Abstract
Adhesives, which also comprise of terms like bonding agents, glues and gums, are substances which possess the ability to attach to various substrates and hence can be used to join materials. They find use for bonding of various substrates such as wood, metals, plastics and find applications in automotive, construction sectors, electrical or thermal conductivity applications, and dentistry among others. Adhesives are mainly composed of polymers and different additives, of which, one is fillers. Fillers are solid substances which are insoluble in the adhesive, and they are incorporated in adhesives to reduce cost, modify mechanical properties, increase viscosity, improve electrical and/or thermal conductivity, obtain better adhesion, etc. Some types of fillers utilized are bio-based (cellulose nanoparticles, tree bark powders); carbon-based (carbon black, carbon nanotubes, diamond, graphene); ceramics (aluminium oxide, boron nitride, iron carbide, silicon carbide); metallic (aluminium, aluminium oxide, copper, silver, titanium dioxide); silicon-based (layered silicate, silica); or hybrid (consisting of more than one type of filler). Both the type and the amount of filler added will affect the properties of the adhesive. Several adhesives having diverse uses have been considered in this study. The paper reviews in detail the effect of a number of fillers on the properties of the different adhesives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adhesives are substances which can permanently attach to surfaces and can be used to join surfaces of materials [1]. They are usually may be in liquid or semi-liquid forms [1, 2]. Adhesives include commonly used terms like glue, paste, gums, adhesive cement and bonding agents [2, 3]. The materials being joined using adhesives are called as substrates and adhesion is the interaction between the adhesive surface and substrate surface [1, 3]. Several theories exist which explain the mechanism of adhesion [2].
Adhesives happen to be important components in several applications. Some areas where adhesives find use include automobile and aerospace industry, bonding of numerous materials (glass, metals, plastics, rubber, wood), conductivity applications, construction industry, footwear, furniture, healthcare sector (biomedical and dentistry), packaging, and others [1, 2]. Figure 1 represents the various types of adhesives based on their properties and application sectors.
The formulations of adhesives consist of mainly polymers which may be natural or synthetic, or monomers and pre-polymers which can form polymers [1, 2]. These basic raw materials determine the adhesion and cohesion [2]. In addition to the raw materials, auxiliaries or additives are used in adhesive and sealants formulations. These may have a significant effect on curing, performance and final properties of the product. Some additives which are incorporated are fillers, plasticizers, rheology control additives, stabilizers, adhesion promoters, anti-agers, antioxidants, hardeners and others depending on the required properties and end use [2,3,4].
Fillers are solid and relatively non-adhesive substances which are insoluble in the adhesive binder. Rheology and other properties of the adhesive can be modified by adding fillers. They offer benefits like reducing the cost of the formulations, formulating adhesives with desired consistency, enhanced strength, give body to liquid adhesives to improve workability and other properties which include preventing over penetration of adhesive into the substrate, increasing thermal or electrical conductivity, obtaining better heat resistance, improving solvent resistance, reducing moisture absorption and others depending on the requirements for specific applications [2, 4,5,6]. Since adhesives shrink as they harden, fillers can be used to lessen the danger of failure at interface of adhesive and interface by preventing contraction of the glue [7]. The type and amount of filler used should be chosen carefully depending on the substrate and application. Organic as well as inorganic fillers exist [6]. In most cases, with rising filler content the general trend in the properties of adhesives is an initial increase and subsequent decline after reaching a maximum value. In this paper, different types of fillers used in adhesives are discussed.
Fillers in wood adhesives
Adhesives are much used compounds in the wood industry. Large number of adhesive systems exist for wood and the type and amount of fillers in the adhesive mixture affects the properties and performance of the adhesive and hence of the wood [6, 8, 9]. The area of application of adhesives covers a large scale from fibres and small particles to veneers, laminates and lumbers. They add value to residues like chips, small logs and pieces [10]. Addition of fillers to wood adhesives can be to increase viscosity, control rheology, achieve higher thermal and dimensional stability, get better mechanical properties, etc. [9, 11]. Fillers fill the pores present on the wood surface which leads to less undesired adhesive flow, better bonding between components and less resin penetrates in the pores, thus decreasing amount of resin required [6, 8, 11]. So the choice of filler should be appropriate as it will help in reducing the cost and amount of adhesive used and also improve performance of the wood [6]. Thus, fillers are one of the most important components of adhesives for wood [8]. Figure 2 represents the various fillers used in wood adhesive application.
Inorganic fillers
Colloidal hydrophilic silica (SiO2)
Colloidal silica is used as a filler for 2-component polyurethane adhesive formulations. Addition of colloidal silica leads to higher viscosities than non-filled adhesive as shown in Table 1, hence producing thixotropic behaviour and minimizing over penetration of the polyurethane adhesive. As a result of hydrogen-bonding between polyol molecule of adhesive and SiO2 filler, reactivity of polyurethane adhesive decreases and curing slows down. It also affects the thermal degradation of polymers. Presence of dispersed silica improves mechanical properties of the adhesive such as the bonding shear strength for adhesive having low innate strength. The shear strength of polyglycerol polyol-based adhesive having low innate strength filled with SiO2 filler is about 7.2–7.5 MPa as against 1.8–2.8 MPa value of bond line strength for the same adhesive not containing any filler. Use of SiO2 leads to lower water contact angle and increased work of adhesion. So, the interaction of polyurethane adhesive with hydrophilic wood surface containing large number of hydrophilic groups is enhanced. This leads to better adhesion between adhesive and wood surface [12].
Calcium carbonate (CaCO3)
Polyvinyl acetate (PVAc) is used in wood adhesives due to excellent adhesion of polyvinyl acetate emulsions to cellulosic materials. They are commonly referred to as white glues and are used for gluing of wood and wood-based materials, furniture and other timber industries. The calcium carbonate filler can be added to water and mixed till a proper dispersion of filler is achieved. The filler dispersion can be mixed with the PVAc emulsion. Addition of CaCO3 as filler affects the mechanical properties, there is increase in the tensile modulus of the polymer film below the glass transition temperature. The shear strength of dry films increases over that of the original PVAc emulsions and the shear strength develops as setting time increases. Using CaCO3 as filler is to decrease in depth of adhesive penetration in wood substrate and for its gap filing properties. The hardness of the developed film is also improved but greater hardness of adhesive could lead to more tool wear rate [2, 13,14,15].
Polymer isocyanate emulsions are another class of adhesives used in production of solid wood panels of different types, plywood, furniture, window frames, parquet, etc. They have good adhesion to wood, high heat and moisture resistance, low creep and wide temperature range for curing resulting in a wide range of applications. Similar to PVAC, calcium carbonate is most commonly used filler in polymer isocyanate emulsions to reduce cost of the adhesive, improve gap filing properties of the formulation and for better heat resistance of the cured adhesive. CaCO3 particle size should be in the same range as that of the emulsion particles to get a smooth adhesive, to prevent settling of the CaCO3 particles during storage and to get a proper glue line [16]. Two component polyurethane (2C PUR) adhesives are used to bond hardwood. Resin component (polyester-polyether polyol) can be filled by CaCO3 powder to modify mechanical properties, the other component being isocyanate-containing compounds. The 2C PUR-based adhesives can be used to form lap joints of hardwoods like beech wood. 2C PUR adhesives give sufficient spontaneous wetting of beech wood. Elastic modulus (E-modulus) of adhesive films for different filler contents shows that as compared to unfilled adhesive, the E-modulus is lower for adhesive with 15 weight% CaCO3 while it is higher for 30 weight% and 60 weight% filled adhesives. The tensile strength of CaCO3 containing adhesives is less than that of 2C PUR without fillers. 60 weight% filled PUR adhesive shows higher E-modulus and comparable tensile strength to commonly used phenol resorcinol formaldehyde (PRF) adhesives for wood bonding [17].
Nanofiller
Alumina nanoparticles
Alumina nanoparticles (ANP) can be added as filler to polyvinyl acetate (PVAc) polymer-based wood adhesives for better bonding strength under wet conditions and at elevated temperatures. Alumina nanoparticles (γ phase) can be also be introduced in Phenol–formaldehyde (PF) resin-based adhesives as filler. The viscosity of adhesive increases and its curing time decreases with increase in ANP loading in the adhesive. Increased viscosity due to nanoparticle filler addition results in uneven resin distribution on the wood surface [18]. Owing to the thermal properties of the nanoparticles, the heat transfer of the adhesive increases [19]. This leads to faster curing because ANP result in better heat transfer rate of PF resin during ultrasonic treatment and there could be some interaction between the surface of nanoparticles and the resin by formation of Al-O-Phenol bond, so curing could start even at room temperature. Increased manufacturing efficiency and lower energy costs are advantages associated with lesser curing time. With addition of more alumina nanoparticles, the wet shear strength gets better and the strength distribution becomes uniform [18]. ANPs can also be incorporated in urea formaldehyde (UF)-based adhesives. Similar to the case of PVAc-based adhesives, ANPs modify the viscosity of UF adhesives. As more amount of filler is added, the viscosity of adhesive becomes higher due to interaction between filler particles and polymer resin. When the nanoparticles are present in UF adhesive, the curing becomes faster. Also, the presence of ANPs lead to less formaldehyde emissions when medium density fiberboard (MDF) panels with adhesives are heat cured [20].
Nano-clay
Nanoclay is a nano-filler for polyvinyl acetate (PVAc). Hydrophilic nanoclay such as montmorillonite clay can be added to PVAc adhesive formulation used for wood like bonding of wood joints. Use of nanoclay affects the thermal stability of the PVAc adhesive. The thermal stability of PVAc improves when nanoclay is added for temperatures less than 200 °C, nanocomposites do not show much increase in thermal stability than pure PVAc for temperatures between 200 and 360 °C while it is more than that of pure PVAc for temperatures higher than 360 °C. Heat resistance of PVAc improves as nanoclay loading increases (upto 2%) as shown in Fig. 3 for nanoclay named “Lit.G-105116” and Fig. 4 for nanoclay named “Nanofil 116” [21]. Montmorillonite can be included as filler in other adhesives like UF-based adhesive. In case of PVAc-based as well as UF-based adhesives, when montmorillonite modified with benzalkonium chloride is added, the viscosity increases due to addition of filler. The viscosity change depends on the extent of exfoliation of the nanoclay in polymer matrix. In wet conditions, the shear strength of adhesive glueline becomes higher when PVAc and UF adhesives are filled with clay. In dry conditions, the bond shear strength for PVAc adhesive increases when 1.5 weight% filler is added while there is no significant improvement in the property for UF resin at 1 weight% or 1.5 weight% filler loading [22].
Thermal stability of PVA and its blends with Lit.G-105 [Reprint from Composites Part A: Applied Science and Manufacturing, Vol 42, Effects of adding nano-clay on performance of polyvinylacetate (PVA) as a wood adhesive, Alireza Kaboorani, Bernard Riedl, pp. 1035, 2011, with permission from Elsevier] [21]
Thermal stability of PVA and its blends with Nanofil 116 [Reprint from Composites Part A: Applied Science and Manufacturing, Vol 42, Effects of adding nano-clay on performance of polyvinylacetate (PVA) as a wood adhesive, Alireza Kaboorani, Bernard Riedl, pp. 1035, 2011, with permission from Elsevier] [21]
Polymeric fillers
Polyurethane powder
Polyurethane powder when used as fillers in urea–formaldehyde and phenol–formaldehyde adhesives, improves the physical properties and water resistance of the adhesive mixture [9]. Polyurethane adhesives are another class of widely used adhesives in wood and furniture industries [12]. Powder of recycled polyurethane- polyisocyanurate foam can be used as a filler for polyurethane adhesives applied on wood. For a one component moisture curing polyurethane adhesive, particle size and amount used affects the viscosity of the adhesive and contact angle of the adhesive and the wood surface which is used to determine the wetting properties of the adhesive. Both the viscosity and contact angle increase with increase in filler from 0 to 15%. The filler content also affects the shear strength, thermal stability and resistance to cold water of bonded joints. Addition of polyurethane powder as filler increases strength of bonded joints boiled in water while the strength decreases if the system is immersed in cold water. The thermal stability becomes better and resistance to cold water decreases with increases in filler content [9].
Rubber powder
Waste tire rubber is produced in large numbers in automobile industry. Accumulation of waste tire rubber has environmental issues, so it is minimized by reusing or recycling it. Waste rubber powder can be used as filler in Melamine-Urea–Formaldehyde (MUF) adhesive formulation for plywood panels. Addition of filler reduce formaldehyde emission. It may be due to presence of carbon phases in the waste rubber powder which enable the wood panel to absorb formaldehyde. The effect of addition of rubber powder on the properties of adhesives is shown in Table 2 [11].
Bio-based fillers
Birch bark
Most plywood adhesives are formaldehyde-based adhesives, like urea–formaldehyde, phenol–formaldehyde, and melamine-urea–formaldehyde. There is a breakdown of these formaldehyde-based resins in wood panels resulting in emission of formaldehyde which poses a hazard [11]. Urea–formaldehyde (UF)-based adhesives are used in plywood as well as other wood-based composites. Fillers are added to UF adhesives to decrease undesired fluidity, excessive penetration of adhesive into the substrate and release of free formaldehyde from the adhesive [6, 8].
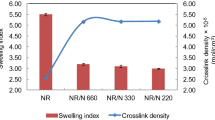
Birch bark is a type of bark powder utilized as filler in UF adhesives. Bark is obtained from tree trunks and branches and also as a by-product of plywood mills. So, use of birch bark solves problem of waste management for wood process industry. The birch bark filler also acts as scavenger to reduce free formaldehyde emission from plywood panels due to presence of lignin and tannin content which also lead to adhesive properties. Viscosity of the adhesive rises due to the addition of birch bark filler. For adhesive with 10 weight parts of bark powder per 100 weight parts of liquid UF resin (Adhesive- BB10), the average dynamic viscosity is 1058.4 mPa⋅s; while for adhesive with 15 weight parts of filler per 100 weight parts of resin (Adhesive- BB15), the value is 2878.4 mPa⋅s and the viscosity further rise to 6427.8 mPa⋅s for adhesive sample with 20 weight parts of birch bark is added per 100 weight parts UF resin (Adhesive- BB20) [8]. The effect of birch bark on mechanical properties is shown in Fig. 5.
Variation of properties in different adhesives [Blue: Bending strength (MPa); Orange: Shear strength (MPa) (× 10–2); Grey: Modulus of Elasticity (MPa) (× 102)] [8]
Western red cidar tree bark powder
Another polyurethane adhesive system used as wood adhesive is polymeric diphenylmethane diisocyanate. Use of bark as a bio-based filler is possible in this system is studied. Bark is rich in hydroxyl groups which can react with polymeric diphenylmethane diisocyanate. Western red cidar tree bark powder is added as a reactive functional filler to the adhesive. The resulting adhesive mixture is thicker, has more consistent bond line, and the bark powder helps in retention of adhesive at the bonding interface. So polyurethane adhesive consisting of cidar bark powder as reactive functional filler can be used in wood products like plywood which require consistent bond line. The effect of this filler on shear bonding strength of adhesive is shown in Table 3 [23].
Nanocellulose
Inclusion of fibrous fillers in liquid adhesives is known as fibre-reinforced polymer and can lead to increased strength and other mechanical properties which are important in wood bonding for especially structural applications [10, 24]. The cellulose nanofibrils can be prepared from wood pulp like beech tree pulp, and also from bacterial cellulose. Urea formaldehyde adhesive powder is mixed with fibrillated cellulose dispersion in water to get cellulose-filled adhesive. Toughening effect and bond strength increases due to presence of cellulose nanofibrils. Due to high viscosity of the filled adhesive, bond line thickness also increases. But increase in viscosity of the adhesive as a result of cellulose addition acts as a limiting factor to the amount of nanofibrils that can be added [10, 25].
Silanised nanocellulose can also be used as filler in Urea–formaldehyde adhesives to reduce formaldehyde emissions and improve mechanical properties [25].
Nanofibrillated cellulose suspension, where cellulose is produced from birch kraft pulp can be added to polyvinyl acetate adhesive and starch glue as filler. Similar to urea–formaldehyde adhesive filled with cellulose nanofibrils, polyvinyl acetate adhesives and starch glues with higher nanofibrillated cellulose filler content are more viscous. Both PVAc as well as starch adhesive show increase in shear strength by addition of nanocellulose but only upto a certain nanocellulose content after which the viscosity increases exhibits penetration of adhesive into the wood surface and hence strength decreases. One potential reinforcement mechanism is the cross linking between methylene groups of PVAc molecules and methylol groups of starch with the hydroxyl groups of nanocellulose [10, 24].
Palm kernel
Palm kernel meal (PKM) is a by-product of the palm oil industry and can be used as a filler for MUF-based wood adhesives. PKM contains about 21–23% of crude fibre, 14–21% of protein and 8–17% of lipid. The crude fibres which are rich in cellulose, hemicellulose and lignin facilitate the bonding interactions between the MUF adhesive and the wood surface. Also, there can be hydrogen bonding between amino groups in the crude protein and melamine and urea in MUF resin, and also with crude fibres for bonding strength of MUF resin. The lipid present in PKM can reduce water intrusion to disrupt the hydrogen bonding between MUF resin and also the wood surface. Hence PKM can be considered as a filler material alternative to industrial flours for MUF-based adhesives [26, 27].
Fillers in electrically conductive adhesives
There exists a large area of application for adhesives in electronics industry [2, 3]. Adhesives are used in electronics assembly, conducting or insulating electricity, etc. TV, radio and electronics assembly is a major area of adhesive use [1, 2]. In the electronics industry, lead-based solders are being used as interconnection materials to obtain electrical pathways between circuit elements. To minimize pollution by electrical and electronic wastes, lead-based solder can be replaced by lead-free solders and electrically conductive adhesives (ECAs) [28, 29].
ECAs are being viewed as next generation interconnect material for electronic packaging. They are composite materials consisting of insulating adhesive binder resin and a conductive filler [28, 29]. Epoxy, silicone, phenolic, polyurethane, polyimides are few resins used in these adhesives [1, 3, 28]. Some conductive fillers include aluminium, copper, nickel, gold, silver, tin, zinc, SnBi coated Cu, etc. [2, 30, 31]. The resin matrix provides physical and mechanical properties to the adhesive while the conductive fillers lead to electrical conductivity of the ECA by forming shortest conductive path for electric currents. The conductive fillers are physically in contact creating a short conductive path for electric current [28, 31].
ECAs can be divided on the basis of volume percentage of conductive fillers as shown Fig. 6. ICAs can provide electric current in all directions and find applications in electronic industry when high temperature soldering processes cannot be used. ACAs provide current in one direction only, these adhesives are used in LCDs as they can resolve small gaps between conductor tracks and solder is not viable for joining glass substrates [2, 32].
The different fillers explored in electrically conductive adhesive is enlisted in Fig. 7.
Inorganic fillers
Silver
Silver (Ag), either in powder or flake form is used as a filler for electrically conductive adhesives. Ag can also be processed easily. Copper, aluminium, nickel are other fillers which can provide electrical conductivity to the adhesive and have lower costs than silver filler but these fillers show electrical instability especially after exposure to high temperatures. Also, there is a possibility of oxide formation on the filler surface which decreases the conductivity as particle-to-particle contact is reduced. Silver has advantage over these fillers since the oxide layer formed on the silver flakes is also conductive. It is one of the most common conductive filler used in ICAs. Silver filled ICAs can be seen as an alternative to solder in surface mount technology (SMT), chip scale package (CSP) and ball grid array (BGA) applications [2, 29,30,31, 33]. Resistance to conduction in ECAs containing silver flakes as filler is due to tunnelling effect between neighbouring Ag flakes [34, 35].
Ag microfillers, both in particle and flake form, can be added to resin matrix for instance in epoxy resin-based adhesives. The Ag microfillers can be sintered in the polymer matrix leading to formation of microstructure giving improvement in conductivity. Ag flakes (3–10 µm) and microparticles (2–3 µm) when used as filler in ECAs having polyurethane-based binder, leads to low electrical resistivity of below 10 µΩcm after curing at 150 °C [36].
Use of nano-scale conducting materials in ECAs as dopants can fill the gaps between the conductive filler and develop more conductive paths. Modified carbon nanotubes (CNTs); carbon blacks (CBs); hybrid of silver flakes, silver nanoparticles, and silver nanowires; are some fillers added to function as bridges connecting adjacent Ag flakes [34, 35].
Nano-sized silver powder can lead to increased electrical conductivity of epoxy composite-based ECAs [37]. Hence, nanofillers like silver nanoparticles (NP) and silver nanowires (NW) can also be added to ECAs. These conductive fillers possibly decrease the constriction and tunnelling resistances of the ECAs thus increasing their electrical conductivity [28, 37]. Another theory of conductive mechanism is the percolation process which suggests that at the percolation threshold value, the nanoparticles form electrically conductive paths which result in rise of conductivity value of the adhesive [37].
When the metal filler particles in the resin of adhesive are fused together to form metallic joints, the joint resistance of the ACA decreases. But the melting temperature (Tm) of Ag is around 960 °C and printed circuit boards (PCBs) on which adhesive is used cannot withstand such high temperatures. Bridging between surface atoms of Ag NPs can be obtained by sintering. Decreasing size of the filler particles can reduce the Tm and sintering temperature required significantly. Thus, the depressed melting point effect results in metallurgical contact between the nanoparticles by bonding of the NPs being possible at low temperatures. Hence, the use of nano-size Ag particles is beneficial. When ECAs contain 92 weight% of spherical Ag nanoparticles (< 10 nm), they have low bulk resistivity of 8 × 10−6Ω cm and with 80 weight% of Ag NPs in the adhesive the resistivity is 5 × 10 −5Ω-cm. But with higher loading of nanoparticle filler in the ECA, mechanical properties of the adhesive decline [28, 29].
High aspect-ratio silver nanowires can be used over silver nanoparticles. Even at very low loading of Ag nanowires in the ECA, the NWs can establish large number of electrical paths. When the Ag nanowire content of the ECAs is 56 weight% the bulk resistivity of the adhesive has a low value of 1.2 × 10 −4Ω cm. ECAs containing Ag NWs have better mechanical properties than ECAs filled with Ag NPs, shear strength of nanowire filled adhesive is 0.3 MPa higher than nanoparticle filled adhesive with a value of 17.5 MPa when NWs are used [28].
Nano-silver coated copper particles
Fabrication of copper nanoparticles (Cu NPs) can be done at a lower price than silver nanoparticles, so Cu NPs can be used over Ag NPs. Copper also has low resistivity and good electro-migration performance. However, copper gets oxidised easily in a corroding environment like presence of oxygen and moisture causing deterioration in electrical properties of the adhesive. Also, unlike silver which can be sintered and bonded to other materials at lower temperatures (< 300 °C), copper cannot be sintered at such low temperature. By the use of chemical plating method, the surface of micro-copper can be coated with nano-silver particles to perform sintering joint between adjunct particles for lower electrical resistance. The modified nano-silver coated copper particles can be added to epoxy resins, phenolic resins used in ECAs as fillers. The electrical resistance of the adhesive with silver-coated copper filler is much lower than that of adhesive with pure copper as filler irrespective of the shape of the silver-coated copper particles (whether spinous or spherical). On high temperature exposure for 100 h, for the pure copper particle filler the copper oxide formed remains near the surface of the copper particles affecting the electrical resistance. However, for the silver-coated copper particle filler, the oxygen concentration near the surface does not rise much, thus for the adhesive filled with silver coated copper particles the conductivity remains almost stable [28, 38, 39].
When the ECA contains 23.5 volume % of nano-silver coated copper particles filler, higher curing temperature is preferred. For lower bulk resistivity of the ECA since the electrical resistivity of the ECA varies with curing temperature, the electrical resistivity at 110 °C is five times greater than that at 200 °C. The electrical resistivity also depends on the curing time [28]. The effect of the filler on shear strength (MPa) is shown in Fig. 8. The electrical resistivity of ECA on glass and Copper (Cu) pad substrates is depicted in Table 4.
Change in shear strength of adhesive with filler concentration [28]
Low-melting-point alloys and low-temperature transient liquid phase fillers
Addition of transient liquid phase sintering metallic fillers can increase electrical conductivity. The filler consists of a low melting point alloy powder (LMPA) and a high melting point metal powder. When the adhesive is cured, the low-melting-alloy powder component of the filler (like Sn–Pb or Sn–In) melts at its melting point and the liquid phase dissolves the high-melting-point metal powder component of the filler (like Cu). The liquid forms an alloy and solidifies quickly. Thus, a metallurgical connection is formed from the two components of the filler giving electrical conductivity. Also, an interpenetrating polymer network develops giving adhesion. Another class of conductive adhesives consist of two filler types, silver flakes filler and low-melting-point alloy filler (LMPA). The LMPA filler melts on curing of the adhesive (can be epoxy resin based) and there is formation of metallurgical interconnections between the silver flakes filler and the metallization of the substrate [29, 40].
Carbon fillers
Graphitic fillers like carbon nanotubes (CNT) and graphene can be used in ECAs due to their excellent electrical and mechanical properties. Single wall (SWCNT), double wall (DWCNT), and multi wall (MWCNT) CNTs are the types of CNTs [31]. MWCNTs when added to adhesive as filler improve the mechanical and electrical properties of the adhesive. MWCNTs exhibit excellent electrical conductivity, good tensile strength and Young’s Modulus. Graphene nanosheet consists of a flat monolayer of sp2-bonded carbon atoms arranged in the form of a two-dimensional honeycomb network [31, 41, 42]. Apart from being the thinnest and strongest material, graphene has several desirable properties including high electrical conductivity of around 6000 S/m, high Young’s modulus (∼1.0 TPa), high strength of about 130 GPa (its mechanical strength is comparable to that of carbon nanotubes), so graphene contributes to the mechanical and electrical characteristics of polymers [41, 43]. Also, producing large number of graphene sheets can be done at a lower cost than carbon nanotubes [42]. Both CNTs and graphene fillers have a large surface area and aspect ratio, so they can form electrical networks even when their loading in the ECA is less. These fillers need to be dispersed effectively in the polymer matrix to obtain significant improvement in the adhesive properties [31, 44]. The graphite nanofillers can also be used as auxiliary fillers along with other fillers like silver flakes. They can create electrical pathways in the network to increase conductivity [31].
Reduced graphene oxide
Reduced graphene oxide (rGO) is a graphene derivative having high electrical conductivity and good mechanical and thermal properties. Thus, electrically conductive adhesives (ECAs) can be loaded with rGO as a functional nanofiller. Epoxy-based adhesives are ECAs which can be filled with rGO filler. As the filler content of the adhesive increases from 10 to 50 weight%, the volume conductivity (conductivity through the adhesive thickness) as well as the surface conductivity (in-plane conductivity) gradually rises. The insulating epoxy gets converted into an electrically conductive material due to the development of electrical pathways by the filler layers throughout the polymer matrix. The initial increase in the shear strength can be attributed to interfacial interaction between rGO and the epoxy resin leading to effective load transfer between the filler and the polymer. With further filler loading, viscosity of the adhesive increases decreasing its flow rate which leads to formation of micro-voids reducing the shear strength. Similar to the trend of shear strength, the impact strength of the adhesive (both notched and unnotched values) improves with increase in the filler content of formulation and after reaching a maximum value the impact strength declines. However, with addition of rGO, the tensile strength of the adhesive is less than that of the base resin [41, 43].
Polymeric fillers
Polypyrrole (PPy) nanoparticles and nanotubes
Conjugated polymers have good electrical properties so they can be used in the form of nanoparticles as fillers for ECAs. For instance, polyaniline can be used as conductive filler. Polypyrrole nanoparticles (PPy NPs) can be added into ECAs containing silver flakes as filler to get better electrical conductivity. The bulk resistivity values with PPy NPs nanoparticles based ECA is shown in Table 5. The PPy NPs fall under semiconducting materials and have lower conductivity than silver flakes and hence after a certain amount of nanoparticle addition the conductivity begins to decrease [32, 35].
Polypyrrole nanotubes (PPy NT) can also be added as filler to silver flake-filled ECAs due to their high aspect ratios and proper dispersion can be obtained in many organic solvents. By the use of PPys in ECAs filled with silver, continuous 3D conductive networks can be formed leading to increase in conductivity. Ag-filled ECAs such as thermoplastic polyurethane (TPU)-based adhesives show reduction in electrical resistivity as visible from Table 6. It decreases from 4500 × 10−5 Ω-cm to 346 × 10−5 Ω-cm when 0.5 weight% of properly dispersed PPy nanotubes are added. The resistivity decreases as the PPy NT content in the ECA increases to 3 weight% where the resistivity is minimum at 5.8 × 10–5 Ω-cm. If the nanotube filler is further added beyond 3 weight% content, the resistivity begins to increase because the excessive PPy nanotubes begin to aggregate in the TPU matrix. The high aspect ratio nanotubes act as bridges to connect adjacent Ag flakes decreasing the tunnelling resistance and thus increasing conductivity of the adhesive. Similar to PPy nanoparticles, the nanotubes also lead to high-performance flexible ECAs, hence the PPy NTs can be incorporated in ECAs for flexible electronics [34]. Dopamine (DA) modified polypyrrole nanostructures represented as DA-PPy finds use as a co-filler in epoxy-based ECAs consisting of siler micro-sized flakes as filler. When considering ECAs with 60 weight% silver flakes, addition of pure PPy show that PPy begins to form aggregates and decreases contact between the silver flakes thus decreasing conductivity. But DA-PPy shows increased conductivity because DA could result in good dispersibility. So, for ECA with 60 weight% silver flakes, DA-PPy obtained using 0.064 DA/PPy mole ratio leads to better dispersibility and thus higher conductivity than DA-PPy prepared with 0.032 DA/PPy mole ratio. Proper and uniform dispersion of the DA-PPy throughout the adhesive can act as bridges to develop contact between separated silver flakes forming pathways for conductivity. There is synergistic effect of silver flakes and DA-PPy fillers leading to increased conductivity of epoxy-based ECAs. But as the amount of DA-PPy filler added rises, the electrical conductivity of the adhesive decreases. This is because with more DA-PPy filler, the silver flakes get separated and contact points in forming bridges between them resulting in increased contact resistance which negatively affects the conductivity [45].
Fillers in thermally conductive adhesives
On the basis of their applications, adhesives can be required to have either high or low thermal conductivity for use at above ambient temperature [46]. Heat dissipation is important in some electronic devices where with every 2 °C rise in temperature the device stability can decrease by 10%. Thermally conductive adhesives (TCAs) are used for electronic capsulation and heat dissipation is essential [47].
In TCAs, usually the polymeric materials used in adhesives have low thermal conductivity, hence they are not be able to remove the heat generated in electronic devices sufficiently though they contribute to the physical and mechanical properties. So, some materials may be added when dissipation of heat is required, that is, for better heat transport [46, 48, 49]. Fillers can be added leading to development of connected thermal transport pathways in the adhesive as shown in Fig. 9 [47].
Formation of thermally conductive paths (shown in blue) by filler particles in the adhesive [48]
The concentration of fillers used is desired to be above the percolation threshold at which all filler particles are in contact with each other to develop a three-dimensional conductive network [48, 50]. There is shrinkage reaction of the resin matrix during curing as a result of which the contact between the filler particles increases. Hence, the actual thermal conductivity value is known after the curing is complete [50].
Fillers that are added to increase the thermal conductivity of TCAs can be metallic (like aluminium, copper, gold, silver, zinc); carbon-based (such as carbon black, carbon fibre, carbon nanotubes (CNTs), diamond powder, graphite powder, graphene); and ceramics (including aluminium oxide (Al2O3), aluminium nitride (AlN), boron nitride (BN), silicon dioxide (SiO2), silicon carbide (SiC)) [47, 49]. The fillers are dispersed in the polymer matrix and can be micro- or nano- sized and in shapes of balls, flakes, wires, fibres, etc. [48]. The shape of filler particles affect the thermal conductivity of the adhesive. This is because as filler is added, there can be formation of filler aggregates which connect with each other leading to formation of a continuous conductive scaffold in the adhesive. For spherical filler particles, the contacts are points while for flake shaped fillers there may be surface contact which decreases the thermal contact resistance (TCR) [51]. The thermal contact resistance between the filler particles limits the heat conductivity of the adhesive, hence with reduction in the TCR there is a rise in thermal conductivity [50, 51]. Figure 10 represents the various fillers to induce the thermal conductivity in adhesives.
Inorganic fillers
Particulate fillers like metal powders and their oxides have high conductivity and hence can be used to improve the thermal conductivity of adhesives for instance epoxy adhesives [46]. Aluminium (Al) powder, Copper (Cu) powder, Nickel (Ni) powder and Silver (Ag) powder are examples of metals added as fillers [47, 51]. Metal fillers can be used for applications where dielectric isolation is not necessary. Thus, the thermally conductive adhesives are also electrically conductive adhesives [33].
Copper
Copper powder can increase the thermal conductivity of an adhesive. The particle size of copper also affects the thermal conductivity of the adhesive. When copper powders with particle sizes of 50 µm, 75 µm and 100 µm are added to different adhesive samples, generally the more finely sized filler leads to most increase in the thermal conductivity. For all these sizes of the filler particles, the thermal conductivity increases as the copper powder volume fraction in the adhesive increases. But its rate of increase is higher for lower filler concentrations and it decreases as the loading of copper powder in the adhesive increases [46]. Another factor affecting thermal conductivity is the morphology of the copper particles. For micro-sized copper, flakes are better in forming thermally conductive networks than sphere-shaped particles. This is due to more contact area of the Cu flakes where contact of particles is face-to-face as against Cu spheres which exhibit point-to-point contact. When added to epoxy adhesives, microflakes result in higher viscosities of TCAs as compared to microspheres since the flakes have more specific surface area which means there is greater friction between the copper flakes and epoxy resin [52].
Aluminium
Aluminium powder is another filler for heat transport in adhesive. Changes in the thermal conductivity of aluminium powder filled epoxy adhesive with temperature, filler volume concentration, particle size of filler are all similar to the trends for these parameters in copper filled adhesive, although the change in particle size of aluminium filler does not affect the thermal conductivity as much as in case of copper filler [46].
Silver
Silver, other than having use in electrically conductive adhesives due good electrical conductivity also has high thermal conductivity and hence can be used as a thermal filler. So micro-sized silver filled ECAs can as well be applied as thermally conductive adhesives in applications where the electrical conductivity of silver will not be an issue [48]. For instance when used in adhesives for electronic packaging, silver nanoparticles contribute to the thermal conductivity as well as shear strength of the joint [53]. The silver particles though mostly in the form of thin flakes, can be of various shapes and the particle shape affects the thermal contact resistance [48]. Filler nanoparticles and epoxy resin form a heat conductive network in the adhesive giving twice the thermal conductivity of unfilled epoxy adhesive [53].
Ceramic fillers
Ceramic fillers are electrically non-conductive and thermally conductive. So ceramic materials can be filled in TCAs for electronic devices which will prevent short-circuits while also providing heat dissipation [49]. Fillers like boron nitride, aluminium nitride, silicon carbide etc. can be added to the polymer matrix [54].
Aluminium oxide (Al2 O 3)
Al2O3 is a cost-effective filler with good bulk thermal conductivity of 12 W/mK along with good electrical insulation, which can be useful in electronic devices for heat management. Hence this filler finds application in light emitting diodes to avoid voltage drops or short circuits [33, 49, 53]. Higher the loading of alumina in the TCA, more will be the thermal conductivity obtained and more viscous the adhesive becomes [33]. Mechanical properties of epoxy-based adhesives also get better due to addition of alumina nanoparticles as filler [53].
Aluminium nitride (AlN)
Aluminium nitride is an important filler since it is non-toxic and has high thermal conductivity, low dielectric constant, low coefficient of thermal expansion and low cost [48, 49, 54]. Properties of AlN loaded epoxy adhesives depends on the size of the filler particles [53]. At temperature of 30 °C, as the AIN content in epoxy-based adhesive increases from 0 to 62% volume fraction the thermal conductivity of the adhesive rises from 0.2 W/mK to 4 W/mK [55]. When AlN particles of 5 µm size are filled in epoxy adhesives, 70 weight% filler in the adhesive gives thermal conductivity which is about 10.8 times the conductivity of pure unfilled epoxy adhesive [53].
Boron nitride
The thermal conductivity of boron nitride is higher than that of Al2O3 and also has a higher cost than alumina. This thermal filler can form aggregates in the polymer matrix of the adhesive leading to rise in heat conductivity. Good thermal properties can be obtained at low content and hence there is not much change in viscosity [33].
Silicon carbide (SiC)
Owing to properties such as good heat transfer ability, excellent stability, no degradation at high temperatures of up to 1000 °C, good strength and hardness, resistance to corrosion and oxidation, etc.; SiC is a thermal filler for epoxy-based adhesives [49, 53, 56]. 3 weight% of SiC nanowires in epoxy adhesives increases thermal conductivity of pure epoxy adhesive by 1.06 times to 0.449 W/mK [53]. Silicon carbide can be added along with graphite as filler in epoxies for synergistic effect to get higher thermal conductivity than the conductivity of adhesive consisting of these fillers individually [49, 56].
Carbon-based fillers
Carbon-based materials can be used in TCAs due to their low density and high conductivity. Examples of thermal fillers include CNTs, carbon fibres, graphite nanoflakes, natural graphite powder, diamond, etc. [49, 57].
Graphite powder
Graphite has high inherent thermal conductivity and low cost; hence lamellar graphite is an important filler in thermally conductive adhesive [47, 49]. It has layer structure which is advantageous as the filler gets connected to form heat pathways in the polymer matrix by a smaller number of contact points meaning that there is low thermal contact resistance and hence better conductivity. Another benefit in the formation of heat pathways is that the layered graphite filler does not aggregate much and gets distributed properly in the resin matrix. For pure unfilled epoxy resin, the thermal conductivity is about 0.17 Wm−1 K−1 and the value rises as graphite powder filler is added to a maximum of about 1.68 Wm−1 K−1 at 44.3 weight% filler content [47].
Graphene sheet
Graphene has high thermal conductivity values in the range of 600–5000 Wm1K−1 and very less resistance [49, 57]. So it can be used as a thermal filler. The heat conductivity of graphene sheet filled adhesive is higher than that of adhesives containing some other carbon-based fillers like natural graphite powder and graphite nanoflakes as represented in Table 7. This is due to the very thin layered graphene structure which can efficiently form three dimensional pathways for heat transport [57]. Heat conductivity of epoxy-based glues can be improved by addition of graphene as thermally conductive filler [53].
Carbon nanotubes (CNTs)
Carbon nanotubes improve heat transport of composites since they exhibit high thermal conductivity of about 2000–3000 W/mK for multi-wall carbon nanotubes (MWCNTs). Hence, they can be added as thermal filler to adhesives to improve thermal conductivity [48, 58]. Due to the high aspect ratio of CNTs, they can conduct over long distances without requiring particle to particle transition [49, 53]. But owing to their low dispersibility in the resin, CNTs start forming aggregates when added in higher concentrations. Adhesives filled with CNTs are utilized in joining high performance components such as wings and fuselage parts [53].
Carbon fibres
Carbon fibres are less considered as fillers as compared to CNTs due to bigger diameter, higher density and inferior mechanical properties of carbon fibres. But the fibres cost less and are more easily available than CNTs [49, 53]. Carbon fibres possess a high aspect ratio and good thermal conductivity, so can be used as filler in adhesives to improve thermal conductivity (especially for heat dissipative TCAs) even when the filler content in adhesive is less. Another advantage is that while the polymers used usually have a high positive value of coefficient of thermal expansion (CTE), carbon fibre has a negative CTE (~ 1.5 ppm/K in the longitudinal direction) thus leading to dimensional stability [49]. The aspect ratio and thickness of the fibres affect the overall thermal conductivity of the adhesive [53].
Diamond filler
Synthetic diamond powder, owing to its very high thermal conductivity can be used as a filler in TCAs. The conductivity could be highest for specially purified synthetic diamonds with a value of about 2000 W/m·K or higher. But the conductivity of diamond decreases with increase in temperature or increase in impurities like nitrogen. The microstructure and grain size of the filler also affect the conductivity. For extremely pure filler, significant increase in thermal conductivity of TCAs can be obtained. In spite of its high cost, diamond filler can be used in adhesives for high power devices, metal heat sinks, etc. [48, 59].
Hybrid fillers
A thermally conductive adhesive can contain a combination filler of various sizes and shapes for improvement in thermal conductivity, referred to as a hybrid filler system or multi-component fillers [33, 48, 49]. Use of hybrid fillers can be to minimize the cost, to combine the advantages of different fillers or for better packing of conductive filler particles due to their varying particle sizes [33, 60].
Copper nanowires and graphene oxide
Copper nanowires and graphene oxide nanosheets can be used as a hybrid filler system for epoxy-based adhesive. Copper nanowires have good thermal conductivity and low cost as compared to silver nanowires. Graphene oxide has excellent thermal properties along with many oxygen-containing groups such as –O–, –OH, –COOH on its surface and hence can be easily modified to be compatible with organic resin of the TCA. The copper nanowires form heat transport networks while the graphene filler with its large surface area acts as a bridge between the non-continuous networks to develop a proper continuous conductive network. Table 8 represents the thermal conductivity values at various concentrations of these fillers [60].
Aluminium nitride and boron nitride (BN)
Hybrid polymer composites consisting of aluminium nitride and boron nitride as fillers show improvement in the heat conductivity. Micro-sized aluminium nitride and hexagonal boron nitride (hBN) can be dispersed in epoxy-based adhesives, where the smaller 1 µm sized BN flakes occupy interstitial gaps in between the AIN particles which are large granular particles with 10 µm size. Due to the different sizes of the filler particles, there is a greater possibility of the conductive particles to be in contact with each other thus developing complex heat conduction network. Another factor affecting the thermal conductivity of the adhesive is the amount of AIN and BN particle fillers added to the epoxy resin [61].
Alumina nanoparticles, multi-walled carbon nanotubes (MWCNTs) and micro-sized alumina particles
TCAs containing micro-sized as well as nano-sized fillers can be formulated. Micro filler-spherical alumina particles and nano fillers-alumina nanoparticles (with thermal conductivity value of 36 W/mK) and multi-walled carbon nanotubes (having high thermal conductivity of about 3000 W/mK) can be added to epoxy-based adhesive. The MWCNTs form a continuous heat transport network in the epoxy matrix, thus improving the thermal conductivity of the adhesive [62].
Fillers in pressure sensitive adhesives
Pressure sensitive adhesives (PSAs) remain tacky and have flowability even after curing, pressure is applied for some time so that the adhesive can wet the surface properly and adhere to it without covalent bonding or activation [3, 4, 63]. Tack and hold are the parameters considered while formulating the PSAs [7]. Tack allows the adhesive to get adhered to the surface in less contact time when some pressure is applied for proper surface wetting. [4] Hold is the property of an adhesive to resist creep when subjected to any dead loads [7].
These adhesives are available in the form of films, labels, rolls, tapes as well as liquids. PSAs may require both adhesive strength and cohesive strength depending on their use. For permanent label application, adhesive strength of the adhesive will be more important. The PSA will need high cohesive strength in protective films, and weak adhesive strength to remove the film after use such that there are no adhesive residues on the surface. Adhesive as well as cohesive strength will be required for the adhesive in packaging tapes which could be used in high temperatures and under stress [4].
Acrylic ester copolymers, ethylene–vinyl acetate copolymers, natural rubber, polyisobutylene, polyvinyl ethers, silicones, styrene-butadiene rubber, etc. are some polymers used in PSAs [3, 4].
Fillers can be utilized in PSAs to decrease costs, increasing viscosity to a proper value for coating the adhesive on a substrate etc. [64]. For instance, Acrylic PSA tapes find extensive use in automotive, electrical and electronic industries [65]. Fillers commonly incorporated in acrylic PSAs include calcium carbonate, glass microbubbles, graphene, hydrophobic fumed silica, polymeric microspheres, etc. [64, 66].
Protective tapes find use in electronics where electrostatic discharge is necessary so that the device does not get damaged during the tape removal by electric discharge. Thus, the PSAs used in these tapes are composed to have electrical conductivity property. Metal powders and carbon-based fillers like carbon black, carbon nanotubes can impart electrical conductivity [66]. When the conductive filler concentration in the PSA is above the percolation threshold, the composite shows transition from insulator to conductor. The filler content affects the peel adhesion, shear strength, and tack in addition to electrical conductivity [67]. If the filler loading is low and the filler is properly dispersed, it can get insulated by the non-conductive polymer matrix. The fillers require higher loading at which important properties of PSAs such as tack deteriorate and there is risk of the polymer drying out by the conductive filler. So, the appropriate concentration of carbonaceous filler needs to be determined to get a good balance of all properties [68]. Figure 11 shows the different fillers explored in PSAs applications.
Inorganic fillers
Layered silicate
Fillers have been extensively used in various acrylic PSAs [64]. Another example is layered silicate or clay-based nanocomposite filler which gives better barrier properties, rise in heat distortion temperature, increase in strength, etc. [69]. Naturally occurring sodium montmorillonite (Na-MMT) as well as organo-modified MMTs (which can be obtained by replacing Na+ counter ions in MMTs by quaternary ammonium cations having hydrophobic tails) can be potential filler for PSAs such as polyisobutylene (PIB)-based adhesives [70].
A significant property of acrylic PSAs is transparency. At low loadings of the MMT filler, the optical clarity is not affected much, but at higher loadings of about 5 weight% of MMT there is scattering by the filler aggregates thus decreasing the clarity. There is strong interfacial interaction between the filler and polymer matrix, hence the storage modulus in the rubber plateau increases and glass transition temperature (Tg) rises by addition of MMT filler in the poly (butyl acrylate) based PSA [69]. Sodium montmorillonite when dispersed in acrylic PSA to obtain acrylic polymer/ MMT clay nanocomposite adhesive, shows decrease in gel phase with increasing amount of MMT clay filler in the adhesive. This is because entanglement of polymer chains in the reaction mixture is hindered by the filler particles during adhesive formulation. The shear strength of the PSA increases [71]. 5 weight% is too high filler loading in the PSA which though gives increase in holding strength, but also results in decrease in peel strength and tack [69]. The fall in peel strength results from the increase in cohesive strength. Due to this, the degree of deformation is affected which means less force is required for debonding [71]. 3 weight% MMT filler content gives proper balance of holding strength, peel strength and tack properties [69]. Figure 12 shows the effect of addition of MMT filler on mechanical properties of adhesives.
Changes in adhesion properties for different coated PSA sample tapes [Blue: Holding strength (Hour); Orange: Peel strength (N/25 mm); Grey: Tack (Ball number)] [69]
MMT-type nanofiller can also be added to PSAs based on polyisobutylene (PIB). Viscosity of the PSA increases by addition of MMT filler. There is decrease in tack of the PSA similar to other compositions due to the incorporation of clay filler, though the shear adhesion properties show significant increment. But at very high filler loadings, the MMT clay fillers could form aggregates which can occupy space in PSA-substrate boundary acting like defects and leading to failure [70].
Silica
Modified silica nanoparticles- Modified silica nanoparticles can be used as filler to acrylic copolymer-based PSAs. The silica can be modified by reacting the filler with γ-methacryloxypropyl trimethoxysilane (MPS) to get vinyl groups (carbon double bonds) in the surface of nanoparticles which can possibly be UV cured with acrylic copolymers of the PSA [72]. Figure 13 shows the reaction scheme for this modification.
Schematic diagram of UV crosslinking of acrylic copolymer/MPS modified silica composite PSA [Reprint from Materials Science and Engineering: B, Vol 178, Improvement of thermal stability of UV curable pressure sensitive adhesive by surface modified silica nanoparticles, Beili Pang, Chong-Min Ryu, Hyung-Il Kim, pp. 1214, 2013, with permission from Elsevier] [72]
The modified nanoparticles can then be added to the acrylic PSA and it can be coated on poly (ethylene terephthalate) (PET) film [72].
MPS modified silica nanoparticles gives better dispersion in the polymer matrix and form lesser aggregates as compared to silica particles dispersed in the matrix. Similarly, on UV curing, the gel content rises with increasing modified filler content in the PSA indicating more cross linking by reaction of the modified silica and acrylic copolymer. Again, the gel content is more when modified silica is used instead of unmodified silica filler. Thermal stability of the PSAs also improves with increasing addition of modified silica nanoparticles due to strong and extensive bonding between the filler and polymer. The tack and peel strength decrease due to the filler since cross linking restricts polymer chain mobility [72].
Polymer-silica nanocomposites- Hard inorganic particles such as silica (SiO2) can modify the viscoelastic properties of organic polymer containing PSAs [65, 73]. So an organic–inorganic nanocomposite will give properties of both organic polymers (such as toughness, optical properties, reduction in brittleness) and inorganic compounds (like strength) to the PSA [74]. A nanocomposite consisting of a soft polymer such as poly (n-butyl acrylate) (PBA) as core in a hard silica SiO2 shell is a filler for improving adhesion properties of water-based PSAs. The nanocomposite particles in PBA homopolymer latex particles can be used to prepare adhesive films. The stability of PBA-SiO2 nanocomposite colloid can be increased by Poly (N-vinyl pyrrolidone) (PNVP). But PNVP becomes hard in the dry state so these particles on the surface can lead to decrease in tack. Blend PSA adhesive films can also be prepared by using Ammonium persulfate (APS)-PBA particles along with SiO2 particles or with PNVP-PBA-SiO2 particles in different ratios [73].
The interaction between PBA and SiO2 particles means there is restriction in the mobility of PBA giving increase in cohesive strength. Adhesion energy also rises. When 10 weight% of the PNVP-PBA-SiO2 nanocomposite is added, the cohesive strength increases and hence maximum stress increases from 0.39 to 0.48 MPa. At 20 weight%, the maximum stress reaches the highest value 0.52 MPa, the value is 0.50 MPa for 30 weight% and then it decreases rapidly. At higher loading, the filler nanocomposite particles aggregate giving decrease in efficiency of cohesive strength [73].
It also affects the optical performance as shown in Fig. 14. This could be because the SiO2 filler particles may agglomerate in the PBA or there could be light scattering by the SiO2 particles’ surface. Hence among these PSA adhesive films, adhesives composed of PNVP-PBA-SiO2 composite filler can lead to highly transparent films [73].
Effect of filler particle amount on transmittance of the adhesive films. The films were prepared from mixture of APS-PBA latex particles and PNVP-PBA-SiO2 nanocomposite particles (solid line (Big black circle)), mixture of APS-PBA latex particles and SiO2 particles (D: 180 nm) (broken line (Small black circle)), and mixture of APS-PBA latex particles and PNVP-PS-SiO2 nanocomposite particles (broken line (Black diamond)) [Reprint from Polymer, Vol 70, Soft polymer-silica nanocomposite particles as filler for pressure-sensitive adhesives, Yusuke Yamamoto, Syuji Fujii, Kohei Shitajima, Kazuko Fujiwara, Shigeki Hikasa, Yoshinobu Nakamura, pp. 30, 2015, with permission from Elsevier] [73]
Halloysite nanofiller & Wollastonite microfiller
Halloysite is an aluminosilicate clay mineral with chemical formula Al2Si2O5(OH)4·nH2O. It has high pore volume, surface area and thermal resistance; low cytotoxicity and low cost. Halloysite nanoparticles can be filled in PSAs to give nanocomposites. The filler leads to better mechanical and thermal properties of the adhesive. When excessive wollastonite microfiller is added to the SAT and coated on polyester foil, cohesion deteriorates because the filler microparticles occupy space and decrease the active contact area between the PSA and the substrate. The effect of addition of filler is shown in Table 9. The higher Tg value when the nanofiller is used can be attributed to the smaller sized particles of halloysite than wollastonite which affects the mobility of the polymer chains. The shear strength also decreases by 6% compared to unfilled SATs though the deterioration in this property is less than wollastonite filled SAT. [75]
Wollastonite fibres coated with silver using electroplating are included in PSAs which are electrically conductive. Silver-coated wollastonite fibres with an average diameter of 12 µm can be mixed with acrylic based PSAs. The fibrillar nature of wollastonite leads to development of electrical contact in the PSA even at low levels of loading, resulting in decrease of volume electrical resistance of the adhesive. The strength of aluminium joint bonded with the adhesive shows slight reduction with rising filler content. The joint strength is 0.18 MPa when unfilled adhesive is used to 0.15 MPa when the adhesive contains 23 volume% of coated wollastonite fibres [76].
Ceramic fillers
Iron carbide
Iron carbide in a carbon matrix (Fe3C, C) is a filler that can be added to solvent based acrylic PSAs. Methane is subjected to catalytic decomposition and then at temperatures of about 650 °C, the methane is used to carbonize nano-crystalline iron giving iron carbide filler in carbon matrix. Dry grinding of the iron carbide results in carbon matrix having iron carbide nano-particles [77].
Iron carbide filler in carbon matrix does not consist of any functional groups and there cannot be any reaction between the inactive filler surface and the acrylic PSA. Hence with the increase in filler content of the PSA, the tack value decreases. The peel adhesion property of the PSA shows a similar trend as tack in variation with filler content that is, the peel adhesion reduces as more and more iron carbide filler is added. Peel adhesion of thicker 90 g/m2 layer of the adhesive is less than the value for 60 g/m2 layer by about 2 N. Shear strength also decreases with increase in filler concentration. But the use of iron carbide filler leads to very good removability of the PSA from substrates like glass and steel. Also, the adhesion does not build up with ageing time. Filler concentration of about 1.3% give good balance of the properties like adhesion, removability, shear strength and tack. Hence, iron carbide filler in carbon matrix can be used in acrylic PSAs in removable and repositionable applications in double sided tapes, labels, memo notes, protective foils, etc. [77].
Aluminium nitride
Acrylic PSAs find use for thermal applications owing to low cost, durability and resistance to chemicals, sunlight and temperature [78]. Thermal conductivity of the PSAs can be improved by addition of heat conductive fillers like aluminium nitride (AlN) (micro-sized as well as nano-sized particles) which are also electrically insulating which acts as an added advantage [78, 79].
The untreated particles lead to PSAs having rough surface resulting from formation of agglomerates. There is no compatible functional group in the untreated AlN particles that can interact with the polymer, hence proper dispersion of the filler in polymer matrix is not obtained. 3-aminopropyl triethoxysilane (3-APTES) is a silane that can be used for modification of aluminium nitride. In silanization, there is a reaction of the -OH groups on the surface of AlN particles with the ethoxy functional groups of the APTES resulting in formation of AlN-O-Si bonds. Silanized micro-sized as well as nano-sized AlN particles give effective dispersion in acrylic polymer. The APTES gets attached to surface of AlN particles and its functional groups react with active groups on acrylic polymer forming continuous networks which restrict mobility of the chains during thermal treatment. This improves the thermal stability of the filled adhesive. When filler contains both micro-sized and nano-sized filler particles, the nanoparticles fill the gaps in between microparticles thus ensuring continuous conductive network. So, addition of treated filler reduces heat resistance of the PSA. The thermal conductivity also rises significantly. It is highest for PSA filled with treated micro-sized AIN particles with 316% increase in conductivity value as compared to PSA with no filler [78].
Molybdenum disulphide
Molybdenum disulphide (MoS2) is an inorganic filler that can be reinforced in organic polymer-based PSAs. MoS2 nanoplatelets (two-dimensional) is a possible filler in polyurethane (PU) / methacrylate waterborne PSAs. MoS2 filler exists as a layered structure with van der Waals forces holding the layers together, and S-Mo-S covalent bonds in the layers in the form of hexagonal structure. The weak forces connecting the layers means monolayers can be exfoliated [80].
The MoS2 particles can be dispersed in poly(vinylpyrrolidone) (PVP) aqueous solution and the dispersions can be added to PU/(meth)acrylic latexes while stirring. The PVP prevents the MoS2 monolayers from restacking and acts as a bridge to improve interaction between the inorganic filler and organic polymer matrix. The interaction between MoS2 nanoplatelets and the polymer restricts mobility of polymer chains resulting in rise of Tg with increase in filler loading upto 0.25 weight%. For nanocomposite containing filler more than 0.25 weight%, there is decrease in Tg due to aggregation and restacking of MoS2 nanoplatelets in the PSA composite [80].
PSA nanocomposites show better tack adhesion on 0.1–0.25 weight% filler loading as compared to unfilled PSA but with further addition of filler, the adhesive stiffness increases (due to increase in Young’s modulus with increasing filler concentration) too much such that it loses dissipative properties and so the tack decreases. At 0.25 weight% filler content of the PSA, tack adhesion energy is 3 times higher than for unfilled PSA. Decrease in tack energy at higher loading of filler is a result of large elastic modulus and strain hardening. Storage modulus of the nanocomposites is also more than the value for PSA without the MoS2 nanoplatelets [80].
Carbon-based fillers
Graphene
Graphene filled acrylic PSA coats can be applied on different films and fabrics for use as protective films, films to shield electro-magnetic interference (EMI), etc. When a layer of the filled adhesive is coated on the conductive fabric, there is conduction of electric current through PSA to the fabric due to reduction in the surface resistivity. A single graphene layer absorbs very less light (about 2.3% light intensity) and hence is transparent. This means that the optical clarity of the PSA won’t be influenced much by the addition of graphene though the peel strength decreases slightly [66].
When an electrically insulative PET film is coated with PSA containing graphene prepared by in situ polymerization, the dispersed graphene in polymer matrix can form electrically conductive channels at filler loadings higher than 1.0 part of graphene per 100 parts of PSA solid (phr). Hence the surface resistivity will significantly decrease though there is much deterioration in peel strength. For PET film coated with PSA, where the graphene is mixed in the PSA by physical mixing, there is decrease in surface resistivity with increase in filler content, but the peel strength doesn’t fall as much as in the above case [66].
With addition of layers of graphene filler, there is a decrease in the transparency of the adhesive due to agglomeration or stacking of the graphene layers. Rather than homogeneously dispersing the graphene filler in PSA, embedding the filler in the PSA as separate layers yields less decrease in the peel strength along with fall in surface resistivity [66].
Van der Waals forces exist between graphene layers making them difficult to disperse. Hence the graphene can be acidified to modify its surface. Then it can be filled in polyolefin hot melt PSAs (HMPSA). HMPSA have thermal conductivity of 0.3 W/mK. Graphene addition brings about formation of network for heat conduction which is not properly developed at low filler content but 10 weight% graphene in the PSA increases the conductivity of the adhesive by nearly 10 times the value of unfilled HMPSA. Thermal conductivity keeps on rising as more filler is added but subsequently due to high viscosity of the adhesive, graphene forms agglomerates contributing to internal thermal resistance and so there is not much rise in conductivity. Presence of graphene also improves the hardness of HMPSA. Graphene filled HMPSA can be used as a thermal interface material (TIM) [81].
Carbon nanotubes
Carbon nanotubes (CNTs) can be used as filler in PSAs to increase its stiffness and at the same time increase its ability to dissipate energy during deformation. Also, the use of CNTs doesn’t affect the optical transparency while also contributing to the electrical conductivity of the adhesive [63].
The nanotubes can be functionalized by grafting polymers onto them. The filler can then be added in the form of polymer-CNT nanocomposites where the polymer-CNT interface provides energy dissipation and affects the viscoelasticity as well. For instance, hydrophilic poly(vinyl alcohol) (PVA) can be grafted on single-walled CNTs (SWNTs) to get nanocomposites referred to as PVA–SWNT which can be added as filler to poly(butyl acrylate) latex dispersions. There is improvement in tack properties as loading of the PVA-SWNT composite in the adhesive increases from 0 to 0.3 weight% where it is maximum and then begins decreasing with further increment in adhesive filler content. At higher loading of the filler, the PSA can become very stiff due to which its adhesion, ability to dissipate energy and tack can get affected [63].
When metallic fillers are used for electrical conductivity of PSAs, the storage modulus and transparency decreases. But PVA-SWNTs gives electrical conductivity along with optical clarity. Hence the use of CNT based nanocomposite fillers in PSAs is advantageous over metallic fillers [63].
MWCNTs which are modified using polydopamine (PDA-CNTs) for better adhesion to the substrates can be added to PSAs based on polyurethane/polysiloxanes as filler and the filled adhesive can be used to coat a PET film. Owing to the hydrophobicity of the fillers, the PSA becomes more hydrophobic seen as increase in contact angle of the PSA. When grafted with PDA, the dispersion of CNTs in the matrix improves and the CNTs form network of conductivity pathways also. As a result of these along with the high electrical conductivity of CNTs, the PSAs become more conductive. Storage modulus and loss modulus values of the PSAs show increment due to hydrogen bonding between PDA-CNTs and polyurethane. Friction between the nanotubes and polymer chains during tensile process contributes to rise in adhesive’s shear strength when attached to stainless steel. PDA-CNTs as filler result in higher shear strength than CNTs in the PSA [82].
Carbon black
Considering its lower cost, low density, good electrical conductivity etc.; carbon black is a common filler used to impart conductivity to PSAs for example acrylic PSAs to get electrically conductive PSAs. Nano carbon black can be used along with carbon nanotubes as conductive fillers in these adhesives. When these fillers are added to acrylic PSA, the polymer begins to crosslink making the structure compact [67, 83]. Due to this, the tack and peel strength of the adhesive decreases as the filler concentrations increase. But there is less decrease in tack and peel adhesion with increase in filler content of PSA when carbon black is the filler as compared to the reduction in these properties for PSA consisting of carbon nanotubes. The surface resistivity decreases due to the addition of carbon black filler leading to increase in the electrical conductivity of the acrylic PSA. The percolation threshold for nano-carbon black filled acrylic adhesive is about 25weight% of filler concentration at which the electrical conductivity value is high (about 38–45 S/cm) [67].
For solvent-borne acrylic PSAs based on 2-ethylhexyl acrylate and acrylic acid, modified with resins, and crosslinked with aluminium acetylacetonate; carbon black filler with particles of size 30 nm were added in different amounts from 3 to 40 weight%. Similar to the above example, crosslinking makes the adhesive compact leading to deterioration in tack and peel adhesion with increasing content of carbon black in the adhesive. Presence of filler leads to better cohesion, hence the shear strength at 20 °C and 70 °C is improved. The electrical conductivity of PSA rises substantially with 15 weight% or more filler loading due to reduced surface resistivity as seen before [83].
Polyethylene wax
Various solid fillers are used to improve properties of PSAs. But their incorporation results in large increase in viscosity of the adhesive which affects the formation of a good adhesive joint. So, polyethylene wax (an oligomeric hydrocarbon) can be used as an alternative to these fillers [84, 85].
Polyethylene wax filler can be added to blend of different polyisobutylene polymers by melt mixing. The solubility of wax in polymer of PSA does not exist at 25 °C, and solubility increases as temperature rises and the solubility is reversible; as the temperature decreases, the wax recrystallizes [84].
The wax performs dual function in the PSA; since wax crystallizes upon cooling resulting in formation of structures like dispersed solid filler, while the amorphous part has low viscosity and is soluble in the polymer hence acts as plasticizer by affecting the rheological properties of the PSA. So, PSA filled with 10% of the filler has lower viscosity as compared to unfilled adhesive as the wax dissolves in the polymer giving equal decrease in the storage as well as loss modulus which are almost equal for PSA without any wax. This leads to worsening of adhesion and cohesion strengths. PSA composite with 20% and more filler loading gives increase in viscosity due to structures formed by insoluble polyethylene wax. The storage modulus becomes higher than loss modulus. At 40% polyethylene wax addition, there is a threefold increase in the storage and loss modulus values. When the filler added is 60%, along with increase in the viscosity and both moduli, there is loss of tack. But when pressure is applied to a PSA composite with 60% polyethylene wax, the spatial structure of wax gets destroyed and the adhesive acts as a liquid, so it can still form adhesive bond [84].
Bio-based fillers
Cellulose nanocrystals
Cellulose nanocrystals (CNCs) can be extracted from wood pulp, cotton and other plant-based products by controlled acid hydrolysis using hydrochloric acid, sulfuric acid, etc. Several desirable properties such as high aspect ratio, biodegradability, low density, ease of chemical modification, non-toxicity, high strength, etc. lead to the use of CNCs as nanofillers in PSAs [86, 87].
As the CNC content in the adhesive increases, the cross linking of the polymer chains increases, there can also be graft polymerization of the filler (via hydroxyl groups on its surface) with the polymer chain. The cross linking of polymer chains also restricts the mobility of the adhesive, hence the Tg and the elasticity of the PSA increases slightly and the cohesive strength (shear strength) also rises with addition of CNC filler. Peel strength, shear strength and tack- all these properties improve as the filler content increases. The nanocrystals lead to rise in hydrophilicity of the PSA meaning decrease in water contact angle. This results in better tack [86].
CNC can also be added after it is modified by reaction with alkoxy silanes like 3-methacryloxypropyl trimethoxysilane (γ-MPS) as depicted in Fig. 15. This modification can improve the compatibility between CNCs and hydrophobic polymers. This functionalized CNC (fCNC) can be introduced in PSAs such as acrylic PSAs by similar procedure as CNC that is, by in situ emulsion polymerization. The fCNC-acrylic PSA shows more gel content and wetting capability, and hence has better peel strength and other adhesive properties as compared to CNC-acrylic PSA [87]. Table 10 shows various fillers explored for PSAs adhesives and their effect on various properties.
Schematic of grafting γ-MPS onto the surface of CNC [87]
Fillers in dental adhesives
Acid etching of tooth gives superficial decalcification of enamel and dentin. This leads to loss of tooth inorganic minerals causing formation of porosities. Dental adhesives are applied that infiltrate the porosities and the resin monomers of the adhesive take the place of the lost minerals. Then, the adhesive gets micromechanically retained (interlocked) in the porosities when cured in-situ. This process is referred to as hybridisation [88,89,90].
Apart from the adhesion of the dental adhesive on the tooth surface, the adhesive also requires to form bond with the overlying resin composite [88, 89]. So one function of dental adhesive is to act as intermediate and bond the resin composite fillings or composite cements to the tooth substrate [89, 91]. This means that how the composite resin performs is affected by its adhesion to the enamel and dentin achieved by the dental adhesive [92]. Further, dental adhesives withstand contraction stress exerted by the resin composite and also ensure there is no leakage from the restoration’s margins [89]. Decayed/fractured teeth reconstruction is another area where dental adhesives are utilized [90]. Dental adhesives also find application in other artistic and restorative dentistry for example in aesthetic role [92]. Adhesive restorative dentistry forms a part of many dental procedures such as bonding of orthodontic brackets, dental sealant replacement, direct composite restorations, root canal obturation, etc. [93].
Other than resin monomers, dental adhesives are composed of resin monomers, solvent, initiator, inhibitor and additionally fillers [88, 89, 94]. The resin monomers in the adhesive and resin composite are similar to obtain good bonding. Monomers in dental adhesives can either be cross-linking (resulting in adhesive with good strength and hydrophobic properties so that there is no water sorption by the cured adhesive) such as bisphenol A-glycidyl methacrylate and triethylene glycol dimethacrylate (TEGDMA); or functional (giving demineralization of the tooth and adhesion to calcium in hydroxyapatite (HAP)) like 4-methacryloxy-ethyl trimellitate anhydride, dipentaerythritol pentaacrylate monophosphate and 10-methacryloyloxydecyl dihydrogen phosphate [88].
Fillers are used in dental adhesives as a reinforcement to fortify its strength (elastic modulus, bond strength and bond durability) since they form a vital link between the tooth substrate and the composite [88, 89]. Fillers in dental adhesives lead to hybrid layer which has better hardness, less chances of degradation, less water sorption, dimensional changes become low and crack propagation also decreases [95, 96]. Fillers are added to the adhesives to modify their viscosities. The adhesive layer gets thickened when loaded with filler, which prevents over-thinning and gives more elasticity. The higher elasticity means that the shrinkage stress due to the overlying composite can be relieved in a better way [89, 94]. Incorporation of fillers can also be for specific properties such as therapeutic use, for instance better radiopacity, release of fluoride from the adhesive, antimicrobial properties, etc. [88, 89]. Silanization of fillers can give better bonding of filler with the adhesive resin, so the stress can be transmitted between the filler and resin efficiently. Silane coupling also prevents premature degradation of the dental adhesives [89].
The filler size and content are the parameters governing their addition to dental adhesives [94]. The filler particle size should be small enough so that they are able to infiltrate into the dentinal tubules and interfibrillar spaces of demineralised collagen along with the resin monomers [88, 94]. The filler content in dental adhesives should be low considering that high filler content leads to increase in viscosity and negatively affects surface wettability of the adhesive [89, 94]. High viscosity of the adhesive can prevent penetration of the resin and filler particles into the demineralised substrate. It can also influence the polymerization kinetics of the adhesive resin and thus the extent of curing of the adhesive [97]. Type and colloidal stability of the fillers in the adhesive are important parameters to be considered [92].
Various types of dental adhesives are: etch-and-rinse adhesives (where acid etching of enamel and dentin is followed by application of primer and the adhesive, either separately in two steps or in a single step as a mixture), self-etching adhesives (where there is no acid etching step, and self-etching primer and adhesive can be added separately or in a single step as self-etching adhesives- in the form of mixed/two bottle adhesives or unmixed/single bottle adhesives) and glass ionomer adhesives (where the adhesive consists of glass ionomer which is self-adhering, it is modifies by resin and added as two step etch-and-rinse adhesive) [88, 90, 93]. Figure 16 shows the diverse fillers explored in dental adhesives.
Inorganic fillers
Wollastonite
Wollastonite is a commonly used compound in dental applications on account of its biocompatibility, sufficient strength and less shrinkage properties [98]. Being a CaSiO3 based inorganic filler, wollastonite can release Ca2+ ions which leads to mineral precipitation and collagen remineralization, inducing apatite formation. This bio-interactivity favours adhesion of the dental adhesive containing wollastonite to dentin which improves bond durability and prevents biodegradation of dentin. Consequently, wollastonite is used as filler for protecting the tooth tissues substrate and develop therapeutic ability of the adhesive [99].
The wollastonite filler can be added to photocuring dental adhesives, and the dispersion consists of needle-like filler particles. Filled adhesives exhibit good microtensile bond strength even after one year of ageing. Mechanical properties for example ultimate tensile strength (UTS) improves from 34.9 MPa for unfilled adhesive to 46.0 MPa for adhesive with 2 weight% filler. [99]
Montmorillonite
Montmorillonite (MMT) which is a nanoclay having plate-shaped particles called platelets with high aspect ratios is used to improve mechanical properties of dental adhesives [91, 100, 101]. Sodium montmorillonite (Na-MMT) can be treated by graft polymerization of methyl methacrylate monomer [100], methacrylic acid monomers, [91] acrylic acid, [101]. etc. to modify the surface of the nanofiller, and then used in dental adhesives. Grafting introduces functional groups on the filler particles’ surface [91]. Interlayer spacing in the nanoclay filler increases after intercalation of PMMA chains in the clay. This is because the grafted PMMA chains prevent the clay layers from stacking together. Na-MMT and PMMA-grafted-nanoclay can be dispersed in the dental adhesive solution [100].
The elastic modulus of adhesive increases and so does its viscosity [100]. The effect of filler on Microshear bond strength is shown in Fig. 17.
Microshear bond strength of adhesives containing different percentages of PMMA-g-nanoclay [Reprint from Dental Materials, Vol 25, PMMA-grafted nanoclay as novel filler for dental adhesives, Mohammad Atai, Laleh Solhi, Azizollah Nodehi, Seyed Mojtaba Mirabedini, Shahin Kasraei, Khatereh Akbari, Samal Babanzadeh, pp. 344, 2009, with permission from Elsevier] [100]
Incorporation of poly (methacrylic acid)-grafted-nanoclay as reinforcing filler in dental adhesives also results in properties alike to grafting of PMMA on the nanoclay. There is improved bond strength and mechanical properties, and the colloidal stability of grafted nanoclay in the adhesive is higher as compared to unmodified filler particles. Better properties are obtained, namely diametral tensile strength, flexural modulus, flexural strength and microshear bond strength [91].
Similarly, poly (acrylic acid) (PAA) monomers can be grafted on Na-MMT particles. These modified particles can also be used as reinforcing filler in dental adhesives. As is the case in PMMA-grafted nanoclay, stacking of the filler layers is prevented by the grafted polymer chains. The dispersion of filler in the adhesive system is stable and sedimentation time rises due to the modification [101].
Niobium pentoxide
Niobium pentoxide with chemical formula Nb2O5 can be considered as a promising filler for dental adhesives. It is a material with bioactive properties such as hydroxyapatite crystal growth when it comes in contact with human saliva [95]. Its use can lead to deposition of phosphate layer over the adhesive resin surface that can stimulate mineralization of the tooth surface [102].
The filler in the adhesive exists as a single crystalline monoclinic phase resulting in it being chemically stable in oral environment. When used as a filler in the applied dental adhesive, Nb2O5 particles can diffuse into the hybrid layer and Knoop microhardness and radiopacity of the adhesive improve. Kinetics of polymerization of the resin monomers in the adhesive are also affected. With the addition of filler, the increased viscosity affects radical mobility thereby decreasing the chances of bi-radical termination. Also, the filler being a transition metal oxide acts as catalyst accelerating polymerization. Thus, the filler addition results in faster polymerization reactions of the monomers in the dental adhesives [102].
Tantalum oxide
Tantalum is biocompatible, radiopaque and has affinity to phosphate which brings about deposition of apatite on its surface. Tantalum oxide (Ta2O5) as a filler has inert and high oxidation state meaning it has very less toxicity and good chemical inertness, and apart from this, it results in bioactivity by inducing hydroxyapatite growth and osteoblasts attachments, has good fracture toughness and workability [103, 104]. It can be silanized by γ-methacryloxypropyl trimethoxysilane. The radiopacity of the dental adhesive increases with Ta2O5 content in the adhesive from 0 to 10 weight%. Since the filler can avoid crack propagation in the adhesive, it improves the ultimate tensile strength. 10 weight% loading of nanoparticles in the dental adhesive could give better resistance to hydrolytic degradation and good microtensile bond strength [103].
Silica
Silica as a filler can give better bonding and bond strength, less polymerization shrinkage and improved mechanical properties of dental adhesives. Silica particles may facilitate formation of calcium-phosphate precursors which are important for mineralization of the substrate [105]. It is an emulsifier resulting in stable dispersions. Since silica is hydrophilic, it can be subjected to surface treatment by a silane coupling agent for better interaction of the filler with the resin and other organic components of the adhesive [106]. This interaction also decreases the surface energy of the adhesive [107].
Viscosity of the dental adhesive increases with addition of silica filler [106]. Mechanical properties such as flexural strength and microtesile bond strength (µTBS) show improvement due to presence of silica nanoparticles [108]. For both these properties, as filler content in the adhesive rises, the property increases and then reduces [105, 108, 109].
Thermal sintering at about 1300 °C gives silica nanoparticles which are porous and so have lower surface area, consequently more filler loading in adhesives is possible and the surface porosity enables micromechanical interlocking between the filler particles and cured adhesive matrix. The sintered silica filler can be silanised using 3-(methacryloyloxy) propyl trimethoxy silane (γ-MPS). Diametral tensile strength of sintered nanosilica filled dental adhesive is similar to that of micro-sized silica filled adhesive. The elastic modulus, flexural modulus, flexural strength and fracture toughness of dental adhesives containing sintered silica is significantly higher than adhesive loaded with silica microparticles [110].
Inspite of resulting in stable dispersions and improvement in properties of dental adhesives, silica does not contribute to radiopacity or required refractive index of the adhesive. Mixed fillers like BaO/SiO2, Ta2O5/SiO2, Yb2O3/SiO2, YbF3/SiO2, etc. can be incorporated to achieve radiopacity along with desired properties and interaction with resin [111].
Nanoparticles-based fillers
Hydroxyapatite nanoparticles
Hydroxyapatite (HAp) is an inorganic compound with chemical formula Ca10(PO4)6(OH)2. HAp is a form of chemical phosphate found in teeth (it is an important constituent in enamel and dentin) and is very useful as a biocompatible material [92, 112, 113]. Hence HAp can be added in the form of nanoparticles to dental adhesives as reinforcing filler [92]. Incorporation of the filler as nanoparticles is advantageous over micro-sized filler because smaller particles can penetrate into the dentin tubules along with adhesive resin more easily to form composite adhesive [92, 114]. Growth of the HAp nanofiller in one direction yields nanorods which improves their mechanical properties [92].
The HAp nanorods have high colloidal stability in the dental adhesive even after significant amount of time [92]. But agglomeration of the nanoparticles can have undesired impact on the mechanical properties. [113] After photocuring of the adhesive, it is observed that 0.2 to 0.5 weight% loading of the filler in dental adhesive gives enhances the diametral tensile strength which is maximum (34 MPa) at 0.2 weight% and then decreases as more filler is added. The steep decline in strength after initial increase can be due to presence of filler agglomerates and incomplete curing of the adhesive owing to the opacity of filler to irradiations of photocuring system. Other mechanical properties of the adhesive such as flexural strength and microtensile bond strength show similar trend [92, 113].
Thus, dental adhesives with low amounts of HAp nanorods (0.2–0.5 weight%) can lead to improved mechanical properties and microshear bond strength [88, 92]. A filler is said to be bioactive if it results in a biological response at the adhesive-tooth interface giving formation of bond between the adhesive and dental tissues [115]. The HAp filler exhibits high bioactivity, that is, the filler particles interact with the tooth’s collagen and the decayed dentin at the adhesive-dentin interface gets remineralized [88, 92, 112]. This means that HAp can be an important filler in mineral releasing dental adhesives [113]. HAp nanoparticles can be silanised by reacting with γ- methacryloxypropyl trimethoxysilane to obtain good adhesion interface with the adhesive resin matrix [114]. Quaternary polyethyleneimine-hydroxyapatite (QPEI/HAp) nanofiller can also be included as filler in dental adhesives [115].
Zirconia nanoparticles
Zirconia-based materials are commonly used ceramics in dental adhesives apart from alumina, leucite, and silica-based fillers. Zirconia/ zirconium dioxide (ZrO2) is biocompatible; has good fatigue resistance, toughness, strength and excellent wear properties. Hence it can be used in dental applications [116]. Spherical zirconia nanoparticles can be incorporated as filler in dental adhesives such as 3 step etch-and-rinse adhesive and a dispersion of the filler in the resin can be obtained. The filler acts as a reinforcement to the dental adhesive improving its tensile strength [117]. The dentin-adhesive bond gets stronger due to the presence of zirconia [97]. But ZrO2 particles have high values of hardness and this can result in antagonist wear and fatigue wear. Zirconia-silica hybrid nanofibers can also be used as filler in dental adhesives to get improved properties [112].
Gold nanoparticles
Gold nanoparticles can be incorporated as fillers in dental adhesives in the form of colloid because of high chemical stability and inertness as well as biocompatibility and antibacterial and antifungal properties [118, 119]. They can also heal bone defects since they can enhance bone regeneration [119]. As the amount of nanoparticles in the adhesive increases, its flexural strength increases since at higher concentration the nanoparticles prevent crack growth, diametral tensile strength also improves upto some concentration but then reduces as a result of agglomeration of filler particles where cracks can initiate [118].
Boron nitride nanotubes
Considering highly stable boron-nitrogen bond; their bioactivity (they can induce mineral deposition), good physicochemical and mechanical properties, boron nitride nanotubes (BNNTs) are nanofillers for dental adhesives. Also, they have fibrillar nature and are hydrophobic, hence avoid water on demineralized collagen which prevents hydrolytic softening of the adhesive. This can lead to more durable bonding of adhesive on tooth substrate [120, 121]. Solvent resistance of adhesives increase due to presence of BNNTs in them [121]. Increment in degree of conversion, polymerization rate and microtensile bond strength is also observed [120].
Glass fillers
Pre-reacted glass-ionomer
Pre-reacted glass-ionomer (PRG) is a filler that can be used in dental adhesives. The filler can be obtained by carrying out acid–base reaction of polyalkenoic acids (such as polyacrylic acid) and glasses comprising of fluoride (such as fluoroaluminosilicate glasses (FASG)) in presence of water. When the glass-ionomer particle fully reacts with the acid, full pre-reacted glass-ionomers (F-PRG) are obtained while reaction of only the surface of particles give rise to surface pre-reacted glass-ionomers (S-PRG) [122,123,124]. Wet siliceous gel is produced which is desiccated to form xero-gel which can be milled. After silanization process, PRG filler is obtained [122]. When the dental adhesive has 17 weight% of PRG, the adhesive adheres well to the enamel or dentin substrate. Fluoride is liberated from the adhesive which reaches the dentin through the interface between adhesive and substrate [124]. Calcium type glass filler (PRG-Ca) can be incorporated in light-cured dental adhesives. Fluoride released from the adhesives increase with increasing filler content in them. The polymerization of resin monomers in the adhesives is not affected significantly due to the presence of PRG-Ca particles, and good copolymerization of the monomers can be expected. But more PRG-Ca addition does result in a decrease of energy of polymerization. Considering accelerated ageing, the shear bond strength (SBS) values of adhesives containing filler loading between 7 and 27 weight% are similar [122].
Bioactive glass
Bioactive glass (BAG) filler can be synthesized by utilizing alkoxides, calcium methoxy ethoxide and triethyl phosphate. After air drying, the solvent is completely evaporated by heating to high temperatures and stable glass filler is obtained. The composition of BAG is about 65 mol% SiO2, 31 mol% CaO and 4 mol% P2O5. The filler can remineralize dental tissues present adjacent to it. It releases calcium and phosphate ions that have harmful effect on bacteria and cause neutralization of the acidic environment by increasing the pH at which oral bacteria are not able to survive. For these reasons, bioactive glass is a potential filler for dental adhesives [125].
Phosphate-based glass is another example of filler for dental adhesive. They are bioactive hence their inclusion leads to remineralisation of the tooth by releasing calcium and phosphate ions. Also, this type of glass filler containing adhesives can be subjected to controlled degradation based on their composition. They can also be doped by metal oxides like copper oxide for antibacterial action. Copper-doped phosphate glass filler can be added to acrylic-based dental adhesives as microparticles. High weight% of the filler increases the degree of conversion in the adhesive, but the particle size should not be closer to the wavelength of curing light, else it would yield low degree of conversion. Another property surface roughness of the adhesive is not affected much [126].
Fillers in construction adhesives & adhesive joints
Construction adhesives find a variety of uses in construction industry which include improving strength of concrete and metal structures; and bonding composites to existing structures [127]. Adhesives can bond to several types of substrates such as ceramics, polymers, metals and composites [128]. Mechanical and shear properties of the adhesives need to be considered for these applications [127]. Anchors are used in construction structures and can be installed using post-installed method. Here adhesives are used to act as a bond between the anchor and concrete. Steel bars in the holes of concrete are held to the concrete using adhesives. Epoxy adhesives are economical and exhibit good adhesion strength to concrete as well as steel, hence are commonly used [129]. But low mechanical properties such as shear properties means fillers are required to be incorporated in the adhesives to yield composites having improved mechanical properties [127]. Another application of epoxy adhesives is bonding of carbon fibre reinforced polymer composites to steel structures for improving strength [130]. Glasses are used in different structural applications including overhead, safety and walkable glazing. These glasses can be included in buildings using adhesives by point fixed glazing. Fillers are required in these adhesives to reduce costs, obtain better rigidity, decrease shrinkage of the adhesive during its curing, increase operating temperature range of the adhesive [131]. Alumina, carbon black, carbon nanotubes, graphene, lime, metal particles, nanoclay, talc are some fillers that can be included in adhesives for construction applications [127, 128, 131]. Type, size and properties of the filler are important parameters to be considered as they affect various properties including bond strength and bond durability of resulting adhesive [132].
Adhesive joints can be considered as an alternative to methods of forming structural joints like mechanical fastening, soldering and welding [128, 133]. Adhesive joints constitute ease of joining materials, lighter structures, neat and simple designs, less points where stress is concentrated, superior resistance to fatigue, corrosion and environment [133, 134].
Carbon fibre/epoxy (CFE) composites used in several applications has less joint ability to other composites and metals, hence the use of adhesives for bonding is preferable [135]. Fillers can be utilized in adhesive joints as well. Photo-Activated Adhesive Work-holding (PAAW) with fillers are used to support work-pieces. UV curable adhesives can be filled with glass fibres, glass spheres, etc. (which could be micro-sized to nano-sized) to reduce volumetric shrinkage [136]. Other fillers like alumina and silica also find use in adhesive joints considering their high mechanical and thermal stability [135].
Inorganic fillers
Silica
Epoxy adhesives containing silica (SiO2) filler can be prepared using silica microparticles or nanoparticles. The filled epoxy adhesive can be employed in anchoring systems. This can be done by filling the spaces between concrete and steel rods by adhesives and letting it cure on its own [129]. Microsilica (MS) filled epoxy adhesives can also be used to form glued joints [137].
Microparticles of the filler added can be of a single size such as 25 µm, 50 µm etc., or it can be as a hybrid of different sizes like 25 µm and 50 µm particles can be used together in the adhesive. The flexural modulus and tensile modulus of the adhesive improve, while the flexural strength and tensile strength decrease as a result of filler addition to the adhesive. Smaller particle size of filler (that is, greater surface area) means more interfacial interaction between filler particles and epoxy matrix, and this results in higher compressive modulus, compressive strength and pull-out strength. Hybrid fillers with various particle sizes gives some improvement in these properties. Additionally, tensile strength and modulus; and flexural properties show higher values when hybrid filler is used [129].
Spherical silica nanoparticles can be added to epoxy adhesives for lap-shear joints involving stainless steel substrates. For cross linking of the filler with the polymer and to prevent formation of silica agglomerates, the surface of silica particles is modified. The tensile strength of lap-shear joints increased by the use of filler but at higher filler contents of 40 weight%, the wettability of steel by the adhesive declines as a result of increased viscosity. The silica nanoparticles whose surfaces have been modified with hydroxyl groups absorb water molecules on their surface during hygrothermal treatment. These water molecules help to form bridges between epoxy polymer chains improving the storage modulus of adhesive joint. Hence, there are benefits of utilizing silica as filler even after hygrothermal treatment [138].
Silica nanoparticles can even be added to microsilica containing adhesives but this results in decrease of mechanical properties like compressive, flexural and tensile strength and even adhesive properties like pull-out strength. The reason is that OH groups on the filler particles’ surface become so excess which make the filler incompatible with the epoxy matrix. Silanization of the nanoparticles could improve the effect of nanofiller on properties of the adhesive [129].
Alumina
Alumina nanoparticles (Al2O3) have use in structural adhesives to improve properties of adhesive joints (such as bond strength) and give more resistant joints [128, 134]. The filled adhesive can be used in a metal-to-metal (aluminium-to-aluminium) single lap joint. There is improvement in peel strength when alumina is added to the adhesive. The maximum shear strain is also affected by filler amount, and its value rises as filler content in adhesive increases from 0 to 0.5 weight%, then for the range of 0.5 to 1.5 weight% filler there is a negative correlation with the shear strain. The adhesion properties of the adhesive depend on the efficiency of stress transfer between the polymer matrix and alumina nanoparticles. Elastic modulus of the adhesive increases up to 1.5 weight% filler loading after which there is a slight reduction in the property [134]. Overall, as a result of the nanofiller addition to epoxy adhesives, the strength of adhesive joint is enhanced [128]. In another case, nanoparticles in the form of rods or spheres were fillers for epoxy adhesive forming a single lap joint of two aluminium alloy surfaces. The shear strength of flat tensile (FT) joints improved by loading of nanospheres as well as nanorods for 0.5 to 2.0 weight% filler content. Neat epoxy has shear strength of 4.39 MPa, nanorods gave maximum shear strength of 6.83 MPa at 1.0 weight% loading while the highest value when the filler was alumina nanospheres was 6.68 MPa at 1.5 weight% content. The betterment in in shear strength is due to better stress transfer in presence of filler between the alumina and polymer [139].
Limestone
Mineral fillers are important in tile adhesives. Carbonate based fillers obtained from limestone, marble, etc. and silica fillers produced from enriched quartz sand are the main types of mineral fillers incorporated. With increasing limestone content in epoxy-based adhesive, its bulk density decreases from 1440 kg/m3 at 2.5 weight% filler content to 1370 kg/m3 at 10weight% limestone in epoxy adhesive. The water retention of adhesive doesn’t change significantly due to presence of filler or the amount of filler added. Initial adhesive strength and adhesion strength after heat ageing also improve while the adhesive strength after water immersion reduces for fine-sized limestone containing adhesive. Apart from that, slip of ceramic tile from the concrete substrate rises when adhesive is loaded with limestone. There is a decrease in flexural strength of the adhesive on addition of limestone powder as filler [140].
Iron powder
Fillers in the form of powders can be added to epoxy-based adhesives used for aluminium bonding [141, 142]. Iron ore powder with Fe percentage of 80% and particle size being in the range of 100–200 µm is one filler that can be used. The iron powder can increase resistance of the adhesive joint to crack propagation. At low filler volume fraction in the adhesive, the mode of failure is cohesive fracture while it is adhesive fracture at higher filler volume fractions. The peel strength and shear strength are highest at 30% volume fraction of iron; with values of 2.48 MPa and 9.38 MPa, respectively. Lowest values of these properties can be at volume fraction 60%; with peel strength being 0.43 MPa and shear strength being 0.23 MPa. Overall, the shear strength of the adhesive joint is higher than its peel strength as shown in Fig. 18 [141]. Use of iron powder in epoxy glues for aluminium joints does not lead to much electrical conductivity of the adhesive joint [142].
Variation in shear and peel strength with filler volume fraction [141]
Titanium dioxide
Titanium dioxide (TiO2) nanoparticles is a filler for epoxy-based adhesive which has application in forming adhesive joints of copper substrate. A single lap joint of copper surfaces (either chemically or mechanically treated before use) can be formed using the filled adhesive. When chemically treated substrates are used, wettability of adhesive containing 10 weight% TiO2 filler has better wettability as compared to pure epoxy adhesive. Greater toughness of adhesive joint is obtained by the use of TiO2 nanoparticles [143].
The viscosity of adhesive becomes higher as filler is added and hence its bond line thickness in the joint also increases, with impact on the bond line thickness being significant beyond 10 weight% filler contents. The lap shear strength of the joint is affected by the bond line thickness. It increases significantly as filler concentration rises to 10 weight%, then it begins to show a decrease with further increase in the amount of filler to 15 weights%. The initial increase in joint strength till 10 weight% TiO2 content may be due to cohesive fracture resistance provided by the TiO2 particles. But the subsequent decrease in strength with filler content rising to 15 weight% maybe because the inter particulate distance between filler particles decreases leading to fast interaction of stress generated around the particles [143].
TiO2 can also be utilized as filler in acrylic adhesives for binding of steel and glass/epoxy composite beams. When TiO2 is added to a 2-component acrylic structural adhesive and the adhesive used to join steel and composite structures, the shear strength and tensile strength of the joints improve by 47% and 59%, respectively, from the values for neat epoxy for 3 weight% filler content in the adhesive. The betterment in these properties could be a result of efficient stress transfer between filler and the resin. Beyond 3 weight% of nanoparticles, these properties decline as a result of increased viscosity not allowing the filler to be distributed uniformly (leading to agglomeration of the particles), and poor interaction of TiO2 particles with the polymer (causing decline in matrix infiltration by nanoparticles). The nanoparticles restrict mobility of the polymer chains and hence the use of fillers decreases peel strength of the adhesive [144].
Solder powder
63/37 Sn–Pb soft solder powder is a filler for epoxy adhesives included to increase the mechanical strength of steel-to-steel joints. The adhesive joint strength is higher when solder powder is used as filler in the adhesive when compared to aluminium powder. This could be due to different particle shapes of the two fillers. The aluminium particles are spherical, so during the tensile shear tests the particle surface can easily get separated from the adhesive. On the other hand, non-uniform shape of solder particles causes clamping with the adhesive and delayed separation from adhesive. But the strength of the joint reduces when the solder content in adhesive exceeds 5 weight% [145].
Zirconium oxide
Adhesive composites for forming adhesive joints of aluminium sheets can have zirconium oxide (ZrO2) nanoparticles as filler. The filler particles can be dispersed in epoxy adhesives by mechanical mixing process. The filled adhesives are used to bond aluminium surfaces by forming lap adhesive joints. ZrO2 has similar effects on the adhesive properties as TiO2. Amount of ZrO2 has an influence on the adhesive viscosity and thus also the bond line thickness. Both these properties increase on more addition of filler. Joint strength and lap shear strength show similar variation in properties. For a particular bond line thickness, the adhesive joint strength improves as filler content in adhesive increases from 10 to 15 weight% and then decreases as the amount of filler added increases to 20 weight%. The increase in strength is caused by the positive influence of ZrO2 particles on resistance of the joint to fracture and the ensuing fall in strength results from filler particle aggregation giving adverse interactions between the particles [146].
Carbon-based fillers
Carbon nanotubes
The failure mode of carbon fibre reinforced polymer (CFRP) composites attached to steel structures depends on the epoxy adhesive used for bonding the two by double strap joints or single shear lap joints. The epoxy adhesives can be filled by multi walled carbon nanotubes (MWCNTs) and used for double strap joints. The tensile strength and Young’s modulus increase when nanotubes are uniformly dispersed in the epoxy matrix. The stiffness of joints bonded with epoxy adhesive having CNT content is similar to joint bonded with unfilled epoxy adhesive. The bond strength of filled adhesive is greater than that of pure adhesive because the effective bond length of joints is shorter for adhesive containing nanotubes. Ultimate axial strain along the CFRP laminates is also higher for joints where the adhesive used is filled with MWCNTs [130].
Graphene nanoplatelets
Graphene nanoplatelets (GNPs) can be used as reinforcement for epoxy-based construction adhesives [127]. They have large specific surface area, good Young’s modulus and high ultimate tensile strength [128]. The shear strength of adhesive rises with increasing amount of nanofiller. Other shear properties like shear modulus, shear strain at failure and toughness also show similar improvement as more GNPs are added. Hence, nanocomposites with graphene filler result in better mechanical properties of construction adhesives [127].
Fillers in automotive adhesives
In the automotive industry, adhesive bonded items are being utilized in place of spot-welded parts considering welding of coated and galvanized sheets is difficult. Adhesives can also separate galvanized and non-galvanized steel components in automotive bodies which can avoid galvanic corrosion. These structural adhesives are mostly epoxy-based. Epoxy adhesives have the ability to bond to various metal surfaces and have low shrinkage on curing [147]. Epoxy-foam adhesives can be inserted between different parts where foaming can fill the gap. Calcium carbonate is a filler which can be added to these epoxy-foam adhesives [148]. Structural bonding tapes are a type of structural adhesives which can be used for bonding the rear-view mirror buttons, sensor brackets and other hardware with automotive glass. Fillers affect the mechanical properties and curing of epoxy-based adhesives used in these applications [149]. Higher rigidity of most filler particles as compared to epoxy resin in the adhesive results in better modulus of the adhesive joint by addition of filler. The microhardness of a dried, cured adhesive bonded joint is influenced by the composition, type and amount of filler used in the adhesive and the extent of dispersion of the filler in the adhesive. Interfacial bonding of filler and resin and the shear degradation of adhesive that takes place when fillers are added are other factors which affect the hardness value [150]. Apart from that, when automotive epoxy adhesives are used to bond steel substrates, more the filler content of adhesive, less is the extent of adhesive delamination. Calcium oxide (CaO), calcium carbonate (CaCO3), calcium silicate (CaSiO4), magnesium aluminium silicate (MgAlSiO4), silicon dioxide (SiO2), talc clay are some fillers that are explored for such applications [151]. Nano-fillers including nano-Al2O3, nano-CaCO3, carbon nanotubes, nano-SiO2 are also important fillers to increase strength of epoxy based adhesive joints owing to their large surface areas and high value of elastic moduli [152].
Inorganic fillers
Glass flakes and wollastonite
1-D type microfillers like glass flakes and 2-D type microfillers such as wollastonite can be utilized in structural bonding tapes. The structural adhesive can be composed of epoxy resin and epoxy acrylate copolymers. The filled adhesive composite can then be applied on silicone paper or polyester film, UV-cured and used for forming adhesive joint of steel or aluminium substrates. When either glass flakes or wollastonite particles are included as fillers in the structural adhesive tapes (SATs), the properties tack and adhesion to steel mostly deteriorate. This is due to decreased molecular mobility of polymer chains after UV curing for microfiller containing adhesive which negatively affects wetting of steel surface. Along with that, the presence of filler particles causes contact area between adhesive and substrate to decrease. Conversion of epoxy groups during heat curing at 140 °C for 15 min is also lower for filled adhesives when compared with pure adhesives [149].
Considering mechanical properties of aluminium joints using the structural adhesive tapes, the overlap shear strength is lower when adhesive used is filled with glass flakes or wollastonite microparticles. But the microfiller gets uniformly dispersed in the adhesive and formation of cracks that is, delamination between the polymer matrix and filler particles isn’t observed [149].
Epoxy-based adhesives with high lap-shear tensile strengths can be used to bond automotive coach bodies, for more durability of cars during operations, etc. Micro-sized glass powders are one type of fillers that can be loaded in these adhesives also. The major reason of using glass microparticles is to reduce costs. Though the use of glass powders in the adhesives can lead to decreased shear strengths, at lower loading of about 5–10 volume%, the shear strength is not affected significantly. Depending on the application, the filled adhesives can be used where high strength is not necessary. The adhesive becomes more resistant to wear as a result of adding glass powder leading to their application in forming resistant films over machines [153].
In automotive, different joints such as aluminium and aluminium alloy joints can be bonded by adhesives and these can also be used in assembly of supplementary items like windows and windscreens. Glass soda-lime powder is a possible filler in adhesive joints of aluminium alloys. When 140 μm sized glass particles are added to the adhesives, adhesives with 10% volume fraction of filler showed 9.77% increase in tensile joint strength as compared to unfilled adhesive. The improvement decreases as more glass powder is added since higher loading of glass particles in the matrix cause adhesive failure on the bonded area. 15% and 20% volume fraction of filler containing adhesives lead to less increment in the joint strength while adhesive with 25% volume fraction exhibits lesser strength than unfilled adhesive [154].
Carbon-based fillers
Carbon filler
Inspite of various advantages, epoxy-based adhesives have poor adhesion and cohesive strength when subjected to humid conditions [152]. Adhesive bonded joints can get affected as a result of such environmental exposure (moisture). The water on reaching a joint can have negative outcome on physical and chemical properties of the joint [147]. Working conditions of adhesive joints can be wet conditions or high temperatures where the joints undergo hygrothermal ageing. This results in unfavourable changes of physical properties and strength of the adhesive affecting its performance [152].
To minimize the effect of moisture absorption, carbon nanofillers such as carbon nanotubes (CNTs), carbon nanohorns (CNHs), graphene nanoplatelets (GNPs) are added to reinforce epoxy adhesives. The resulting adhesive can be employed in forming adhesive joints. Carbon nanofillers increase decomposition temperature of the adhesive, with GNP showing most increase that is, high thermal stability. Even 0.5 weight% of any carbon nanofiller gives improved lap shear strength of adhesive joints which is shown in Table 11. Ageing performance of adhesive with GNPs is the best followed by adhesive with CNT and CNH fillers. The reason is that unlike porous particles of CNT and CNH, graphite nanoparticles are plate-like which prevents diffusion of water to a greater extent; so water absorption of GNP filled adhesive is the least. Also, GNP has better strengthening efficiency leading to more swelling resistance and reduction in strain at interface. All carbon nanofillers used in the adhesive leads to satisfactory Weibull modulus after ageing indicating good performance of adhesive joint along with lower degradation as against pure adhesive [152].
Carbon nanotubes as filler in epoxy adhesives result in increased strength of adhesive joints, and good electrical conductivity which rises significantly after amount of CNTs in the adhesive exceeds the percolation threshold value. Considering that CNTs have large aspect ratios, they tend to agglomerate and are expensive. Carbon black (CB) as filler has benefits as it is cheaper and its ellipsoid particles give better dispersion in the polymer matrix though they do not contribute much to the electrical conductivity or mechanical properties of the adhesive. A hybrid filler consisting of both CNTs and CB can balance properties and cost. CNT/CB dual filler can be added as a dispersion to epoxy adhesives employed for forming adhesive joints in automotive parts. An example is the use of such adhesives in SUV hatchback which has an inner panel of steel, outer panel of CFRP laminate and some load bearing components. The inner and outer panels can be bonded using these adhesives [155].
For a particular amount of total filler content, the resulting properties of the adhesive will depend on the proportions of the two fillers. Carbon black particles and carbon nanotubes get properly blended when observed at primary particles size leading to creation of a co-supporting network of both the fillers. Proper dispersion of CNTs and CB fillers give a collective outcome and electrical conductivity of hybrid filler containing adhesive is higher than adhesive with individual fillers. Mechanical properties of the joint such as tensile strength and elastic modulus increase when dual filler is included in the adhesive. A car hatchback may be subjected to some static as well as dynamic loads. Considering static performance, torsion and bending are important properties for a hatchback; and the use of CNTs and CB lead to better torsion and bending stiffness. Maximum intrusion which is one indicator of resistance to crashes, can be evaluated for dynamic performance. When adhesive with hybrid filler is used, there is very little effect on the intrusion of hatchback as against unfilled epoxy adhesive [155]. Table 12 enlists the values of various properties of adhesive without filler and after adding 2 weight% of filler (wither CNTs or CBs or CNTs and CBs both in the ratio 1:3).
Fillers for adhesives in aircraft and aeronautics
Adhesives are increasingly being utilized in aviation industry [156]. In aircraft structures, joints are very critical where application of adhesive bonding is increasing [157]. Thin-walled composite structures can be joined by adhesive bonding as well [158]. Manufacturing of aircraft fuselage and wing structures employ structural adhesive bonding [159]. Patch repairs of aircraft also uses adhesive bonding [157]. Adhesives are advantageous in aircrafts considering their light weight, ability to yield aerodynamically smoother surfaces, sealing property, cost effectiveness, ability to prevent galvanic corrosion, avoidance of stress concentration, resistance to fatigue, etc. [156,157,158]. Cyanoacrylates, epoxies, phenolics, polyurethanes, toughened acrylics, silicones are some resins for adhesive manufacturing [157, 159]. Of these, epoxy-based adhesives are high performance structural adhesives commonly used in aero-structures. This is because, in aircrafts, smooth and evenly stressed bonding of aluminium sheets is required and epoxy-based adhesives have affinity for aluminium alloy surfaces [133]. Manufacturing of aircraft cabin materials can require fillers in the adhesives [156]. Adhesive prepregs used in aircraft construction are composed of epoxy resin containing fabric fillers like glass and fibre fabrics [160].
Carbon-based fillers
Graphene oxide
Bonding property of structural epoxy adhesive for aircraft depends on shear strength and T peel strength. Graphene oxide (GO) can improve mechanical properties of epoxy adhesive but it has less compatibility with the polymer matrix so it can be modified by grafting compounds on its surface for better interface between the filler and epoxy matrix. One type of modification is by treating graphene oxide with toluene diisocyanate (TDI) initially and then grafting amino terminated poly(butadiene-acrylonitrile) (ATBN) to obtain TDI-GO grafts and ATBN-TDI-GO grafts [161].
Carbon nanofiller
Nanofilled adhesives for aeronautic materials are being increasingly used. Vapour-grown carbon nanofibers (CNFs) and exfoliated graphite (EG) are carbon nanofillers that can be included in epoxy adhesives. Tensile butt joint and single lap joint can be formed using the adhesives. Nano-sized filler results in strengthening of adhesive joint resistance. Young’s modulus of the adhesive has positive correlation with its filler content. On the contrary, tensile strength values decrease as filler loading in the adhesive increases. Significantly high bond strength of adhesive joints can be achieved by using nanoparticles. High amount of filler reinforcement has negative effect on bonded joints which may be due to aggregation of the nanoparticles and their improper dispersion in epoxy matrix [162].
Carbon nanotubes (CNTs) can be incorporated as fillers in aerospace epoxy based structural adhesives [163, 164]. The large aspect ratio of CNTs implies that they overcome percolation threshold value for electrical conductivity at small volume fractions. So significant electrical conductivity of the adhesives is obtained with little impact of the presence of filler on its structural properties. This leads to the use of CNTs as fillers to obtain adhesives for aircrafts showcasing both electrical conductivity and structural properties. SWCNTs are one type of nanotubes loaded into aerospace type epoxy adhesives. A dispersion of SWCNTs in solvents like tetrahydrofuran (THF) or acetone is mixed with epoxy resin. The obtained electrical conductivity of the adhesives increases as the weight% of SWCNTs in them increases. Ultimate tensile strength (UTS) is higher for 0.5 and 1.0 weight% filler content as compared to unfilled adhesive, but UTS decreases for 2 weight% filled adhesives. 1weight% of SWCNTs gives enhanced peel strength but some decline is seen in lap shear strength of the adhesive joint [163]. Another class of nanotubes incorporated in adhesives for aircrafts are MWCNTs. Adhesion efficiency increases as a result of presence of MWCNTs in the adhesive due to delay in shear failure. Even the galvanic behaviour of adhesive is affected as the CNTs decrease rate of corrosion. This means CNTs result in corrosion control suggesting their use in adhesives to form reinforced composites for patch repair of aircrafts. Hence CNTs are a promising filler which can be added to adhesives for structural repair of aircrafts [164].
Miscellaneous examples of fillers in adhesives
CNT/SP1
Toughening of epoxy adhesives can be achieved by incorporating nanoparticles which can also increase the stiffness of the adhesive. Carbon nanotubes (CNTs) is an example of nanofiller with high mechanical properties. But poor dispersion of the CNTs significant improvement in properties is not observed. Grafting of large molecules such as proteins on the nanotubes’ surface may prevent agglomeration and result in a better dispersion of the filler in epoxy matrix. SP1 is a highly stable protein which self assembles to form a ring shape dodecamer of size 11 nm. Using genetic engineering, the SP1 can be tailored to fuse specific peptides of CNTs to their N terminus. This results 12 CNT binding sites per SP1 ring. A stable CNT/SP1 complex is obtained. After addition of the filler followed by curing, single walled CNTs (SWCNTs) get effectively dispersed in the SP1 [165].
High energy is required to break adhesive filled with CNT/SP1. Adhesive reinforced with only SP1 and no CNTs shows only slight improvement in adhesive strength while adhesive with even less than 1 weight% of CNT/SP1 filler content shows much improvement in shear strength, T-peel strength and tensile adhesion strengths that is, overall adhesion strength. The adhesive hardness also shows increment [165]. Peel strength of epoxy-based adhesive filled with MWCNT/SP1 peptides on aluminium substrate is 48% more than that of pure unfilled epoxy adhesives on aluminium [166]. The filler leads to higher glass transition temperature (Tg) of the adhesive with an increase of 2 °C in Tg value when CNT/SP1 loading is 0.7 weight%. Electrical conductivity of 0.35 weight% CNT/SP1 containing adhesive is better than adhesive with 0.7 weight% of filler. This may be because the CNTs are able to disperse more at lower concentrations [165].
Nano-sized SiO2
The water-borne polyurethane (WPU) adhesives are preferred over solvent-borne PU adhesives to be applied on non-polar polyolefin films like polyethylene (PE) laminated soft packaging of items like food and medicine. A hybrid emulsion of fluorine acrylics with water-borne PU’s give fluorinated water-borne polyurethane (FWPU) hybrid emulsion which has advantages over WPU of better wettability of lower surface energy surfaces and greater surface properties of the polyurethanes [167].
SiO2 whose surface is modified using silane coupling agents such as 3-aminopropyl triethoxysilane (APTES) is often added to FWPU adhesive. The alkoxy group of silane reacts with silanol groups present on SiO2 surface while its functional groups interact with –NCO groups of polyurethane. This linkages between nano-SiO2 and PU chains result in increase of mechanical properties and heat and radiation resistances [167].
Nano-SiO2/fluorinated waterborne polyurethane (SiO2/FWPU) nanocomposite emulsions can be reinforced with 2,2,3,4,4,4-hexafluorobutyl methacrylate (HFBMA) filler. The resulting nanocomposite can be cast on PTFE mould for obtaining nanocomposite films. HFBMA leads to decreased surface tension and better wetting of the nonpolar polyolefin films. Improvement in wettability between adhesive and polyolefin film means adhesion strength of the adhesive increases with increasing HFBMA filler content. Water swelling of SiO2/FWPU nanocomposite films decreases significantly due to HFBMA inclusion, so water resistance improves [167].
Application of polyurethane adhesives in footwear industry includes joining styrene-butadiene-rubbers (SBR). For achieving better adhesion of PUs on the rubbers, the rubber surface can be chlorinated [168].Fumed silica comprising of spherical nanoparticles is a filler which is utilized in the PU adhesives though they are expensive [169, 170]. The filler is hydrophilic considering presence of silanol groups on particles’ surface. Another advantage of using fumed silica as filler here is that these silanol groups and the urethane groups of PU can give hydrogen bonding [168, 169].
For one type of samples, the total solid content of the adhesive is kept constant at about 18 weight%. So with increase in the amount of silica (viscosity of adhesive rises), the PU content of adhesive is decreased (leading to decrease in viscosity). Net effect is that the Brookfield viscosity at 25 °C is not affected significantly by changing amounts of silica and PU. The viscosity at 25 °C when measured after a month is almost same as the initial values meaning that silica doesn’t change the thixotropy of adhesive with time. For another sample type where the PU content is same for all adhesives, the viscosity becomes higher as filler is loaded in the adhesive. Also, the Brookfield viscosity at 25 °C after a month shows an increment of about 1 Pa⋅s. The effect of silica on thixotropic properties of adhesive depends on extent of its dispersion in the adhesive [168].
The presence of silica in the adhesive results in smaller contact angle of polar as well as nonpolar liquids with the adhesive films and thus better wettability by the film giving better adhesion. Also, the resistance of PU in the adhesive to degradation by the chlorine present in chlorinated rubber is enhanced due to silica filler [168].
Precipitated calcium carbonate
Some areas where thermoplastic polyurethane adhesives are used include footwear and textile industry. Calcium carbonate is a commonly included filler in adhesives with an aim to reduce costs, use less colour additives, get better initial adhesion and ageing resistance. Nanoparticles of precipitated calcium carbonate (PCC) can be added to thermoplastic polyurethane adhesives [170, 171]. At higher amount of filler addition to the adhesive, there is formation of clusters of the filler nanoparticles, and length of the filler clusters increases as more PCC is loaded in the adhesive. Considering that there is not much compatibility between PCC particles and PU, the filler nanoparticles migrate to the surface of PU negatively affecting its wettability [169].
The solid content and viscosity of adhesive become higher when PCC filler is present. Storage and loss modulus values increase as calcium carbonate content in the adhesive increases. There is improvement in the immediate peel strength value of the adhesive. The nanofiller causes glass transition temperatures to rise. This is because, though interaction between PCC filler and polyurethane matrix is weak, van der Waals interaction between the filler particles and polymer chains favours separation of soft and hard segments. Apart from that, the nanoparticles interfere in interaction between polyurethane chains leading to increased phase separation. Hence the melting enthalpy and crystallinity of PU decrease by addition of filler [169, 171]. The weak interactions between PCC and PU also mean that elastic modulus becomes only slightly better [171].
Conclusion
Adhesives are used to join substrates by the mechanism of adhesion and they can be utilized in various fields. Several categories of adhesives have been discussed in this study; namely wood adhesives, electrically conductive adhesives, thermally conductive adhesives, pressure sensitive adhesives, dental adhesives, adhesives in construction industry, adhesives in automotive and aircraft applications etc. Fillers, which are solid and non-adhesive substances are additives for adhesives which serve several purposes including lowering of adhesive cost; enhancing mechanical properties for instance shear strength, tensile modulus and flexural strength; preventing over penetration of adhesives in the substrates. The fillers can also be included for achieving specific properties, such as conductivity in case of thermal or electrically conductive adhesive or to achieve remineralization of the decayed dentin substrate in dental adhesives. Different types of fillers are covered in this work- metallic, ceramic, bio-based, carbon-based, silicon-based to name a few. More than one type of filler can also be added to the adhesive known as hybrid fillers. Modification of the fillers can be done for better compatibility of the filler with polymer of the adhesive to obtain their effective dispersion in the polymer matrix. Silanization of the fillers can preferentially modify the filler particles. An important observation regarding the effect of filler content in the adhesive on its properties is that in most cases, the properties initially improve with increasing amount of filler. Then after attaining a maximum value, with further addition of filler to the adhesive, the properties deteriorate. So, the concentration of filler plays a major role in deciding the properties of adhesives. Large number of fillers and adhesives exist and numerous combinations of the fillers and adhesives can be done to obtain desired characteristics of the adhesives.
References
Dinte E, Sylvester B (2018) Adhesives: Applications and Recent Advances. In: Applied Adhesive Bonding in Science and Technology. InTech. https://doi.org/10.5772/intechopen.71854
Gierenz G, Karmann W (2001) Adhesives and adhesive tapes. Adhesives. https://doi.org/10.1002/9783527612802.ch01
Kinloch AJ (1987) Adhesion and adhesives. Springer, Netherlands, Dordrecht
Dunn DJ (2003) Adhesives and sealants: technology, applications and markets
Sellers T, Miller GD, Smith W (2005) Tool wear properties of five extender/fillers in adhesive mixes for plywood. For Prod J 55(3):27–31
Cao L, Zhou X, Du G (2020) Wood Adhesive Fillers Used during the Manufacture of Wood Panel Products. https://doi.org/10.5772/intechopen.91280
Skeist I (1990) Handbook of adhesives. Springer, US, Boston, MA
Réh R, Krišák Ľ, Sedliačik J et al (2021) Utilization of birch bark as an eco-friendly filler in urea-formaldehyde adhesives for plywood manufacturing. Polymers 13:1–21. https://doi.org/10.3390/polym13040511
Hýsek Š, Šedivka P, Böhm M et al (2018) Recycled PU in wood glue. Bioresources 13:2592–2601. https://doi.org/10.15376/biores.13.2.2592-2601
Jiang W, Tomppo L, Pakarinen T et al (2018) Nanocellulose in adhesives. BioResources 13:2283–2292. https://doi.org/10.15376/biores.13.2.2283-2292
Ong HR, Khan MR, Yousuf A et al (2015) Effect of waste rubber powder as filler for plywood application. Pol J Chem Technol 17:41–47. https://doi.org/10.1515/pjct-2015-0007
Mamiński M, Wiecław-Midor AM, Parzuchowski PG (2020) The effect of silica-filler on polyurethane adhesives based on renewable resource for wood bonding. Polymers. https://doi.org/10.3390/POLYM12102177
Mohsen RM (1992) Effect of calcium carbonate filler on polyvinyl acetate emulsion as wood adhesive. Pigm Resin Technol 21:10–11. https://doi.org/10.1108/eb042981
Qiao L, Easteal AJ, Bolt CJ et al (1999) The effects of filler materials on poly(vinyl acetate) emulsion wood adhesives. Pigm Resin Technol 28:326–330. https://doi.org/10.1108/03699429910302300
Dunky M (2003) Handbook of adhesive technology. Third Edition 70. https://doi.org/10.1201/9780203912225.ch47
Grøstad K, Pedersen A (2010) Emulsion polymer isocyanates as wood adhesive: a review. J Adhes Sci Technol 24:1357–1381. https://doi.org/10.1163/016942410X500981
Bockel S, Harling S, Konnerth J et al (2020) Modifying elastic modulus of two-component polyurethane adhesive for structural hardwood bonding. J Wood Sci. https://doi.org/10.1186/s10086-020-01917-9
Zhang R, Jin X, Wen X et al (2018) Alumina nanoparticle modified phenol-formaldehyde resin as a wood adhesive. Int J Adhes Adhes 81:79–82. https://doi.org/10.1016/j.ijadhadh.2017.11.013
Kumar A, Gupta A, Sharma KV, Nasir M (2013) Use of aluminum oxide nanoparticles in wood composites to enhance the heat transfer during hot-pressing. Eur J Wood Wood Prod 71:193–198. https://doi.org/10.1007/s00107-013-0664-9
de Cademartori PHG, Artner MA, Alves de Freitas R, Magalhães WLE (2019) Alumina nanoparticles as formaldehyde scavenger for urea-formaldehyde resin: rheological and in-situ cure performance. Compos B Eng. https://doi.org/10.1016/j.compositesb.2019.107281
Kaboorani A, Riedl B (2011) Effects of adding nano-clay on performance of polyvinyl acetate (PVA) as a wood adhesive. Compos A Appl Sci Manuf 42:1031–1039. https://doi.org/10.1016/j.compositesa.2011.04.007
Moya R, Rodríguez-Zúñiga A, Vega-Baudrit J, Álvarez V (2015) Effects of adding nano-clay (montmorillonite) on performance of polyvinyl acetate (PVAc) and urea-formaldehyde (UF) adhesives in Carapa guianensis, a tropical species. Int J Adhes Adhes 59:62–70. https://doi.org/10.1016/j.ijadhadh.2015.02.004
Chen H, Yan N (2018) Application of Western red cedar (Thuja plicata) tree bark as a functional filler in pMDI wood adhesives. Ind Crops Prod 113:1–9. https://doi.org/10.1016/j.indcrop.2018.01.005
Veigel S, Müller U, Keckes J et al (2011) Cellulose nanofibrils as filler for adhesives: effect on specific fracture energy of solid wood-adhesive bonds. Cellulose 18:1227–1237. https://doi.org/10.1007/s10570-011-9576-1
Pinkl S, van Herwijnen HWG, Veigel S et al (2018) Urea-formaldehyde microspheres as a potential additive to wood adhesive. J Wood Sci 64:390–397. https://doi.org/10.1007/s10086-018-1717-9
Bono A, Nur MI, Anisuzzaman SM et al (2011) The performance of melamine-Urea-Formaldehyde resin with palm kernel as filler. Adv Mater Res. https://doi.org/10.4028/www.scientific.net/AMR.233-235.3
Ong HR, Khan MMR, Prasad DMR et al (2018) Palm kernel meal as a melamine urea formaldehyde adhesive filler for plywood applications. Int J Adhes Adhes 85:8–14. https://doi.org/10.1016/j.ijadhadh.2018.05.014
Zhang S, Qi X, Yang M et al (2019) A study on the resistivity and mechanical properties of modified nano-Ag coated Cu particles in electrically conductive adhesives. J Mater Sci Mater Electron 30:9171–9183. https://doi.org/10.1007/s10854-019-01246-8
Yim MJ, Li Y, Moon KS et al (2008) Review of recent advances in electrically conductive adhesive materials and technologies in electronic packaging. J Adhes Sci Technol 22:1593–1630. https://doi.org/10.1163/156856108X320519
Hussien AK, Mahmood ADF (2018) Effect of fillers on the adhesion properties of the cured unsaturated polyester adhesive. Rafidain J Sci 27:65–72. https://doi.org/10.33899/rjs.2018.141187
Meschi Amoli B, Hu A, Zhou NY, Zhao B (2015) Recent progresses on hybrid micro–nano filler systems for electrically conductive adhesives (ECAs) applications. J Mater Sci Mater Electron 26:4730–4745. https://doi.org/10.1007/s10854-015-3016-1
Mir I, Kumar D (2008) Recent advances in isotropic conductive adhesives for electronics packaging applications. Int J Adhes Adhes 28:362–371. https://doi.org/10.1016/j.ijadhadh.2007.10.004
Murray C, Rudman R, Sabade M, Pocius A (2003) Conductive adhesives for electronic assemblies. MRS Bull. https://doi.org/10.1557/mrs2003.127
Cao G, Wang L, Tian Y (2020) Highly dispersed polypyrrole nanotubes for improving the conductivity of electrically conductive adhesives. J Mater Sci Mater Electron 31:9675–9684. https://doi.org/10.1007/s10854-020-03513-5
Wen J, Tian Y, Mei Z et al (2017) Synthesis of polypyrrole nanoparticles and their applications in electrically conductive adhesives for improving conductivity. RSC Adv 7:53219–53225. https://doi.org/10.1039/c7ra09725e
Inoue M, Tada Y, Muta H, Yamanaka S (2013) Microstructural control of electrically conductive adhesives with Ag micro-fillers by binder chemistry. ICSJ 2013 - IEEE CPMT Symposium Japan. pp 1–4. https://doi.org/10.1109/ICSJ.2013.6756122
Tee DI, Mariatti M, See CH, et al. (2006) International Electronic Manufacturing Technology Study on the Electrical Property of Silver (Ag) Nanoparticles Filled Epoxy Composites for the Application of Electrically Conductive Adhesives (ECAs) in Electronic Packaging 496–505. https://doi.org/10.1109/IEMT.2006.4456501
Nishikawa H, Mikami S, Terada N, Miyake K, Aoki A, Takemoto T (2008) Electrical property of conductive adhesives using silver-coated copper filler. Proceedings—2008 2nd electronics system integration technology conference, ESTC. 825–828. https://doi.org/10.1109/ESTC.2008.4684458
Nishikawa H, Mikami S, Miyake K et al (2010) Effects of silver coating covered with copper filler on electrical resistivity of electrically conductive adhesives. Mater Trans 51:1785–1789. https://doi.org/10.2320/matertrans.MJ201020
Wong ICP, Lu D (2000) Recent Advances on Electrically Conductive Adhesives for Electronics Applications pp 121–128. https://doi.org/10.1109/ADHES.2000.860585
Aradhana R, Mohanty S, Nayak SK (2019) High performance electrically conductive epoxy/reduced graphene oxide adhesives for electronics packaging applications. J Mater Sci Mater Electron 30:4296–4309. https://doi.org/10.1007/s10854-019-00722-5
Berger C, Song Z, Li X et al (2006) Electronic confinement and coherence in patterned epitaxial graphene. Science 312:1191–1196. https://doi.org/10.1126/science.1125925
Zhu Y, Murali S, Cai W et al (2010) Graphene and graphene oxide: Synthesis, properties, and applications. Adv Mater 22:3906–3924. https://doi.org/10.1002/adma.201001068
Chew C, Durairaj R, Hwang J, et al (2014) The Effect of Nano Fillers in Electrical and Mechanical Properties of Isotropic Conductive Adhesive. https://doi.org/10.1109/IEMT.2014.7123070
Zhang W, Zhou Y, Feng K et al (2015) Morphologically controlled bioinspired dopamine-polypyrrole nanostructures with tunable electrical properties. Adv Electron Mater. https://doi.org/10.1002/aelm.201500205
Kilik R, Davies R, Darwish SMH (1989) Thermal conductivity of adhesive filled with metal powders. Int J Adhes Adhes 9(4):219–223. https://doi.org/10.1016/0143-7496(89)90064-X
Fu YX, He ZX, Mo DC, Lu SS (2014) Thermal conductivity enhancement with different fillers for epoxy resin adhesives. Appl Therm Eng 66:493–498. https://doi.org/10.1016/j.applthermaleng.2014.02.044
Felba J (2011) Thermally conductive adhesives in electronics. Advanced adhesives in electronics: materials, properties and applications. Elsevier Ltd, Amsterdam, pp 15–52
Singh AK, Panda BP, Mohanty S et al (2018) Recent developments on epoxy-based thermally conductive adhesives (TCA): a review. Polym Plast Technol Eng 57:903–934. https://doi.org/10.1080/03602559.2017.1354253
Falat T, Wymysłowski A, Kolbe J, et al. (2006) Numerical Approach to Characterization of Thermally Conductive Adhesives. pp 1–7. https://doi.org/10.1109/ESIME.2006.1643972
Petrova AP, Abeliov YA, Zuev AV (2014) The influence of fillers on thermophysical properties of adhesives. Polym Sci Series D 7:93–95. https://doi.org/10.1134/S1995421214020154
Ye T, Tian Y, Wang C, et al. (2017) The 18th international conference on electronic packaging technology : Harbin, China, August 16–19, 2017
Alim MA, Abdullah MZ, Aziz MSA et al (2021) Recent advances on thermally conductive adhesive in electronic packaging: a review. Polymers 13:3337
Xu Y, Chung DDL, Mroz C (2001) Thermally conducting aluminum nitride polymer-matrix composites. Compos A Appl Sci Manuf 32:1749–1757. https://doi.org/10.1016/S1359-835X(01)00023-9
Bujard P, Ansermet JP (1989) Thermally Conductive Aluminium Nitride-Filled Epoxy pp 126–130
Kim J, Kim YD, Nam DG et al (2016) Thermal Properties of epoxy composites with silicon carbide and/or graphite. J Korean Phys Soc 68:551–556. https://doi.org/10.3938/jkps.68.551
Fu YX, He ZX, Mo DC, Lu SS (2014) Thermal conductivity enhancement of epoxy adhesive using graphene sheets as additives. Int J Therm Sci 86:276–283. https://doi.org/10.1016/j.ijthermalsci.2014.07.011
Yu A, Ramesh P, Sun X et al (2008) Enhanced thermal conductivity in a hybrid graphite nanoplatelet - Carbon nanotube filler for epoxy composites. Adv Mater 20:4740–4744. https://doi.org/10.1002/adma.200800401
Bolger JC (1992) Prediction and Measurement of Thermal Conductivity Diamond Filled Adhesives. Proceedings 42nd electronic components & technology conference, pp 219–224. https://doi.org/10.1109/ECTC.1992.204210
Li M, Tang C, Zhang L et al (2018) A thermally conductive and insulating epoxy polymer composite with hybrid filler of modified copper nanowires and graphene oxide. J Mater Sci Mater Electron 29:4948–4954. https://doi.org/10.1007/s10854-017-8454-5
Permal A, Devarajan M, Huong LH et al (2018) Enhanced thermal and mechanical properties of epoxy composites filled with hybrid filler system of aluminium nitride and boron nitride. Polym Compos 39:1372-E1380. https://doi.org/10.1002/pc.24268
Sanada K, Tada Y, Shindo Y (2009) Thermal conductivity of polymer composites with close-packed structure of nano and micro fillers. Compos A Appl Sci Manuf 40:724–730. https://doi.org/10.1016/j.compositesa.2009.02.024
Wang T, Lei CH, Dalton AB et al (2006) Waterborne, nanocomposite pressure-sensitive adhesives with high tack energy, optical transparency, and electrical conductivity. Adv Mater 18:2730–2734. https://doi.org/10.1002/adma.200601335
Kreckel K, Graichen A, Weigl S (1993) Pressure Sensitive Adhesive With Filler, EP0638096B1
Kim DB (2013) Effect of Acrylic acid contents and inorganic fillers on physical properties of acrylic pressure sensitive adhesive Tape by UV curing. Polym (Korea) 37:184–195. https://doi.org/10.7317/pk.2013.37.2.184
Park GH, Kim KT, Ahn YT et al (2014) The effects of graphene on the properties of acrylic pressure-sensitive adhesive. J Ind Eng Chem 20:4108–4111. https://doi.org/10.1016/j.jiec.2014.01.008
Czech Z, Kowalczyk A, Pełech R et al (2012) Using of carbon nanotubes and nano carbon black for electrical conductivity adjustment of pressure-sensitive adhesives. Int J Adhes Adhes 36:20–24. https://doi.org/10.1016/j.ijadhadh.2012.04.004
Novák I, Florián Š (2003) Pressure-sensitive adhesives for electronic applications. J Mater Sci Lett 22:1237–1239
Li H, Yang Y, Yu Y (2004) Acrylic emulsion pressure-sensitive adhesives (PSAS) reinforced with layered silicate. J Adhes Sci Technol 18:1759–1770. https://doi.org/10.1163/1568561042708340
Brantseva T, Antonov S, Kostyuk A et al (2016) Rheological and adhesive properties of PIB-based pressure-sensitive adhesives with montmorillonite-type nanofillers. Eur Polym J 76:228–244. https://doi.org/10.1016/j.eurpolymj.2016.01.040
Kajtna J, Šebenik U (2009) Microsphere pressure sensitive adhesives-acrylic polymer/montmorillonite clay nanocomposite materials. Int J Adhes Adhes 29:543–550. https://doi.org/10.1016/j.ijadhadh.2009.01.001
Pang B, Ryu CM, Kim H (2013) Improvement of thermal stability of UV curable pressure sensitive adhesive by surface modified silica nanoparticles. Mater Sci Eng B Solid-State Mater Adv Technol 178:1212–1218. https://doi.org/10.1016/j.mseb.2013.08.005
Yamamoto Y, Fujii S, Shitajima K et al (2015) Soft polymer-silica nanocomposite particles as filler for pressure-sensitive adhesives. Polymer 70:77–87. https://doi.org/10.1016/j.polymer.2015.06.006
Khalina M, Sanei M, Mobarakeh HS, Mahdavian AR (2015) Preparation of acrylic/silica nanocomposites latexes with potential application in pressure sensitive adhesive. Int J Adhes Adhes 58:21–27. https://doi.org/10.1016/j.ijadhadh.2014.12.007
Kowalczyk A, Kowalczyk K, Gziut K et al (2019) Influence of a wollastonite microfiller and a halloysite nanofiller on properties of thermally curable pressure-sensitive structural adhesives. Int J Adhes Adhes. https://doi.org/10.1016/j.ijadhadh.2019.102397
Novák I, Florián Š, Pollák V (2007) Behavior of pressure-sensitive adhesives filled with metallized inorganic particles. Int J Polym Mater Polym Biomater 56:841–849
Czech Z, Arabczyk W, Hełminiak A, Kowalczyk A (2013) Influence of iron carbide filler in carbon matrix on the adhesive properties of acrylic pressure-sensitive adhesives. Int J Adhes Adhes 40:210–214. https://doi.org/10.1016/j.ijadhadh.2012.07.010
Mittal G, Park SJ, Rhee KY (2020) Acrylic pressure-sensitive adhesive reinforced with aluminum nitride and its thermal properties: effect of surface treatment and particle size. Coatings. https://doi.org/10.3390/coatings10020188
Kim JK, Kim JK, Kim MI, Song MS, Thermal Conductivity and Adhesion Properties of Thermally Conductive Pressure-Sensitive Adhesives
Daniloska V, Keddie JL, Asua JM, Tomovska R (2014) MoS2 nanoplatelet fillers for enhancement of the properties of waterborne pressure-sensitive adhesives. ACS Appl Mater Interfaces 6:22640–22648. https://doi.org/10.1021/am506726f
Cui T, Li Q, Xuan Y, Zhang P (2015) Preparation and thermal properties of the graphene-polyolefin adhesive composites: application in thermal interface materials. Microelectronics reliability. Elsevier Ltd, Amsterdam, pp 2569–2574
Xu CA, Qu Z, Meng H et al (2021) Effect of polydopamine-modified multi-walled carbon nanotubes on the thermal stability and conductivity of UV-curable polyurethane/polysiloxane pressure-sensitive adhesives. Polymer. https://doi.org/10.1016/j.polymer.2021.123615
Czech Z, Pełech R, Kowalczyk A et al (2011) Electrically conductive acrylic pressure-sensitive adhesives containing carbon black. Pol J Chem Technol 13:77–81. https://doi.org/10.2478/v10026-011-0053-2
Kostyuk A, Ignatenko VY, Makarova V et al (2020) Polyethylene wax as an alternative to mineral fillers for preparation of reinforced pressure-sensitive adhesives. Int J Adhes Adhes. https://doi.org/10.1016/j.ijadhadh.2020.102689
Lamb J, Buffet JC, Turner ZR et al (2020) Metallocene polyethylene wax synthesis. Macromolecules 53:5847–5856. https://doi.org/10.1021/acs.macromol.0c00990
Dastjerdi Z, Cranston ED, Dubé MA (2018) Pressure sensitive adhesive property modification using cellulose nanocrystals. Int J Adhes Adhes 81:36–42. https://doi.org/10.1016/j.ijadhadh.2017.11.009
Yu Q, Yang W, Wang Q et al (2019) Functionalization of cellulose nanocrystals with γ-MPS and its effect on the adhesive behavior of acrylic pressure sensitive adhesives. Carbohyd Polym 217:168–177. https://doi.org/10.1016/j.carbpol.2019.04.049
Nassif M, Elaskary F (2012) Chapter 7. Nanotechnology and Nanoparticles in Contemporary Dental Adhesives, pp 131–160. https://doi.org/10.13140/2.1.2681.2800
van Landuyt KL, Snauwaert J, de Munck J et al (2007) Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 28:3757–3785. https://doi.org/10.1016/j.biomaterials.2007.04.044
Yoshida Y, Inoue S (2012) Chemical analyses in dental adhesive technology. Japanese Dental Sci Rev 48:141–152. https://doi.org/10.1016/j.jdsr.2012.03.001
Solhi L, Atai M, Nodehi A, Imani M (2012) A novel dentin bonding system containing poly(methacrylic acid) grafted nanoclay: Synthesis, characterization and properties. Dent Mater 28:1041–1050. https://doi.org/10.1016/j.dental.2012.06.004
Sadat-Shojai M, Atai M, Nodehi A, Khanlar LN (2010) Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: synthesis and application. Dent Mater 26:471–482. https://doi.org/10.1016/j.dental.2010.01.005
Bedran-Russo A, Leme-Kraus AA, Vidal CMP, Teixeira EC (2017) An overview of dental adhesive systems and the dynamic tooth-adhesive interface. Dent Clin North Am 61:713–731. https://doi.org/10.1016/j.cden.2017.06.001
Giannini M, Mettenburg D, Arrais C, Rueggeberg F (2011) The effect of filler addition on biaxial flexure strength and modulus of commercial dentin bonding systems. Quintessence international (Berlin, Germany: 1985) 42:39–43
Leitune VCB, Collares FM, Takimi A et al (2013) Niobium pentoxide as a novel filler for dental adhesive resin. J Dent 41:106–113. https://doi.org/10.1016/j.jdent.2012.04.022
Khosravi K, Mirmohamadi H, Kashani K, Professor A (2012) Evaluation of effect of adding silica fillers to adhesive on microleakage of composite restorations in different times. J Islam Dental Assoc Iran (Jidai) 24(3):105–110
Belli R, Kreppel S, Petschelt A et al (2014) Strengthening of dental adhesives via particle reinforcement. J Mech Behav Biomed Mater 37:100–108. https://doi.org/10.1016/j.jmbbm.2014.05.007
Shamsudin R, Atiqah Abdul Azam F, Abdul Hamid MA, Ismail H (2017) Bioactivity and cell compatibility of β-wollastonite derived from rice husk ash and limestone. Materials. https://doi.org/10.3390/ma10101188
Bendary IM, Garcia IM, Collares FM et al (2020) Wollastonite as filler of an experimental dental adhesive. J Dent. https://doi.org/10.1016/j.jdent.2020.103472
Atai M, Solhi L, Nodehi A et al (2009) PMMA-grafted nanoclay as novel filler for dental adhesives. Dent Mater 25:339–347. https://doi.org/10.1016/j.dental.2008.08.005
Solhi L, Atai M, Nodehi A et al (2012) Poly(acrylic acid) grafted montmorillonite as novel fillers for dental adhesives: synthesis, characterization and properties of the adhesive. Dent Mater 28:369–377. https://doi.org/10.1016/j.dental.2011.11.010
Collares FM, Portella FF, da Silva Fraga GC et al (2014) Mineral deposition at dental adhesive resin containing niobium pentoxide. Appl Adhes Sci. https://doi.org/10.1186/s40563-014-0022-0
Garcia IM, Leitune VCB, Ferreira CJ, Collares FM (2018) Tantalum oxide as filler for dental adhesive resin. Dent Mater J 37:897–903. https://doi.org/10.4012/dmj.2017-308
Chan DCN, Titus HW, Chung K-H et al (1999) Radiopacity of tantalum oxide nanoparticle filled resins. Dental Mater Off Publ Acad Dental Mater 15:219–222. https://doi.org/10.1016/S0109-5641(99)00039-1
Alhenaki AM, Attar EA, Alshahrani A et al (2021) Dentin bond integrity of filled and unfilled resin adhesive enhanced with silica nanoparticles-An SEM, EDX, Micro-Raman, FTIR and micro-tensile bond strength study. Polymers. https://doi.org/10.3390/polym13071093
Wang J, Yu Q, Yang Z (2018) Effect of hydrophobic surface treated fumed silica fillers on a one-bottle etch and rinse model dental adhesive. J Mater Sci Mater Med. https://doi.org/10.1007/s10856-017-6015-3
Fani Jahromi S, Naimi-Jamal MR, Atai M (2014) Preparation of Dental Polymeric Nano-Adhesives with Silica Nano-Porous (MCM-41) as Novel Fillers for Improving the Adhesive Mechanical Properties: Synthesis and Application. MDPI AG, p d010
Azad E, Atai M, Zandi M et al (2018) Structure–properties relationships in dental adhesives: effect of initiator, matrix monomer structure, and nano-filler incorporation. Dent Mater 34:1263–1270. https://doi.org/10.1016/j.dental.2018.05.013
Kasraei S, Khamverdi Z (2009) Effect of Nanofiller Addition to an Experimental Dentin Adhesive on Microtensile Bond Strength to Human Dentin. J Dentistry (Tehran) 6
Atai M, Pahlavan A, Moin N (2012) Nano-porous thermally sintered nano silica as novel fillers for dental composites. Dent Mater 28:133–145. https://doi.org/10.1016/j.dental.2011.10.015
Carreño NLV, Oliveira TCS, Piva E et al (2012) A dental adhesive resin. Nano-Micro Lett 4:189–196. https://doi.org/10.3786/nml.v4i3
Habib E, Wang R, Wang Y et al (2016) Inorganic fillers for dental resin composites: present and future. ACS Biomater Sci Eng 2:1–11. https://doi.org/10.1021/acsbiomaterials.5b00401
Wagner A, Belli R, Stötzel C et al (2013) Biomimetically-and hydrothermally-grown HAp nanoparticles as reinforcing fillers for dental adhesives. J Adhes Dent 15:413–422. https://doi.org/10.3290/j.jad.a29534
Provenzi C, Cb Leitune V, Collares FM et al (2014) Interface evaluation of experimental dental adhesives with nanostructured hydroxyapatite incorporation. Appl Adhes Sci. https://doi.org/10.1186/2196-4351-2-2
Ibrahim R, Sabry R, Ibrahim A-A, et al. Novel Bifunctional Nanofiller (Bioactive\Antimicrobial) for Improving Dental Adhesives Efficacy. J Oral Dent Health, 3(2):1–11
Bona AD, Pecho OE, Alessandretti R (2015) Zirconia as a dental biomaterial. Materials 8:4978–4991. https://doi.org/10.3390/ma8084978
Lohbauer U, Wagner A, Belli R et al (2010) Zirconia nanoparticles prepared by laser vaporization as fillers for dental adhesives. Acta Biomater 6:4539–4546. https://doi.org/10.1016/j.actbio.2010.07.002
Dadkan S, Salari S, Khakbiz M, Atai M (2014) Mechanical Properties of Dental Adhesives Containing Gold Nano Particles. Proceedings of 5th international congress on nanoscience & nanotechnology (ICNN2014)
Bapat RA, Chaubal T, Dharmadhikari S et al (2020) Recent advances of gold nanoparticles as biomaterial in dentistry. Int J Pharm. https://doi.org/10.1016/j.ijpharm.2020.119596
Degrazia FW, Leitune VCB, Visioli F et al (2018) Long-term stability of dental adhesive incorporated by boron nitride nanotubes. Dent Mater 34:427–433. https://doi.org/10.1016/j.dental.2017.11.024
Degrazia FW, Leitune VCB, Samuel SMW, Collares FM (2017) Boron nitride nanotubes as novel fillers for improving the properties of dental adhesives. J Dent 62:85–90. https://doi.org/10.1016/j.jdent.2017.05.013
Ikemura K, Tay FR, Kouro Y et al (2003) Optimizing filler content in an adhesive system containing pre-reacted glass-ionomer fillers. Dent Mater Off Publ Acad Dent Mater 19(2):137–146. https://doi.org/10.1016/s0109-5641(02)00022-2
Ito S, Iijima M, Hashimoto M et al (2011) Effects of surface pre-reacted glass-ionomer fillers on mineral induction by phosphoprotein. J Dent 39:72–79. https://doi.org/10.1016/j.jdent.2010.10.011
Ikemura K, Tay FR, Endo T, Pashley DH (2008) A Review of Chemical-approach and Ultramorphological Studies on the Development of Fluoride-releasing Dental Adhesives Comprising New Pre-Reacted Glass Ionomer (PRG) Fillers
Khvostenko D, Hilton TJ, Ferracane JL et al (2016) Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent Mater 32:73–81. https://doi.org/10.1016/j.dental.2015.10.007
Abou Neel EA, Kiani A, Valappil SP et al (2019) Glass microparticle- versus microsphere-filled experimental dental adhesives. J Appl Polym Sci. https://doi.org/10.1002/app.47832
Wang Z, Jia Z, Feng X, Zou Y (2018) Graphene nanoplatelets/epoxy composites with excellent shear properties for construction adhesives. Compos B Eng 152:311–315. https://doi.org/10.1016/j.compositesb.2018.08.113
Jojibabu P, Zhang YX, Prusty BG (2020) A review of research advances in epoxy-based nanocomposites as adhesive materials. Int J Adhes Adhes. https://doi.org/10.1016/j.ijadhadh.2019.102454
Bagherzadeh A, Jamshidi M, Monemian F (2020) Investigating mechanical and bonding properties of micro/nano filler containing epoxy adhesives for anchoring steel bar in concrete. Constr Build Mater. https://doi.org/10.1016/j.conbuildmat.2019.117979
Korayem AH, Li CY, Zhang QH et al (2015) Effect of carbon nanotube modified epoxy adhesive on CFRP-to-steel interface. Compos B Eng 79:95–104. https://doi.org/10.1016/j.compositesb.2015.03.063
Kothe M (2014) Epoxy resin adhesives for structural purposes-a new approach. International Conference at Glasstec, Düsseldorf, Germany
Kumar P, Patnaik A, Chaudhary S (2017) A review on application of structural adhesives in concrete and steel–concrete composite and factors influencing the performance of composite connections. Int J Adhes Adhes 77:1–14. https://doi.org/10.1016/j.ijadhadh.2017.03.009
Kahraman R, Al-Harthi M (2005) Moisture diffusion into aluminum powder-filled epoxy adhesive in sodium chloride solutions. Int J Adhes Adhes 25:337–341. https://doi.org/10.1016/j.ijadhadh.2004.10.003
Charitidis PJ (2020) The effect of nanoparticles in single lap joints studied by numerical analyses. Eur J Eng Res Sci 5:1288–1293. https://doi.org/10.24018/ejers.2020.5.10.2194
Jouyandeh M, Moini Jazani O, Navarchian AH, Saeb MR (2016) High-performance epoxy-based adhesives reinforced with alumina and silica for carbon fiber composite/steel bonded joints. J Reinf Plast Compos 35:1685–1695. https://doi.org/10.1177/0731684416665248
Doll K, Xie H, de Meter E (2017) Investigation into the use of adhesive fillers and soft start curing to reduce the distortion of a work-piece supported by PAAW joints. Procedia Manuf 10:147–158. https://doi.org/10.1016/j.promfg.2017.07.041
Szewczak A, Szeląg M (2020) Physico-mechanical and rheological properties of epoxy adhesives modified by microsilica and sonication process. Materials 13:1–20. https://doi.org/10.3390/ma13235310
Zhou H, Liu HY, Zhou H et al (2016) On adhesive properties of nano-silica/epoxy bonded single-lap joints. Mater Des 95:212–218. https://doi.org/10.1016/j.matdes.2016.01.055
Gupta SK, Shukla DK, Kaustubh Ravindra D (2021) Effect of nanoalumina in epoxy adhesive on lap shear strength and fracture toughness of aluminium joints. J Adhes 97:117–139. https://doi.org/10.1080/00218464.2019.1641088
Pustovgar A, Zhuravlev A, Nefedov S et al (2014) Comparison of the effectiveness of fine mineral fillers in cement-based tile adhesives. Advanced materials research. Trans Tech Publications Ltd, Freienbach, pp 1496–1502
Anam K, Purnowidodo A (2018) Effect of filler volume fraction on mechanical strength and failure mode of aluminium bonded with epoxy-based adhesive. In: AIP Conference Proceedings. American Institute of Physics Inc. https://doi.org/10.1063/1.5046287
Yuniwantoro C, Surojo E, Smaradhana DF, Imaduddin F (2020) Effect of Filler on Shear Strength and Electrical Conductivity in Epoxy Based Aluminium Bonded. Mekanika: Majalah Ilmiah Mekanika 19. https://doi.org/10.20961/mekanika.v19i1.39903
Ghosh PK, Patel A, Kumar K (2016) Adhesive joining of copper using nano-filler composite adhesive. Polymer 87:159–169. https://doi.org/10.1016/j.polymer.2016.02.006
Tutunchi A, Kamali R, Kianvash A (2015) Steel-epoxy composite joints bonded with nano-TiO2 reinforced structural acrylic adhesive. J Adhes 91:663–676. https://doi.org/10.1080/00218464.2014.961187
Kavak N, Altan E (2014) Influence of filler amount and content on the mechanical performance of joints bonded with metal powder filled adhesive. Materials science forum. Trans Tech Publications Ltd, Freienbach, pp 226–233
Ghosh PK, Nukala SK (2008) Properties of adhesive joint of inorganic nano-filler composite adhesive. Indian J Eng Mater Sci 15:68–74
Al-Harthi M, Loughlin K, Kahraman R (2007) Moisture diffusion into epoxy adhesive: testing and modeling. Adsorption 13:115–120. https://doi.org/10.1007/s10450-007-9011-y
Back JH, Hwang JU, Lee YH et al (2018) Morphological study and mechanical property of epoxy-foam adhesives based on epoxy composites for automotive applications. Int J Adhes Adhes 87:124–129. https://doi.org/10.1016/j.ijadhadh.2018.09.010
Kowalczyk A, Kowalczyk K, Czech Z (2012) Synthesis and properties of solid structural adhesives modified in-situ using 1D and 2D-type microfillers. Int J Adhes Adhes 32:76–81. https://doi.org/10.1016/j.ijadhadh.2011.10.006
Tai RCL, Szklarska-Smialowska Z (1993) Effect of fillers on the degradation of automotive epoxy adhesives in aqueous solutions Part II The microhardness change and delamination of automotive epoxy adhesives in distilled water and NaCI solutions. J Mater Sci 28:6205–6210. https://doi.org/10.1007/BF00365045
Tai RCL, Szklarska-Smialowska Z (1996) Delamination of filler-incorporated automotive epoxy adhesives from different steel substrates upon exposure to distilled water and NaC! solutions under applied bending stresses. J Mater Sci 31:1925–1935. https://doi.org/10.1007/BF00372209
Jojibabu P, Ram GDJ, Deshpande AP, Bakshi SR (2017) Effect of carbon nano-filler addition on the degradation of epoxy adhesive joints subjected to hygrothermal aging. Polym Degrad Stab 140:84–94. https://doi.org/10.1016/j.polymdegradstab.2017.04.017
Valášek P, Müller M (2016) Possibilities of adhesives filling with micro-particle fillers - Lap-Shear tensile strength. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis 64:195–201. https://doi.org/10.11118/actaun201664010195
Mejbel MK, Allawi MK, Oudah MH (2019) Effects of WC, SiC, iron and glass fillers and their high percentage content on adhesive bond strength of an aluminium alloy butt joint: an experimental study. J Mech Eng Res Dev 42:224–231. https://doi.org/10.26480/jmerd.05.2019.224.231
Zhang C, Sun L, Huang B et al (2019) Electrical and mechanical properties of CNT/CB dual filler conductive adhesives (DFCAs) for automotive multi-material joints. Compos Struct. https://doi.org/10.1016/j.compstruct.2019.111183
Safronava N, Lyon R (2012) Combustion Characteristics of Adhesive Compounds Used in the Construction of Aircraft Cabin Materials. U.S. Department of Transportation. Federal Aviation Administration
Tserpes K (2020) Adhesive Bonding of Aircraft Structures. In: Revolutionizing Aircraft Materials and Processes. Springer International Publishing. https://doi.org/10.1007/978-3-030-35346-9_12
Pantelakis S, Tserpes KI (2014) Adhesive bonding of composite aircraft structures: challenges and recent developments. Sci China Phys Mech Astron 57:2–11. https://doi.org/10.1007/s11433-013-5274-3
Higgins A (1998) Adhesive bonding of aircraft structures. Int J Adhes Adhes 20(5):367–376. https://doi.org/10.1016/S0143-7496(00)00006-3
Petrova AP, Lukina NF (2008) Adhesive technologies in aircraft construction. Polym Sci, Ser D 1:83–90. https://doi.org/10.1134/s1995421208020032
Xu H, Zhang X, Hu G et al (2020) A special filler for epoxy resin to enhance the T peel strength of adhesive. Polym Compos 41:4372–4378. https://doi.org/10.1002/pc.25719
Vietri U, Guadagno L, Raimondo M et al (2014) Nanofilled epoxy adhesive for structural aeronautic materials. Compos B Eng 61:73–83. https://doi.org/10.1016/j.compositesb.2014.01.032
Jakubinek MB, Ashrafi B, Zhang Y et al (2015) Single-walled carbon nanotube-epoxy composites for structural and conductive aerospace adhesives. Compos B Eng 69:87–93. https://doi.org/10.1016/j.compositesb.2014.09.022
Gkikas G, Sioulas D, Lekatou A et al (2012) Enhanced bonded aircraft repair using nano-modified adhesives. Mater Des 41:394–402. https://doi.org/10.1016/j.matdes.2012.04.052
Wolf A, Buchman A, Eitan A et al (2012) Improved adhesives containing CNT/SP1 nano fillers. J Adhes. https://doi.org/10.1080/00218464.2012.660398
Otorgust G, Dodiuk H, Kenig S, Tenne R (2017) Important insights into polyurethane nanocomposite-adhesives; a comparative study between INT-WS2 and CNT. Eur Polymer J 89:281–300. https://doi.org/10.1016/j.eurpolymj.2017.02.027
Fu H, Yan C, Zhou W, Huang H (2014) Nano-SiO2/fluorinated waterborne polyurethane nanocomposite adhesive for laminated films. J Ind Eng Chem 20:1623–1632. https://doi.org/10.1016/j.jiec.2013.08.009
Fernández-García JC, Pastor-Sempere N, Martín-Martínez JM (1992) Addition of silica to polyurethane adhesives. J Adhes 38:31–53. https://doi.org/10.1080/00218469208031266
Donate-Robles J, Martín-Martínez JM (2011) Comparative properties of thermoplastic polyurethane adhesive filled with natural or precipitated calcium carbonate. Macromol Symp 301:63–72. https://doi.org/10.1002/masy.201150309
Sepulcre-Guilabert J, Ferrándiz-Gómez TP, Martín-Martínez JM (2001) Properties of polyurethane adhesives containing natural calcium carbonate + fumed silica mixtures. J Adhes Sci Technol 15:187–203. https://doi.org/10.1163/156856101743409
Donate-Robles J, Martín-Martínez JM (2011) Addition of precipitated calcium carbonate filler to thermoplastic polyurethane adhesives. Int J Adhes Adhes 31:795–804. https://doi.org/10.1016/j.ijadhadh.2011.07.008
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sanghvi, M.R., Tambare, O.H. & More, A.P. Performance of various fillers in adhesives applications: a review. Polym. Bull. 79, 10491–10553 (2022). https://doi.org/10.1007/s00289-021-04022-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-04022-z