Abstract
The usage of active antimicrobial food packaging systems has become inevitable with the globalization of food trade, and in this regards antimicrobial nanocomposites have attracted universal attention. The present study aims to examine the antimicrobial effects of CuO-containing nanocomposite on two important spoilage bacteria, namely gram-positive Bacillus subtilis and gram-negative Enterobacter aerogenes, and comparison of its antibacterial effect with ZnO-containing nanocomposite. To synthesize the nanoparticles of CuO, sonochemical method has been employed. The nanoparticles have been characterized by X-ray diffraction. By melt mixing in a twin-screw extruder, nanocomposite film containing 2 wt% CuO nanoparticles was prepared. CuO-containing nanocomposites had reduced the growth of both bacteria. CuO-containing nanocomposite had a stronger antibacterial effect on both of the microorganisms in comparison with ZnO-containing nanocomposite, which could be ascribed to their small size. Due to the significant antibacterial effect of ZnO- and CuO-containing nanocomposites, they have the potential to be used in active food packaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The tendency to use fresh and minimally processed foods has increased drastically during recent years; on the other hand, the globalization of food trade and the transportation of food over long distances problematize the safety and quality of the food; therefore, there is an increasing attention for the development and usage of novel food packaging systems [1, 2]. Active antimicrobial food packaging systems are developed to protect food products against environmental factors, retarding and additionally hindering microbial growth and food spoilage [3]. The development of new antimicrobial agents has become feasible due to recent advances in nanotechnology and mainly because of the ability to produce metal oxide nanoparticles of any size and shape [4]. As a result, the food packaging industry can be a good market for innovative products of nanotechnology [5]. Materials in nanoscale have a larger surface-to-volume ratio than similar materials in normal molecular size, which is the main reason for the acceleration in antibacterial activity of some nanoparticles [6]. Stability of inorganic materials such as metal and metal oxides at high pressure and temperatures enables them to tolerate severe process condition, and for this reason these materials have attracted universal attention over the past decade [7].
Silver nanoparticles are among the most studied metal nanoparticles with antimicrobial effect in food packaging [8]. Although the antibacterial properties of these nanoparticles are well recognized, their use in the food industry has also some disadvantages such as their high price and toxic nature [9]. As a result, during the recent years, investigators have studied antimicrobial properties of some other metal nanoparticles including ZnO, Fe2O3, TiO, MgO and CuO. Although most of these studies reported promising results, there are still large controversies regarding their antibacterial power [10] and there are only few studies regarding some of these nanoparticles including CuO nanocomposites [11].
Copper is an essential mineral present in most foods in the form of ions or salts and also has remarkable antimicrobial attributes. In February 2008, the US Environmental Protection Agency (EPA) approved copper alloys as compounds to reduce lethal bacteria-linked infections [12]. Owing to the fact that copper is not concentrated by animals and consequently has a negligible adverse impact on higher animals, it is judged as relatively safe [13]. CuO’s efficiency and stability have led to its popularity as the simplest member of the copper compounds. Even though CuO has some of the same properties of noble metals, such as silver and gold, its usage is more cost-effective [14]; moreover, it easily mixes with polymers and is relatively stable, both chemically and physically [15].
In a previous study, we showed the antibacterial properties of ZnO-containing nanocomposite [16]. The objective of the present study is to assess the antibacterial activity of CuO-containing nanocomposite and its comparison with the antimicrobial effects of ZnO-containing nanocomposites on gram-positive Bacillus subtilis, one of the most important spoiling bacteria in the food industry and gram-negative Enterobacter aerogenes, pathogenic bacteria that are found in water, vegetable and meat.
Materials and methods
For synthesizing CuO nanoparticles, 1 g of Cu(NO3)2 was first dissolved in 100 ml of deionized water. 10 ml of NaOH (1 M) solution was then added slowly to the solution under applying ultrasonic waves (30 min, 80 W). A green–blue precipitate, which substantiated the synthesis of copper oxide, was attained. The obtained precipitate was then centrifuged and washed with distilled water. After washing the obtained CuO nanoparticles with hot deionized water, they were centrifuged at 4000 rpm at 25 °C for 15 min. To ensure the complete removal of water-soluble impurities, washing and centrifugation of nanoparticles were repeated four times. Finally, the sediment was dried at 100 °C for 3 h to obtain a black powder of CuO nanoparticles.
The process of synthesizing ZnO nanoparticles has been explained elsewhere [16].
X-ray diffractometer (XRD, Philips, X’Pert) was employed to examine the phase composition and the microstructure of ZnO and CuO nanoparticles. The particle size and morphology of the CuO nanoparticles and its dispersion in the LDPE film were assessed using scanning electron microscopy (SEM, Tescan). To analyze the surface chemical composition, X-ray photoelectron spectroscopy (XPS) using Al-Kα X-ray source at an energy of 1486.6 eV was used. All binding energy values were calibrated by fixing the C (1s) core level to the 285.0 eV. The SDP software was used to deconvolute all of the peaks (version 4.1) with 80% Gaussian–20% Lorentzian.
Using a twin-screw extruder with a screw diameter of 42 mm, nanoparticles were inserted into a low-density polyethylene matrix. Film grade LDPE resin pellets (300 g) were directly mixed with ZnO and CuO nanoparticles (6 g for 2 wt% ZnO- and CuO-containing nanocomposites), and the mixture was fed into a twin-screw extruder machine. The heating profile was set up to six heating zones of the twin-screw extruder including 90 °C, 160 °C, 175 °C, 155 °C, 155 °C and 150 °C. Then, by using a hot press with a pressure of 120 kg/cm2 at 200 °C, the nanoparticle-containing granules were transmuted into the nanocomposite films with an average thickness of 0.1 mm.
The Iranian Research Organization for Science and Technology provided us with the required B. subtilis (American type culture collection 6051) and E. aerogenes (American type culture collection 13,048). To carry out antimicrobial tests, the bottom of the 8-cm plates was coated by the nanocomposite films. Additionally, 100 μl of 106 cfu/ml microorganisms containing suspension which were previously inoculated into 10 ml of tryptic soy broth (TSB Merck, Germany) was poured into plates. The incubation of the plates took place in 37 °C (the optimum temperature for the bacterial growth), and meanwhile the bacterial growth was monitored turbidometrically each 2 h (up to 24 h) at the wavelength of 600 nm via Bioscreen C (FB1100C/Finland). Ultimately, the growth curves of microorganisms over the incubation period were plotted.
In order to perform statistical analyses, SPSS 19.0 (SPSS, Chicago, IL) was used. All statistical tests were two-tailed, and a p value < 0.05 was considered to be significant. Between-group differences were also analyzed using analysis of variance (ANOVA).
Results and discussion
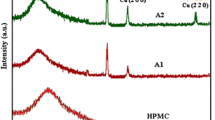
The X-ray diffraction of CuO nanopowders synthesized by the sonochemical method is presented in Fig. 1. Following Scherrer formula (Eq. 1), in this equation, D, λ, θ and β stand for the average grain size, the X-ray wavelength (0.15405 nm), the diffraction angle and the full width at half maximum of an observed peak, respectively.
The average crystalline size of the ZnO and CuO particles is 39.7 nm and 20 nm, respectively
According to the classical theory of nucleation, the construction of nanoparticles has three steps: (1) pre-nucleation, (2) the formation of metal nuclei or clusters, and (3) growth. A sonochemical hydrolysis mechanism has been advanced to explicate the non-oxidative reaction of metal species. The sonochemical synthesis of CuO from Cu+2 can be expressed as
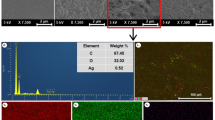
The extreme condition during the sonochemical process provides the means of the formation of a large number of product nuclei. The extreme condition could also assist us in avoiding the accumulation of newly formed nanoparticles. Accordingly, highly dispersed CuO nanoparticles (70 nm) were acquired without using a stabilizing agent (Fig. 2a). Figure 2b shows the SEM micrograph of CuO-containing nanocomposite. The image proves that the CuO nanoparticles are not aggregated.
Figures 3 and 4 present the growth curves of B. subtilis and E. aerogenes in the presence of ZnO- and CuO-containing nanocomposites and control nanocomposites. The curves represent the relationship between microbial population and optical density (OD) versus time of incubation.
Figures 5 and 6 verify this hypothesis that both ZnO- and CuO-containing nanocomposites can have a substantial role in reducing the growth of bacteria. These findings are in accordance with several previous studies. In a study by Emamifar et al. [17], 0.25% ZnO-containing LDPE nanocomposite significantly reduced the numbers of Lactobacillus plantarum. In another investigation, gelatin/ZnO nanocomposite films showed a strong antibacterial effect on food-borne pathogenic bacteria. These nanocomposite films inhibited the growth of both gram-positive Listeria monocytogenes and gram-negative Escherichia coli [18]; furthermore, it has been outlined that the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) films prolong the antimicrobial activity against L. monocytogenes [19]. In a recent study by Beigmohammadi et al. [20], the researchers assessed the antibacterial effect of LDPE films containing silver, ZnO and CuO nanoparticles to see if they could be utilized in active packaging of ultra-filtrated cheese. They conducted an inquiry into different compositions of these nanoparticles to introduce the optimum sample for diminishing coliform load of the cheese with no toxicity. Ultimately, a composition of 0% Ag, 0% ZnO and 1% CuO was introduced to serve this end. Yadollahi et al. [21] have reported that carboxymethyl cellulose/CuO bio-nanocomposite hydrogels have noticeable antibacterial effects on gram-positive staphylococcus aureus and gram-negative E. coli. Further research studies conducted by Llorens et al. [2] have also verified the reduction of 4 log cycles a load of yeasts and molds which are present in pineapple juice by using cellulose/copper composites.
The exact procedure of the antimicrobial activity of metal nanoparticles is still unknown. However, several studies have suggested the formation of reactive oxygen species (ROS) as the main mechanism responsible for the antimicrobial activity of these nanoparticles. The reactive oxygen species include hydrogen peroxide (H2O2), superoxide ions (O2−) and hydroxide (OH−). High reactivity and oxidizing property of ROS incur toxicity to bacteria. These species can pass the bacterial cell membrane and damage the cellular components such as lipids, DNA and proteins [22]. It is likely that the internalization of nanoparticles is another mechanism for their antibacterial activity. Nanoparticles attach to the bacterial cells and subsequently rupture the cell membrane. Once nanoparticles internalize the bacteria, they inhibit the energy metabolism of cells [22, 23]. Several studies have reported that ROS generation by the CuO nanoparticles attached to the bacterial cells causes the toxicity of CuO nanoparticles [24,25,26]. Some other studies list the interaction of copper with the thiol (–SH) groups of bacterial proteins and enzymes as possible antibacterial mechanisms [27]. Furthermore, the antibacterial effect may be due to the penetration of copper ions to the bacteria cell membrane and as a result alteration of the permeability and functionality of the outer cell membrane [28, 29] that ultimately leads to damaging DNA and vital enzymes of bacteria [30,31,32].
As represented in Fig. 3 and Fig. 4, CuO-containing nanocomposites had a stronger antibacterial effect on both B. subtilis and E. aerogenes compared with the nanocomposite films containing the same amount of ZnO. A previous research compared the antibacterial effects of nanocopper oxide (CuO)- and nanozinc oxide (ZnO)-coated orthodontic brackets on Streptococcus mutans. The findings of the study confirmed that CuO and ZnO–CuO nanoparticles-coated brackets were superior to ZnO-coated brackets in terms of the antimicrobial effect on S. mutans [33]. In a more recent study, Duffy et al. [34] compared the antibacterial efficacy of silver, ZnO and CuO nanoparticles against Salmonella and Campylobacter strains; they concluded that the minimum inhibitory concentration (MIC) for all Campylobacter strains was in the order of Ag > CuO > ZnO nanoparticles. Our findings are in consistence with these results; however, in an investigation by Azam et al. [35], it was decided that out of three metal oxide nanomaterials (ZnO, CuO and Fe2O3), ZnO showed the greatest antimicrobial activity against both gram-positive and gram-negative bacteria, while Fe2O3 nanoparticles exhibited the least bactericidal activity. They suggested that the stronger antimicrobial effect of ZnO nanoparticles was due to their smaller size (18 nm) compared to CuO (22 nm) and Fe2O3 (26 nm). In the present study, the sizes of ZnO and CuO nanoparticles were 39.7 nm and 20 nm, respectively. Therefore, the greater efficiency of CuO nanoparticles in inhibiting the bacterial growth could be associated with their smaller size. The size of the nanoparticles is directly correlated with many essential properties, such as surface property, solubility and chemical reactivity, and these properties can exert an influence on the interactions between nanoparticles and biomolecules. A reduction in size results in the enlargement of nanoparticle’s specific surface area which in turn increases reactivity and interactions between nanoparticles and microorganisms [36]. Moreover, the smaller particles perforate the bacterial cells and disorganize the cell membrane [37]. In addition to size, the higher oxidizing power of Cu compared with Zn can also affect the antibacterial efficiency. Ivask et al. [23] reported that CuO nanoparticles can bring high amounts of oxidative damage and can even surpass Ag nanoparticles in this regard. It is known that ions of redox-active metals, including copper, can produce free radicals by using the Fenton-type reaction and inflicting intracellular oxidative stress [38]
In a study on antimicrobial properties of CuO nanoparticles by Ren et al. [15], it was demonstrated that the minimum bactericidal concentrations (MBCs) of CuO nanoparticles for decreasing the amount of seven strains of gram-positive and gram-negative bacteria were lower than MBCs of ZnO nanoparticles.
As shown in growth curves of bacteria (Figs. 3, 4), B. subtilis as gram-positive bacteria is more reactive to both nanocomposites compared to E. aerogenes as gram-negative bacteria. In line with our findings, Tam et al. [39] concluded that ZnO nanorods are more effective against gram-positive bacterium Bacillus atrophaeus compared with gram-negative E. coli. Azam et al. [35] also asserted that CuO nanoparticles have a stronger antibacterial effect on B. subtilis and S. aureus in comparison with E. coli and Pseudomonas aeruginosa as gram-negative bacteria. In another research by Bhuyan et al. [40], the antibacterial effect of Cu-doped ZnO nanorods was more conspicuous for gram-positive bacteria (S. aureus and Streptococcus pyogenes) in comparison with gram-negative bacteria (E. coli).
Different factors such as the cell structure, physiology, metabolism or degree of contact of microorganisms with nanoparticles can determine the variations in sensitivity of nanocomposites between gram-positive and gram-negative bacteria [35]; however, the differences in cell wall structure are considered to be the main determiner of this sensitivity. Gram-positive bacteria have a thick peptidoglycan layer which contains teichoic acids. Phosphodiester bonds link these teichoic acids. The phosphodiester bonds give an overall negative charge to the gram-positive bacteria; meanwhile, gram-negative bacteria lack in teichoic acids and phospholipids and lipopolysaccharides surround a thin peptidoglycan layer [8, 16]. Therefore, positively charged ions are more inclined to attach to gram-positive B. subtilis compared with gram-negative E. aerogenes.
To investigate the presence of nanoparticles on the surface of polymer films, we have used XPS analysis. The results of the study verified the purity of the synthesized films since no other chemicals except for the zinc, copper oxide and carbon-based polymer were observed on the surface of the films. The peak of Cu 2p3/2 at 932.98 eV with two shake-up satellites at higher binding energy and the Zn 2p at 1043 eV is attributed to CuO and ZnO binding on the surface. Besides, the presence of carbon-based polymer on the surface of polymer films is confirmed by peak C (1s) at 284.6 eV. The peak at about 530 eV represents O (1s) level in ZnO and CuO, which is surrounded by Zn and Cu atoms. The XPS results (Figs. 5, 6) also show that the presence of CuO nanoparticles on polymer surface is greater than ZnO nanoparticles. It can be safely deduced that the interaction between CuO nanoparticles and B. subtilis and E. aerogenes increases with the expansion of surface concentration.
The ultimate purpose of the present study is to develop nanocomposites for active food packaging application. In this regard, the effect of incorporation of nanoparticles on the barrier properties of nanocomposites can be an important issue. Although in this study we did not investigate the permeability of CuO and ZnO nanocomposites, there are several studies which show that metal nanoparticles can improve the barrier properties of polymer composites. In a study by Polat et al., the investigators assessed the permeability of Ag and ZnO LDPE nanocomposites and concluded that the addition of nanoparticles reduced the oxygen and water vapor transmission rates. The proposed mechanism by the authors is that impermeable nanoparticles fill the amorphous regions of the polymer matrix and lead to a decrease in the area available for diffusion [41]. In a recent review study by Abbas et al., it has been concluded that the barrier properties of the nanocomposites can be improved by a high aspect ratio, uniform dispersion and low incorporation (up to 5 wt%) of nanoparticles within the polymer matrix [42].
Conclusion
In the present study, both ZnO- and CuO-containing nanocomposites inhibited the growth of bacteria. Furthermore, CuO-containing nanocomposite had stronger antibacterial effects on both gram-positive and gram-negative bacteria compared with ZnO-containing nanocomposite. Our results also substantiated that the gram-positive B. subtilis was more sensitive to both nanocomposites than gram-negative E. aerogenes.
In view of the fact that inorganic antimicrobial materials such as Zn and Cu easily incorporated with different polymers and were stable in the processing procedure, it can be concluded that polymer nanocomposites are highly appropriate materials to be used in active food packaging with the aim of extending the shelf life of foods. However, further studies are needed to investigate the safety aspects of their application in the food industry.
References
Appendini P, Hotchkiss JH (2002) Review of antimicrobial food packaging. Innov Food Sci Emerg Technol 3(2):113–126
Llorens A, Lloret E, Picouet P, Fernandez A (2012) Study of the antifungal potential of novel cellulose/copper composites as absorbent materials for fruit juices. Int J Food Microbiol 158(2):113–119
de Azeredo HM (2013) Antimicrobial nanostructures in food packaging. Trends Food Sci Technol 30(1):56–69
Jones N, Ray B, Ranjit KT, Manna AC (2008) Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 279(1):71–76. https://doi.org/10.1111/j.1574-6968.2007.01012.x
Lee KT (2010) Quality and safety aspects of meat products as affected by various physical manipulations of packaging materials. Meat Sci 86(1):138–150
Emamifar A, Kadivar M, Shahedi M, Soleimanian-Zad S (2010) Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice. Innov Food Sci Emerg Technol 11(4):742–748
Zhang L, Jiang Y, Ding Y, Povey M, York D (2007) Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J Nanoparticle Res 9(3):479–489. https://doi.org/10.1007/s11051-006-9150-1
Gold K, Slay B, Knackstedt M, Gaharwar AK (2018) Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv Ther 1:1700033
Rezić I, Haramina T, Rezić T (2017) 15—Metal nanoparticles and carbon nanotubes—perfect antimicrobial nano-fillers in polymer-based food packaging materials. In: Grumezescu AM (ed) food packaging. Academic Press, Berlin, pp 497–532. https://doi.org/10.1016/B978-0-12-804302-8.00015-7
Kalyani RL, Venkatraju J, Kollu P, Rao NH, Pammi SVN (2015) Low temperature synthesis of various transition metal oxides and their antibacterial activity against multidrug resistance bacterial pathogens. Korean J Chem Eng 32(5):911–916
Tamayo L, Azócar M, Kogan M, Riveros A, Páez M (2016) Copper–polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater Sci Eng C 69:1391–1409
Llorens A, Lloret E, Picouet PA, Trbojevich R, Fernandez A (2012) Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci Technol 24(1):19–29. https://doi.org/10.1016/j.tifs.2011.10.001
Rhim J-W, Park H-M, Ha C-S (2013) Bio-nanocomposites for food packaging applications. Prog Polym Sci 38(10–11):1629–1652
Hoseinnejad M, Jafari SM, Katouzian I (2018) Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit Rev Microbiol 44(2):161–181
Ren G, Hu D, Cheng EWC, Vargas-Reus MA, Reip P, Allaker RP (2009) Characterisation of copper oxide nanoparticles for antimicrobial applications. Int J Antimicrob Agents 33(6):587–590
Esmailzadeh H, Sangpour P, Shahraz F, Hejazi J, Khaksar R (2016) Effect of nanocomposite packaging containing ZnO on growth of Bacillus subtilis and Enterobacter aerogenes. Mater Sci Eng C 58:1058–1063
Emamifar A, Kadivar M, Shahedi M, Soleimanian-Zad S (2010) Effect of nanocomposite packaging containing Ag and ZnO on inactivation of Lactobacillus plantarum in orange juice. Food Control 22(3–4):408–413
Shankar S, Teng X, Li G, Rhim J-W (2015) Preparation, characterization, and antimicrobial activity of gelatin/ZnO nanocomposite films. Food Hydrocoll 45:264–271
Castro-Mayorga JL, Fabra MJ, Pourrahimi AM, Olsson RT, Lagaron JM (2017) The impact of zinc oxide particle morphology as an antimicrobial and when incorporated in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for food packaging and food contact surfaces applications. Food Bioprod Process 101:32–44. https://doi.org/10.1016/j.fbp.2016.10.007
Beigmohammadi F, Peighambardoust SH, Hesari J, Peighambardoust SJ (2018) Inhibition of coliform bacteria in ultra-filtrated cheese packed in nanocomposite films containing Cloisite30B-metal nanoparticles. Nutr Food Sci Res 5(1):25–32
Yadollahi M, Gholamali I, Namazi H, Aghazadeh M (2015) Synthesis and characterization of antibacterial carboxymethylcellulose/CuO bio-nanocomposite hydrogels. Int J Biol Macromol 73:109–114
Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett 7(3):219–242
Ivask A, Juganson K, Bondarenko O, Mortimer M, Aruoja V, Kasemets K, Blinova I, Heinlaan M, Slaveykova V, Kahru A (2014) Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: a comparative review. Nanotoxicology 8(Supp 1):57–71
Applerot G, Lellouche J, Lipovsky A, Nitzan Y, Lubart R, Gedanken A, Banin E (2012) Understanding the antibacterial mechanism of CuO nanoparticles: revealing the route of induced oxidative stress. Small 8(21):3326–3337
Ekthammathat N, Thongtem T, Thongtem S (2013) Antimicrobial activities of CuO films deposited on Cu foils by solution chemistry. Appl Surf Sci 277:211–217
Fu PP, Xia Q, Hwang H-M, Ray PC, Yu H (2014) Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal 22(1):64–75
Slavin YN, Asnis J, Häfeli UO, Bach H (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol 15(1):65
Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K (2014) Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C 44:278–284
Borkow G, Gabbay J (2009) Copper, an ancient remedy returning to fight microbial, fungal and viral infections. Curr Chem Biol 3(3):272–278
Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A (2012) Antimicrobial activity of metal oxide nanoparticles against gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomed 7:6003
Ahamed M, Alhadlaq HA, Khan M, Karuppiah P, Al-Dhabi NA (2014) Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles. J Nanomater 2014:17
Mahapatra O, Bhagat M, Gopalakrishnan C, Arunachalam KD (2008) Ultrafine dispersed CuO nanoparticles and their antibacterial activity. J Exp Nanosci 3(3):185–193
Ramazanzadeh B, Jahanbin A, Yaghoubi M, Shahtahmassbi N, Ghazvini K, Shakeri M, Shafaee H (2015) Comparison of antibacterial effects of ZnO and CuO nanoparticles coated brackets against Streptococcus mutans. J Dent 16(3):200
Duffy LL, Osmond-McLeod MJ, Judy J, King T (2018) Investigation into the antibacterial activity of silver, zinc oxide and copper oxide nanoparticles against poultry-relevant isolates of Salmonella and Campylobacter. Food Control 92:293–300
Azam A, Ahmed AS, Oves M, Khan M, Memic A (2012) Size-dependent antimicrobial properties of CuO nanoparticles against gram-positive and-negative bacterial strains. Int J Nanomed 7:3527
Chang Y-N, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 5(12):2850–2871
Applerot G, Lipovsky A, Dror R, Perkas N, Nitzan Y, Lubart R, Gedanken A (2009) Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv Funct Mater 19(6):842–852
Valko M, Morris H, Cronin M (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12(10):1161–1208
Tam K, Djurišić A, Chan C, Xi Y, Tse C, Leung Y, Chan W, Leung F, Au D (2008) Antibacterial activity of ZnO nanorods prepared by a hydrothermal method. Thin Solid Films 516(18):6167–6174
Bhuyan T, Khanuja M, Sharma R, Patel S, Reddy M, Anand S, Varma A (2015) A comparative study of pure and copper (Cu)-doped ZnO nanorods for antibacterial and photocatalytic applications with their mechanism of action. J Nanopart Res 17(7):288
Polat S, Fenercioğlu H, Güçlü M (2018) Effects of metal nanoparticles on the physical and migration properties of low density polyethylene films. J Food Eng 229:32–42
Abbas M, Buntinx M, Deferme W, Peeters R (2019) (Bio) polymer/ZnO nanocomposites for packaging applications: a review of gas barrier and mechanical properties. Nanomaterials 9(10):1494
Acknowledgements
The authors would like to thank the Research and Technology Council of National Nutrition and Food Technology Research Institute (721391010) and Materials and Energy Research Center (MERC) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Esmailzadeh, H., Sangpour, P., Shahraz, F. et al. CuO/LDPE nanocomposite for active food packaging application: a comparative study of its antibacterial activities with ZnO/LDPE nanocomposite. Polym. Bull. 78, 1671–1682 (2021). https://doi.org/10.1007/s00289-020-03175-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03175-7