins. Likewise, these results were well according to protein solubility of the SPI-MD conjugates as illustrated in Fig. 3A and B. As compared heating method, the greater FC and FS in wet heat-induced Maillard conjugates are due to their better solubility which facilitate their rapid absorption during whipping process to form more foam as compared to the conjugates with higher molecular weight and low solubility as represented in dry heating [31].
Water and oil holding capacity
Water and oil holding capacity (WHC and OHC) indicate the amount of water or oil retention of the protein. There was a gradual decrease in the WHC of the conjugates after dry heating as prolonging the reaction period (Fig. 6A). A reduction in the WHC is associated with an increase in surface hydrophobicity of the conjugates [32]. On the other hand, a dramatic improvement in the WHC was observed for wet heat-induced Maillard conjugate with the SPI:MD ratio of 1:1 and 2:1. An improvement of WHC by wet heating is probably due to a reduction in hydrophobicity of conjugates (Fig. 6C). A decrease in surface hydrophobicity of protein–polysaccharide Maillard conjugates by wet heat treatment was also reported by Hu et al. [33]. However, there is no significant improvement in the WHC for the conjugate with SPI:MD ratio of 1:2 after wet heating.
Conjugation via dry heating did not exhibit a noticeably increase in the OHC. However, the maximum OHC exhibited with the SPI:MD ratio of 1:1 at the 7 days of reaction (Fig. 6B and D). It is likely that modified protein structure occurred during heat-induced Maillard reaction which facilitated the exposure of hydrophobic amino acid group of protein [31]. This result was in consistent to the improvement of emulsifying properties by dry heating Maillard conjugate in which the values of ESI and EAI were relatively high at 7 days of incubation (Fig. 4A and B). Similarly, there is no significant change in the OHC of the SPI-MD conjugates by wet heating (Fig. 6D) as compared to the unheated SPI-MD mixture. This could be attributed that wet heating did not profoundly enhance surface hydrophobicity of the conjugates [26].
Characterization of SPI-MD conjugates
FTIR
According to the infrared absorption of protein, SPI exhibited main characteristic peaks at 1631 cm−1 (Amide I, C=O stretching vibration of peptide bonds), 1516 cm−1 (Amide II, N–H bending vibration) and 1391–1234 cm−1 (Amide III, C–N stretching and N–H bending vibration). The absorption peak in the range of 1180–953 cm−1 is belonging to the saccharide band of sugar which associates with the vibration of C–H bending and C–C/C=O stretching bonds [34]. The absorption peak at 1016–1021 cm−1 of both conjugate samples was stronger than those of native SPI and the mixture of SPI-MD (Fig. 7), referring newly formed C–N covalent bond generated in the conjugates [6]. Indeed, the intensity of this peak was higher for the SPI-MD conjugate by dry heating for 7 days in comparison with the conjugate by wet heating for 60 min. This result was in agreement with the studies of Nooshkam and Varidi which reported that the greater intensity of saccharide band is appeared in the Maillard conjugates with higher glycation degree [20]. The result was well consistent to the DG of the SPI-MD conjugates by dry and wet heating that was 58% and 16%, respectively, as represented in Fig. 2A and B.
A profound increase in hydroxyl group was observed in the SPI-MD conjugates especially by wet treatment. Upon Maillard conjugation, the band in the 3700–3200 cm−1 caused by –OH stretching vibration was more intensive than those of native SPI and the mixture of SPI-MD, indicating a –OH bending vibration in the conjugates which is likely confer to the combination of SPI and MD. Geng et al. also reported that the enhancement of peak intensity at the region of 3600–3000 cm−1 and 1260–1000 cm−1 indicated the formation of covalent bond between protein and polysaccharide [35].
Maillard reaction occurs between amino and carboxyl groups, leading to the loss of –NH2 characteristic band, whereas the new characteristic bands can appear contributing to the Maillard reaction products, such as Amadori compound (C=O) and Schiff base products (C=N). According to the result, the absorption peaks belonging to the amide regions (1631, 1516 and 1391–1234 cm−1) were decreased and disappeared from the spectra of the conjugates prepared via wet and dry heating, respectively. After conjugation, the less intense of these peaks in the amide II and III region was generally happened since amino groups were consumed during Maillard reaction [19]. Particularly, the absence of the peaks in this region of the dry heat-induced conjugate was observed which could be deduced that more amino groups were participated for Maillard reaction. This result was well agreement with the DG reported earlier.
Indeed, the new appearing band at 1642 cm−1 may be ascribed to C=N stretching vibration of the Schiff base products [10]
SDS-PAGE
Figure 8 illustrates the effect of heating method on SDS-PAGE profile of SPI-MD conjugates. Native SPI showed the characteristic bands mainly including glycinin (11S) and β-conglycinin (7S). The 11S fractions consist of A3, acidic and basic subunits represented the bands at 45, 35 and 20 kDa, respectively, whereas the 7S fractions consist of α, α´ and β subunits at 72, 68 and, 52 kDa, respectively. After conjugation by dry and wet heating (lane 3 and 4), the intensity of the bands identified in SPI declined. This signified the participation and interaction of protein and polysaccharide upon Maillard reaction. Another reason could be due to the transformation of the protein molecules occurred in the further stage of the reaction [36]. The finding was well consistent to the report by Hu et al. [37] which a reduction of blue stained bands was observed in the conjugation between SPI and dextran via Maillard reaction in the molecular crowding environment. As compared the variation patterns among the conjugated samples, the characteristic bands of 11S and 7S subunits disappeared in the conjugation by dry heating (lane 3). Xu et al. [38] also revealed that there was none of the clear single band in the SPI-glucose conjugates prepared via dry heating for 24 and 48 h. On the other hand, the darker bands still remained for the wet heat-induced conjugate (lane 4). Thus, the subunits of SPI are more susceptible to Maillard reaction during dry heating (60 °C, 7 days) than those of wet heating (90 °C, 60 min).
Circular dichroism
Figure 9 illustrates the changes in CD spectra of native SPI, mixture of SPI-MD and conjugates of SPI-MD by dry/wet heating. There were remarkably shifts of negative band position and changes in peak intensity, which occurred in different manners among samples. This phenomenon indicates a conformational change of secondary structure of proteins. The main secondary structure of SPI and their conjugates was β-sheet and random coil composition. The β-turn structure of all tested samples was not significantly different. The heating method influenced the secondary structure composition of protein (Table 1). A decrease in α-helix and an increase in β-sheet were distinctively shown after the conjugation formed due to dry and wet heating. The study of Boggione Santos et al. revealed a reduction of α-helix structure when α-lactalbumin conjugates with carboxymethylcellulose via dry heating [39]. Feng et al. [40] reported a shifted of negative bands toward the lower wavelength and a significant decreased in peak intensities of CD spectra in ovalbumin-dextran conjugates by dry heating. Indeed, a decrease in α-helix and an increase in β-sheet content were observed. Liu et al. [41] revealed a gradual decrease in α-helix and random coil structure with an increased in β-sheet content after pea protein isolate-dextran conjugates by dry heating. Although there were a decrease in α-helix and an increase in β-sheet content in both conjugates by dry and wet heating, the dry heat conjugate lost more α-helix and gained more β-sheet composition as compared to the conjugate by wet heating. This affected the distinct changes of the conjugates peak by dry and wet heating as represented in Fig. 9.
XRD and crystallinity
The XRD analysis provides the structural property of molecules whether they are crystalline or amorphous in nature. A pointed peak indicates crystalline fraction, while a broad peak refers to amorphous fraction [42]. According to diffractogram (Fig. 10), the broader shape of XRD pattern of MD indicates highly amorphous structure of MD, whereas other samples exhibited a semi-crystalline structure with different degree of crystallinity. Major diffraction peaks of the native SPI are observed in the 2θ region at 12.3 and 19.4°. The previous study also demonstrated that SPI represented diffraction peaks at 9.1 and 19.4° in the 2θ region, belonging to the α-helix and β-sheet structure of SPI, respectively [43]. A presence of MD in SPI-MD mixture and conjugates weakens the intensity of main diffraction peaks when compared to peaks obtained from the native SPI (Fig. 10). The similar XRD profiles were found for SPI-MD mixture and the conjugate by dry method. On the other hand, there was distinctly decrease in the diffraction peak in a range of 10–15° for wet heat-induced conjugates as compared to the diffraction peak in the same region of the mixture of SPI-MD and dry heat-induced conjugate. This finding was consistent to the crystallinity index (CI) of the wet heat-induced conjugate (18.58%) whereas the CI values of the native SPI, MD, mixture SPI-MD, the dry heat-induced conjugate were 28.48, 17.51, 26.60, and 28.15%, respectively. There was an obvious decline in the CI value of the conjugate by wet heating as compared to other samples. The CI of conjugate by dry heating was not significantly changed compared to that in the native SPI and SPI-MD mixture; however, the crystallinity index of the conjugates by wet heating was lower than the former group. As a consequent, Maillard conjugate by wet method could better reduce the crystallinity of the conjugates. The similar findings have been reported by the studies of Pirestani et al. [17] and Dong and Cui [43].
Conclusions
SPI-MD conjugates were successfully developed via Maillard reaction induced by dry and wet heating. Glycation method, heating period and mass ratio of reactants greatly impacted the degree of glycation, functionalities and structural modification of the conjugates. The DG of dry heat-induced conjugates continuously raised for a certain time of heating (during 3 days), whereas an increase in DG with extended heating duration (up to 120 min) was found in the wet heat-induced conjugates. The conjugates with higher MD ratio prepared by both heating methods exhibited greater DG. The emulsifying properties of conjugates were highly improved as prolonged the heating time during dry heating. Nevertheless, extending the reaction period during dry heating did not show significant effect on protein solubility and foaming properties. In contrary, the conjugation via wet-heating profoundly improved the emulsifying properties, protein solubility and foaming properties. An improvement in functional properties of conjugates could be attributed to the structural modification occurred during Maillard reaction in which the results of conformational change were confirmed by FTIR and CD. The XRD profile showed that the conjugates prepared by dry and wet heating possess a semi-crystalline structure. The conjugation via wet-heating method could better reduce the crystallinity.
References
Li R, Cui Q, Wang G et al (2019) Relationship between surface functional properties and flexibility of soy protein isolate-glucose conjugates. Food Hydrocoll 95:349–357. https://doi.org/10.1016/j.foodhyd.2019.04.030
Saatchi A, Kiani H, Labbafi M (2019) A new functional protein-polysaccharide conjugate based on protein concentrate from sesame processing by-products: functional and physico-chemical properties. Int J Biol Macromol 122:659–666. https://doi.org/10.1016/j.ijbiomac.2018.10.122
Pirestani S, Nasirpour A, Keramat J, Desobry S (2017) Preparation of chemically modified canola protein isolate with gum Arabic by means of Maillard reaction under wet-heating conditions. Carbohydr Polym 155:201–207. https://doi.org/10.1016/j.carbpol.2016.08.054
Du Q, Tang J, Xu M et al (2021) Whey protein and maltodextrin-stabilized oil-in-water emulsions: effects of dextrose equivalent. Food Chem 339:128094. https://doi.org/10.1016/j.foodchem.2020.128094
Kan X, Chen G, Zhou W, Zeng X (2021) Application of protein-polysaccharide Maillard conjugates as emulsifiers: source, preparation and functional properties. Food Res Int 150:110740. https://doi.org/10.1016/j.foodres.2021.110740
Chen W, Wang W, Guo M et al (2022) Whey protein isolate-gum Acacia Maillard conjugates as emulsifiers for nutraceutical emulsions: impact of glycation methods on physicochemical stability and in vitro bioaccessibility of β-carotene emulsions. Food Chem 375:131706. https://doi.org/10.1016/j.foodchem.2021.131706
Qu W, Zhang X, Chen W et al (2018) Effects of ultrasonic and graft treatments on grafting degree, structure, functionality, and digestibility of rapeseed protein isolate-dextran conjugates. Ultrason Sonochemistry 42:250–259. https://doi.org/10.1016/j.ultsonch.2017.11.021
Garcia-Amezquita LE, Martinez-Alvarenga MS, Olivas GI et al (2014) Effect of Maillard reaction conditions on the degree of glycation and functional properties of whey protein isolate-Maltodextrin conjugates. Food Hydrocoll 38:110–118. https://doi.org/10.1016/j.foodhyd.2013.11.006
Xue F, Li C, Zhu X et al (2013) Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. FRIN 51:490–495. https://doi.org/10.1016/j.foodres.2013.01.012
Boostani S, Aminlari M, Moosavi-nasab M et al (2017) Fabrication and characterisation of soy protein isolate-grafted dextran biopolymer: a novel ingredient in spray-dried soy beverage formulation. Int J Biol Macromol 102:297–307. https://doi.org/10.1016/j.ijbiomac.2017.04.019
Zhuo X, Qi J, Yin S et al (2012) Formation of soy protein isolate-dextran conjugates by moderate Maillard reaction in macromolecular crowding conditions. J Sci Food Agric. https://doi.org/10.1002/jsfa.5760
Nasrin TAA, Anal AK (2014) Resistant starch III from culled banana and its functional properties in fish oil emulsion. Food Hydrocoll 35:403–409. https://doi.org/10.1016/j.foodhyd.2013.06.019
Kim S, Shin WS (2021) Formation of a novel coating material containing lutein and zeaxanthin via a Maillard reaction between bovine serum albumin and fucoidan. Food Chem 343:128437. https://doi.org/10.1016/j.foodchem.2020.128437
Wen C, Zhang J, Qin W et al (2020) Structure and functional properties of soy protein isolate-lentinan conjugates obtained in Maillard reaction by slit divergent ultrasonic assisted wet heating and the stability of oil-in-water emulsions. Food Chem 331:127374. https://doi.org/10.1016/j.foodchem.2020.127374
Ma X, Hou F, Zhao H et al (2020) Conjugation of soy protein isolate (SPI) with pectin by ultrasound treatment. Food Hydrocoll 108:106056. https://doi.org/10.1016/j.foodhyd.2020.106056
Jain S, Anal AK (2016) Optimization of extraction of functional protein hydrolysates from chicken egg shell membrane (ESM) by ultrasonic assisted extraction (UAE) and enzymatic hydrolysis. LWT Food Sci Technol 69:295–302. https://doi.org/10.1016/j.lwt.2016.01.057
Pirestani S, Nasirpour A, Keramat J et al (2018) Structural properties of canola protein isolate-gum Arabic Maillard conjugate in an aqueous model system. Food Hydrocoll 79:228–234. https://doi.org/10.1016/j.foodhyd.2018.01.001
Nooshkam M, Varidi M (2020) Maillard conjugate-based delivery systems for the encapsulation, protection, and controlled release of nutraceuticals and food bioactive ingredients: a review. Food Hydrocoll 100:105389. https://doi.org/10.1016/j.foodhyd.2019.105389
Zha F, Dong S, Rao J, Chen B (2019) Pea protein isolate-gum Arabic Maillard conjugates improves physical and oxidative stability of oil-in-water emulsions. Food Chem 285:130–138. https://doi.org/10.1016/j.foodchem.2019.01.151
Nooshkam M, Varidi M (2020) Whey protein isolate-low acyl gellan gum Maillard-based conjugates with tailored technological functionality and antioxidant activity. Int Dairy J 109:104783. https://doi.org/10.1016/j.idairyj.2020.104783
Benjakul S, Lertittikul W, Bauer F (2005) Antioxidant activity of Maillard reaction products from a porcine plasma protein-sugar model system. Food Chem 93:189–196. https://doi.org/10.1016/j.foodchem.2004.10.019
Chen W, Lv R, Wang W et al (2019) Time effect on structural and functional properties of whey protein isolate-gum acacia conjugates prepared via Maillard reaction. J Sci Food Agric 99:4801–4807. https://doi.org/10.1002/jsfa.9735
Mao L, Pan Q, Hou Z et al (2018) Development of soy protein isolate-carrageenan conjugates through Maillard reaction for the microencapsulation of Bifidobacterium longum. Food Hydrocoll 84:489–497. https://doi.org/10.1016/j.foodhyd.2018.06.037
Zha F, Dong S, Rao J, Chen B (2019) The structural modification of pea protein concentrate with gum Arabic by controlled Maillard reaction enhances its functional properties and flavor attributes. Food Hydrocoll 92:30–40. https://doi.org/10.1016/j.foodhyd.2019.01.046
Evans M, Ratcliffe I, Williams PA (2013) Emulsion stabilisation using polysaccharide-protein complexes. Curr Opin Colloid Interface Sci 18:272–282. https://doi.org/10.1016/j.cocis.2013.04.004
Qu W, Zhang X, Chen W et al (2018) Effects of ultrasonic and graft treatments on grafting degree, structure, functionality, and digestibility of rapeseed protein isolate-dextran conjugates. Ultrason Sonochem 42:250–259. https://doi.org/10.1016/j.ultsonch.2017.11.021
Du Y, Shi S, Jiang Y et al (2013) Physicochemical properties and emulsion stabilization of rice dreg glutelin conjugated with κ-carrageenan through Maillard reaction. J Sci Food Agric 93:125–133. https://doi.org/10.1002/jsfa.5739
Li C, Huang X, Peng Q et al (2014) Physicochemical properties of peanut protein isolate-glucomannan conjugates prepared by ultrasonic treatment. Ultrason Sonochem 21:1722–1727. https://doi.org/10.1016/j.ultsonch.2014.03.018
Lia C, Wang J, Shi J et al (2015) Encapsulation of tomato oleoresin using soy protein isolate-gum aracia conjugates as emulsifier and coating materials. Food Hydrocoll 45:301–308. https://doi.org/10.1016/j.foodhyd.2014.11.022
Dong X, Du S, Deng Q et al (2020) Study on the antioxidant activity and emulsifying properties of flaxseed gum-whey protein isolate conjugates prepared by Maillard reaction. Int J Biol Macromol 153:1157–1164. https://doi.org/10.1016/j.ijbiomac.2019.10.245
Shen Y, Li Y (2021) Acylation modification and/or guar gum conjugation enhanced functional properties of pea protein isolate. Food Hydrocoll 117:106686. https://doi.org/10.1016/j.foodhyd.2021.106686
Arogundade LA, Eromosele CO, Eromosele IC et al (2013) Effect of glycosylation via maillard reaction and acylation on African yam bean (Sphenostylis stenocarpa) protein functionality. Food Sci Biotechnol 22:951–960. https://doi.org/10.1007/s10068-013-0169-7
Hu Q, Wu Y, Zhong L et al (2021) In vitro digestion and cellular antioxidant activity of β-carotene-loaded emulsion stabilized by soy protein isolate-Pleurotus eryngii polysaccharide conjugates. Food Hydrocoll 112:106340. https://doi.org/10.1016/j.foodhyd.2020.106340
Gu FL, Kim JM, Abbas S et al (2010) Structure and antioxidant activity of high molecular weight Maillard reaction products from casein-glucose. Food Chem 120:505–511. https://doi.org/10.1016/j.foodchem.2009.10.044
Geng X, Cui B, Li Y et al (2014) Food Hydrocolloids Preparation and characterization of ovalbumin and carboxymethyl cellulose conjugates via glycosylation. Food Hydrocoll 37:86–92. https://doi.org/10.1016/j.foodhyd.2013.10.027
Consoli L, Dias RAO, Rabelo RS et al (2018) Sodium caseinate-corn starch hydrolysates conjugates obtained through the Maillard reaction as stabilizing agents in resveratrol-loaded emulsions. Food Hydrocoll 84:458–472. https://doi.org/10.1016/j.foodhyd.2018.06.017
Hu M, Liu G, Du X et al (2020) Molecular crowding prevents the aggregation of protein-dextran conjugate by inducing structural changes, improves its functional properties, and stabilizes it in nanoemulsions. Int J Biol Macromol 164:4183–4192. https://doi.org/10.1016/j.ijbiomac.2020.09.007
Xu ZZ, Huang GQ, Xu TC et al (2019) Comparative study on the Maillard reaction of chitosan oligosaccharide and glucose with soybean protein isolate. Int J Biol Macromol 131:601–607. https://doi.org/10.1016/j.ijbiomac.2019.03.101
Boggione Santos IJ, Hernandez HL, Cardoso Costa MH et al (2019) Conjugates of α-lactalbumin, β-lactoglobulin, and lysozyme with polysaccharides: characterization and techno-functional properties. Food Res Int 116:492–498. https://doi.org/10.1016/j.foodres.2018.08.065
Feng J, Wu S, Wang H, Liu S (2016) Improved bioavailability of curcumin in ovalbumin-dextran nanogels prepared by Maillard reaction. J Funct Foods 27:55–68. https://doi.org/10.1016/j.jff.2016.09.002
Liu Y, Zhao G, Zhao M et al (2012) Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chem 131:901–906. https://doi.org/10.1016/j.foodchem.2011.09.074
Saatchi A, Kiani H, Labbafi M (2021) Structural characteristics and functional properties of sesame protein concentrate–maltodextrin conjugates. J Food Meas Charact 15:457–465. https://doi.org/10.1007/s11694-020-00655-2
Dong D, Cui B (2021) Fabrication, characterization and emulsifying properties of potato starch/soy protein complexes in acidic conditions. Food Hydrocoll 115:106600. https://doi.org/10.1016/j.foodhyd.2021.106600
Acknowledgements
The authors acknowledge the fellowship awarded by Thailand Graduate Institute of Science and Technology, TGIST (Grant No. TG-NN-AIT-63-026D), and Royal Thai Government Fellowship to Ms. Boonlao. The authors are grateful to Prof. Dr. S. Seraphin, NSTDA, Thailand, for his valuable comments on manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boonlao, N., Ruktanonchai, U.R. & Anal, A.K. Glycation of soy protein isolate with maltodextrin through Maillard reaction via dry and wet treatments and compare their techno-functional properties. Polym. Bull. 80, 8603–8626 (2023). https://doi.org/10.1007/s00289-022-04473-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04473-y