Abstract
In this research copper nanoparticles (Cu NPs) were incorporated in the biodegradable hydroxypropyl methylcellulose (HPMC) matrix using the simple and low cost chemical reduction method for application as food packaging material. The properties of Cu/HPMC bionanocomposites (BNCs) were studied as a function of the CuSO4 concentration. Surface morphology of the film was investigated by scanning electron microscopy. Mechanical analysis and water vapor barrier properties of HPMC/Cu nanocomposites were analyzed. It was observed that mechanical and water vapor barrier properties of the films were improved by the concentration of CuSO4. The antibacterial activity of HPMC/Cu thin films were evaluated based on the diameter of inhibition zone in a disk diffusion test against Gram positive bacteria, ie, Streptococus A., S. epidermidis, S.aureus , B.cereus and Gram negative bacteria, ie, E. coli, E. faecalis, Salmonella, P. aeruginosa using Mueller Hinton agar at different concentration of CuSO4. The results revealed a greater bactericidal effectiveness for nanocomposite films containing 5 % of CuSO4. Packages prepared from HPMC/Cu nanocomposite films were used for meat packaging. The films were filled with meat and then stored at 4 °C. Microbial stability of the meat was evaluated after 3, 7, 10 and 15 days of storage. The results showed that microbial growth rate significantly reduced as a result of using this nanocomposite packaging material.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent global competitive markets, active packaging is an important concept to inhibit or retard the growth of microorganisms that may be present in the packed food or packaging material itself. The researches in antimicrobial packaging have been expanded to extend of the shelf life or enhance quality and safety of the food products. The incorporation of antimicrobials into packaging brought in a new generation of nanocomposites comprising antibacterial nanoparticles blended in the polymer matrix.
Among the cellulose-based materials, Hydroxypropyl methylcellulose (HPMC) is a semisynthetic, inert, viscoelastic biopolymer. HPMC has been examined for many different applications due to its biocompatibility, non-toxicity to mammals, barrier properties, low cost, antimicrobial activity and solubility in aqueous medium (Imran et al. 2010). Moreover, HPMC films are water-soluble, odorless and tasteless, and present moderate resistance to moisture and oxygen permeability (Krochta and Mulder-Johnston 1997). Consequently, they have a variety of current and potential applications in deep frying food products (Balasubramaniam et al. 1997; Kester and Fennema 1986) drug delivery and ophthalmology (De Silva and Olver 2005; Williams et al. 2001) and food processing (Moura et al. 2012; Hiroyasu 2003).
Nanomaterials are being widely used as they offer antimicrobial properties due to their high surface area/volume ratio. Gold and silver nanoparticles are the most widely studied particles. Different inorganic antibacterial materials, including Au and Ag nanoparticles have been expanded and some are in commercial use (Kawashita et al. 2000). In recent years, copper nanoparticles (Cu NPs) have attracted great interest due to their potential applications, such as, conductive films, lubrication, nanofluids, ink, metallic coatings and catalysis (Tilaki et al. 2007; Zhu et al. 2005; Larsen and Noriega 2004; Patel et al. 2005; Wang et al. 2004). The antimicrobial activity of copper nanoparticles (Cu NPs) is a new features issued against different microorganism (SCI Finder Scholar™, American Chemical Society). Since the copper has been known as an antibacterial agent (Faundez et al. 2004; Mary et al. 2009; Young et al. 2012), higher disinfecting effects are expected in nano scales. However, no study has been conducted on application of the Cu NPs for antibacterial food packaging.
In this work, Cu NPs have been embedded in a biodegradable polymer matrix, as a means to combine the antimicrobial properties of copper with the biocompatible and antimicrobial features of HPMC for active food packaging.
Materials and methods
Chemicals
Hydroxypropyl methylcellulose (Methocel E15) was obtained from Dow Chemical Co. (Midland, MI, USA.). CuSO4 (99.98) used as the copper precursor, was obtained from Merck (Darmstadt, Germany) and used without purification. Deionized water was used to prepare all the solutions employed. NaBH4 (98.5 %), used as a reduction agent was obtained from Sigma-Aldrich (St Louis, MO).
Gram positive: Streptococus A. (ATCC 19615), S. epidermidis (ATCC 12228), S.aureus (ATCC 25922) B.cereus (ATCC 11788), and Gram negative: E. coli (ATCC 25923), E. faecalis (ATCC 29212), Salmonella (ATCC 14028), P. aeruginosa (ATCC 27853) bacteria, used for the antibacterial assay were obtained from the Bahar Afshan Ltd, Iran.
Synthesis of (Cu NPs)/HPMC film
Net HPMC films (control films) were obtained by casting from a solution containing 3.0 g of HPMC in 100 ml of distilled water kept under magnetic stirring for 12 h. The HPMC: water ratio was kept at 3:97 (w: w) in all film-forming solutions. Following the usual preparation method for Cu NPs, CuSO4 solutions were added to each soluble HPMC sample under constant stirring for synthesis of the CuSO4/ HPMC solutions. The Cu content of the samples was 0.5, 1.0, 2.0, and 5.0 g Cu/100 g HPMC. Freshly prepared NaBH4 (4 × 10−2 M) solution was then added to the suspensions under continuous stirring to reach a constant CuSO4 /NaBH4 molar ratio (1:4). After the addition of the reducing agent, stirring was continued for another hour. The suspensions of Cu/HPMC BNCs were kept closed during 4 h to prevent micro bubble formation. The solutions were poured into a glass plate (30 × 30 cm) for films formation. Films were obtained at a wet thickness of 0.5 mm using casting bars and the plates were placed on a leveled surface at room temperature and let dry for 25 h. After drying, the films were removed and conditioned in sealed plastic bags, stored at room temperature.
Evaluation of antibacterial activity
The in vitro antibacterial activity of the samples was evaluated by the well diffusion method using Mueller Hinton agar with determination of diameter of the inhibition zone formed around the well, which conformed to the recommended standards of the National Committee for Clinical Laboratory Standards (NCCLS, 2000). Petriplates containing 20 ml Muller Hinton medium were seeded with 24 h culture of bacterial strains. Wells were cut and 20 μl of the BNC solution were added. The plates were then incubated at 37 °C for 24 h. Streptomycin antibiotic was used as a positive control and Dimethyl sulfoxide was used as a negative control.
Characterization methods and instrument
The structures of the Cu/HPMC BNCs produced were examined by powder X-ray diffraction using the Siemens-D5000-1. SEM was performed using the Jeol JXA-840 instrument to study the morphology of Cu/HPMC BNCs. The water vapor permeability (WVP) was determined using a Labthink WVP System to determine the relative humidity (RH) at the films underside, according to Angles (Angles and Dufresne 2001). Three samples from each formulation were chosen and after drying for 24 h cut onto 23 × 23 × 0.1 mm diameter. After initial weighting, samples moved to desicator at 25 °C with 75 % relative humidity. Weights were taken periodically after steady state was achieved and used to calculate the percentage of RH at the films underside using following relation:
Where W0 is initial weight of the sample and Wt is the weights after t seconds. The mechanical properties of the composites were evaluated in rectangular pieces of the film with dimensions chosen in accordance to ASTM (1997) and conditioned at 24 °C for 48 h before measurements. An INSTRON instruments was used to determine the maximum tensile strength (TS) and elongation at break. Films were stretched up using a speed of 5 mm min−1. Tensile properties were calculated from the plot of stress (tensile force/initial cross-sectional area) versus strain (extension as a fraction of the original length). The mechanical properties were analyzed as a function of nanoparticles ratio by weight in the films.
Preparation and storage of meat in packages
To prepare minced, 300 g of fresh fatty beef meat were purchased from the local market in Tabriz, Iran. They were grinned using a semi-industrial meat grinder (Buffalo CB943 Meat Grinder, China), thoroughly washed with detergent and hot water. For perfect mixing of fat and meat the grind was repeated two times. The minced immediately transferred into a sterile glass container under sanitized conditions, sterilized in an autoclave at 121 °C for 15 min and refrigerated at 4 °C until processed. Packages were prepared by a hand heat sealer using Cu/HPMC BNC films and pure HPMC films 15 × 10 cm in size. The packages were immediately wrapped in aluminum foil and sanitized at 95 °C for 2 min. After cooling and under a sterile laboratory hood, 10 g of mince was poured into each package and sealed by the heat sealer. Packages containing mince were stored in dark and cool conditions (4 °C) and microbial counts of the samples were evaluated in duplicate by pour plate method using MRS agar and incubation for 48 h at 37 °C under CO2 atmosphere (5 %), immediately after packaging and after 3, 7, 10 and 15 days of storing.
Results and discussion
X-ray diffraction analysis
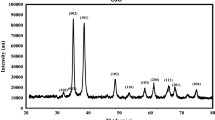
Figure 1 showed the X-ray diffraction pattern of net HPMC and Cu/HPMC BNCs film with different concentration of CuSO4 (0.5 and 5.0 %). The X-ray diffraction pattern of HPMC film showed a broad scattering peak which indicated its highly amorphous structure. The Miller indices are shown above the diffractions. The diffraction patterns for Cu/HPMC BNCs showed peaks of Cu NPs (JCPDS file 04–0836) phase corresponding to (111), (200) and (220) crystallographic planes of the face-centered cubic copper crystals. The intensities of (111), (200) and (220) reflections due to the Cu NP phase were also found to increase along with the increased Cu NPs in the solid support matrix. For all samples, the main crystalline phase was copper, and no other obvious phases were found as impurities in the XRD patterns. The crystallite size of Cu NPs incorporated in the Cu/HPMC BNCs film was calculated by using Scherrer’s formula for the (111) peak for 2θ of 43.37° and was found to be 30 nm.
Morphology
The surface morphology of the synthesized Cu/HPMC BNC film is evaluated by SEM. Figure 2 shows the SEM image of the Cu/HPMC BNC, where the bright particles correspond to the immobilized copper nanoparticles. It is observed that the films are uniform and the nanoparticles are homogeneously distributed in the polymer matrix. The mean diameters of the Cu NPs were about 53.28 ± 15.54 nm.
Mechanical analysis
With regard to the application of these films in packaging, mechanical stability is important to maintain integrity during processing and flexibility during shipping or handling. Tensile strength, elastic modulus, and elongation analyses describe how the mechanical properties of such film materials relate to their chemical structures (Ninnemann 1968). Figures 3 and 4 show the tensile strength and elongation at break of the Cu /HPMC BNCs in different concentration of CuSO4. As it is observed, the incorporation of Cu NPs in polymer matrix increased the mechanical resistance and elongation at break of the film for all concentrations, which leads to film of high toughness. This can be related to increase interaction of the nanoparticles and polymer chain and increase intermolecular bonding accordingly. The maximum tensile strength and elongation was observed for the film with 5 % CuSO4 at 49.3 ± 1.0 (MPa) and 87.6 ± 2.0 (%), respectively.
Water vapor permeability
Water vapor barricading is critical in the packaging of moisture sensitive foods and pharmaceuticals to achieve the required quality, safety, and shelf life. Most edible films are characterized by a high water vapor permeation, which makes them inappropriate for several applications (Torres 1994). The hydrophilic natures of polysaccharide films make them poor water barriers. The copper nanoparticles effect on the relative humidity at film underside is shown in Fig. 5 for pure HPMC film and Cu/HPMC films with different CuSO4 concentration.
Upon addition of nanoparticles in HPMC matrix, a decrease in the WVP values and relative humidity at film underside was observed. The RH at film underside value was 70 % for the native HPMC film. Addition of nanoparticles induced a decrease in WVP values and RH at film underside. The RH values and WVP varied from 68 ± 0.9 and 60 ± 0.1 % for HPMC films containing 0.5 % CuSO4 to 37 ± 0.9 and 41 ± 0.5 % for films containing 5.0 % CuSO4 respectively. The presence of nanoparticles reduced the intermolecular spacing within the films and increase in cohesion structures, thus reducing the water vapor permeability through of film. In the other words, small size nanoparticles have more ability in occupying the empty spaces of the porous HPMC film matrix, and prevent the diffusion of water into the film.
Antibacterial activity
Inhibition zone values were obtained for the synthesized Cu/ HPMC BNCs tested against Gram positive and Gram negative bacteria listed in Table 1.The tests were repeated three times for each treated samples, are presented as average values in Table 1 and Fig. 6, respectively. The result shows that the Cu NPs in HPMC suspension had high antibacterial activity against Gram-positive bacteria and did not show any antibacterial activity against Gram negative bacteria. Inhibition effect of Cu NPs in lower concentrations did not observe except for Strept A, with 13 mm inhibition zone. Maximum inhibition was observed for Streptocucus A and S.epidermis.
The rigid cell walls of Gram negative bacteria limit the entry of hydrophobic and large molecules to reach the nuclear content of bacteria. Several mechanisms argue for the antibacterial activity of nanoparticles, including generation of oxygen species for degradation of cell structure or release of ions from the surface of nanoparticles to binding cell membrane (Morones et al. 2005; Lee et al. 2005; Lok et al. 2006; Sawai 2003). The result reinforces the idea that the lipopolysaccharide proteins in cell wall structures of Gram negative bacteria protect them against Cu NPs attack (Yoon et al. 2007). As well inclination of Cu NPs to amin and carboxyle groups on the cell walls of Gram positive bacteria is the reason for inhibition effect of Cu NPs against these bacteria (Ruparelia et al. 2009). With the regard to the excessive antibacterial effect of HPMC/Cu NPs film against Gram positive bacteria which is responsible to deterioration of foods and clinical infections, it can be used as substitute for commercial chemical antibiotics.
Mean microbial population during refrigerated storage
Beef packaged in a high oxygen modified atmosphere typically retains a shelf-life of ten to 14 days for ground beef and 5 to 7 days for minced beef (Cornforth and Hunt 2008; Belcher 2006; Márquez et al. 2012). The variations in the microbial population during storage are shown in Fig. 7 after 3, 7 and 10 days of storage. Mean initial microbial population immediately after packaging was determined to be 3.7 log cfu/mL in minced. In all of packages, the mean population increased after 3, 7 and 10 days of storage. According to Fig. 7, the level of microbial population increased to 8.91 log cfu/mL after 7 days of storage in HPMC pure packages which is higher than Cu/HPMC BNC 2.0 % and 5.0 %. In all samples (2.0, and 5.0 % Cu SO4), significance decreases were observed over 10 days of storage. However, microbial growth in Cu/HPMC BNC 2.0 %, and Cu/HPMC BNC 5.0 % compared with pure HPMC packages, showed a higher reduction up to 15 days of storage at 4 °C. The microbial population decrease with the increasing in Cu SO4 ratio from 2.0 to 5.0 %. Copper ions released from the surface of these nanoparticles can interact with thiol groups in protein to induce bacterial inactivation, condensation of DNA molecules, and loss of their replication ability (Feng et al. 2000). Based on electron spin resonance (ESR) measurements, the antimicrobial mechanism of nanoparticles, are related to the formation of free radicals and the subsequent free radical-induced membrane damage (Kim et al. 2007).
Conclusion
Cu NPs were successfully prepared from CuSO4/HPMC suspension at different CuSO4 concentrations by using NaBH4 as a chemical reduction agent without any heat treatment or reducing agent. The XRD analysis confirmed that the crystallographic planes of the copper crystals were of the face-centered cubic type. SEM images show that the external morphology of Cu/HPMC BNCs is uniform with shiny points due to the presence of Cu NPs. Tensile strength (mechanical stability) and elongation at break (elasticity) of the synthesized Cu/HPMC BNCs increase by the presence of Cu NPs to 49.3 ± 1.0 MPa and 87.6 ± 2.0 % respectively. The decrease observed in the WVP values for the HPMC/Cu NPs system, in comparison to net HPMC systems. The antibacterial activities of Cu/HPMC BNCs at the different Cu NPs ratio showed strong antibacterial activity against Gram-positive bacteria. These results indicate that Cu/HPMC BNCs can be used in food packaging for certain bacterial inactivation and control. The prepared Cu/HPMC BNCs packages showed a significant antimicrobial activity compared with pure HPMC in minced packaging.
References
Angles MN, Dufresne A (2001) Plasticized starch/ Tunicin Whiskers nanocomposites. Macromolecules 34:2921–2931
Balasubramaniam VM, Chinnan MS, Mallikarjunan P, Phillips RD (1997) The effect of edible film on oil uptake and moisture retention of a deep-fat fried poultry product. J Food Process Eng 20:17–29
Belcher JN (2006) Industrial packaging developments for the global meat market. Meat Sci 74:143–148
Cornforth, D. and Hunt, M. (2008). Low-oxygen packaging of fresh meat with carbon monoxide. Meat quality, microbiology, and safety. AMSA White Paper Series, Number 2, pp. 1-10. American Meat Science Association, Savoy, Illinois, USA.
De Silva DJ, Olver JM (2005) Hydroxypropyl methylcellulose (HPMC) lubricant facilitates insertion of porous spherical orbital implants. Ophthal Plast Reconstr Surg 21(4):301–2
Faundez G, Troncoso M, Navarrete P, Figueroa G (2004) Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and campylobacter. BMC Microbiol 4:19–25
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52(4):662–668
Hiroyasu K (2003) The applications and functions of hydroxypropyl methylcellulose as a new food additive. Foods Food Ingredients J Jpn 208:6
Imran M, El-Fahmy S, Revol-Junelles A-M, Desobry S (2010) Cellulose derivative based active coatings: effects of nisin and plasticizer on physico-chemical and antimicrobial properties of hydroxypropyl methylcellulose films. Carbohydr Polym 81:219–225
Kawashita M, Tsuneyama S, Mijaji F et al (2000) Antibacterial silver containing silica glass prepared by the sol-gel method. Biomaterials 21:393–398
Kester JJ, Fennema OR (1986) Edible films and coatings: a review. Food Technol 40:47–59
Kim JS, Kuk E, Yu K, Kim JH, Park SJ, Lee SJ et al (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine: Nanotechnol Bio Med 3:95–101
Krochta JM, Mulder-Johnston C (1997) Edible and biodegradable polymer films: challenges and opportunities. Food Technol (Chicago) 51:61–74
Larsen G, Noriega S (2004) Dendrimer-mediated formation of Cu- CuOx nanoparticles on silica and their physical and catalytic characterization. Appl Catal A Gen 278:73–81
Lee D, Cohen RE, Rubner MF (2005) Antibacterial properties of Ag nanoparticle loaded multilayers and formation of magnetically directed antibacterial microparticles. Langmuir 21:9651–9659
Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H et al (2006) Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res 5:916–924
Márquez G., Ordóñez J. A., Cambero M. I., Cabeza M. C. Use of E-Beam for Shelf-Life Extension and Sanitizing of Marinated Pork Loin. International Journal of Microbiology. (2012). Article ID 962846, 12 pages.
Mary G, Bajpai SK, Chand N (2009) Copper alginate-cotton cellulose (CACC) fibers with excellent antibacterial properties. J Eng Fiber Fabr 4:24–35
Morones JR, Elechiguerra JL, Camacho A et al (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353
Moura MRD, Mattoso LH, Zucolotto V (2012) Development of cellulose-based bactericidal nanocomposites containing silver nanoparticles and their use as active food packaging. J Food Eng 109:520–524
Ninnemann KW (1968) Measurements of physical properties of flexible films. In: Sweeting OJ (ed) Science and Technology of Polymer Films. Interscience, London, England, pp 546–649
Patel MK, Nagare BJ, Bagul DM, Haram SK, Kothari DC (2005) Controlled synthesis of Cu nanoparticles in fused silica and BK7glasses using ion bean induced defects. Surf Coat Technol 196:96–99
Ruparelia JP, Kumar CA, Duttagupta SP, Diao M, Yao M (2009) Use of zero-valent iron nanoparticles in inactivating microbes. Water Res 43:5243–5251
Sawai J (2003) Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J Microbiol Methods 54(177):182
Tilaki RM, Iraji ZA, Mahadavi SM (2007) Size, composition and optical properties of copper nanoparticles prepared by laser ablation in liquids. Appl Phys A Mater Sci Process 88:415–4192
Torres JA (1994) Edible films and coatings from proteins. In: Hettiarachchy NS, Ziegler GR (eds) Protein Functionality in Food Systems. IFT Basic Symposium Series. Marcel Dekker, New York, pp 467–507
Wang H, Huang Y, Tan Z, Hu X (2004) Fabrication and characterization of copper nanoparticles thin-films and the electrocatalytic behavior. Anal Chim Acta 526:13–17
Williams RO, Sykora MA, Mahaguna V (2001) Method to recover a lipophilic drug from hydroxypropyl methylcellulose matrix tablets. AAPS Pharm Sci Tech 2(2):E8
Yoon K, Byeon JH, Park J, Hwang J (2007) Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ 373:572–5
Young HK, Yu-ri C, Kwang-Mahn K, Se-Young C (2012) Evaluation of copper ion of antibacterial effect on Pseudomonas aeruginosa, Salmonella typhimurium and Helicobacter pylori and optical, mechanical properties. Appl Surf Sci 258:3823–3828
Zhu H, Zhang C, Yin Y (2005) Novel synthesis of copper nanoparticles: influence of the synthesis conditions on the particle size. Nanotechnology 16:3079–3083
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ebrahimiasl, S., Rajabpour, A. Synthesis and characterization of novel bactericidal Cu/HPMC BNCs using chemical reduction method for food packaging. J Food Sci Technol 52, 5982–5988 (2015). https://doi.org/10.1007/s13197-014-1615-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1615-0