Abstract
Currently, biopolymer electrolytes are attracting a great deal of interest as substitute for synthetic polymer electrolytes in electrochemical devices, as they are carbon neutral, sustainable, reduce dependency on non-renewable fossil fuels and easily biodegradable. Some of the biopolymers under research are chitosan, pectin, agar–agar, cellulose acetate and carrageenan. The current work deals with the study of ion conducting polymer electrolyte, pectin with magnesium chloride salt for magnesium battery application. Biopolymer electrolytes of different compositions of pectin with different concentrations of magnesium chloride salt are prepared by solution casting technique and subjected to various studies like by X-ray diffraction (XRD), Fourier transform infrared (FTIR), differential scanning calorimetry (DSC), AC impedance spectroscopy and linear sweep voltammetry (LSV). XRD analysis has been used to identify the amorphous/crystalline nature of the sample. The complex formation between the polymer pectin and the magnesium chloride salt has been analyzed by FTIR spectroscopy. DSC analysis is a thermo-analytical technique which is used to observe the glass transition temperature (Tg) of the samples. AC impedance technique has been used to find the ionic conductivities of the sample. The electrochemical stability of the polymer electrolyte has been analyzed by linear sweep voltammetry. Among the prepared polymer electrolytes, 30 M wt% pectin: 70 M wt% MgCl2 offers the highest ionic conductivity of 1.14 × 10−3 S cm−1. The electrochemical stability of the highest conducting sample is 2.05 V. The primary magnesium battery has been constructed using the highest conducting sample, 30 M wt% pectin: 70 M wt% MgCl2, and the battery performance has been studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At present, the modern society relays on clean, renewable, inexhaustible and eco-friendly energy sources for livelihood. Storing of electrical energy gains more importance in order to meet the growing demand of global energy, and novel battery systems have become a vital area of research. Synthetic solid polymers like PEO, PAN, PVA, PVP and PVDF have been used in the preparation of polymer electrolyte [1,2,3,4,5] for electrochemical devices like batteries, fuel cells, super capacitors, electro-chromic displays, smart window and solar cells owing to its advantage such as no leakage of electrolyte, offers a good mechanical, chemical and thermal stability. Blend polymer electrolytes, salt-doped polymer electrolytes, filler and plasticizer added polymer electrolytes are prepared using these synthetic polymers. The major disadvantage of synthetic solid polymer electrolytes is that they are not biodegradable. Owing to the drawback of synthetic solid polymer electrolytes, the world is seeking for new materials to be used as the electrolytes. Biopolymer electrolytes are biodegradable, sustainable, renewable and eco-friendly. Yulianti et al. [6] synthesized a solid-state polymer electrolyte based on natural polymer, chitosan by incorporating various ion salts (Li, Cu, Ag) in the polymer matrix. Zulkefli et al. [7] prepared a proton-conducting biopolymer electrolyte based on corn starch and NH4SCN salt. Khiar et al. [8] synthesized a proton-conducting polymer electrolyte based on starch and ammonium nitrate (NH4NO3). Moniha et al. [9, 10] synthesized two biopolymer electrolytes based on iota-carrageenan with ammonium thiocyanate and ammonium nitrate (NH4NO3) by solution casting technique for electrochemical devices application. Chitra et al. [11] synthesized and studied the characteristics of a biopolymer electrolyte consisting of iota-carrageenan and LiCl. Christopher Selvin et al. [12] synthesized and studied the characteristics of a proton-conducting solid biopolymer electrolyte (SBE) kappa-carrageenan incorporated with ammonium thiocyanate (NH4SCN). Isa and Samsudin [13] studied biopolymer carboxymethylcellulose (CMC) electrolyte system, prepared by solution casting technique for application in solid-state battery. Rani et al. [14] prepared the biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Raphael et al. [15] prepared a polymer electrolytes based on agar and acetic acid. A biopolymer membrane using tamarind seed polysaccharide (TSP) with various concentrations of lithium chloride (LiCl) was prepared by Kumar et al. [16].
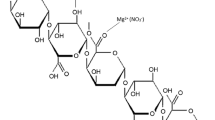
Biopolymer pectin is receiving a lot of attention as it is a naturally occurring substance found in berries, apples, plums, oranges, guavas and other citrus fruit. Pectin consists of a long chain of polygalacturonic acid linked together by α(1–4) glycoside bonds with side chains of arabinans, galactans, rhamnose and xylose. It is commercially available in powder form as white to light brown in color. In food industry, pectin is used as a gelling agent, mainly in jams and jellies. It is also used as thickening agent and stabilizer in food. Some food additives contain about 0.5–1.0% of pectin [17]. In medical field, pectin can ease constipation and softens the stool. It is used to prevent colon cancer and prostate cancer. Pectin is also used to prevent poisoning caused by lead, strontium and other heavy metals. Non-toxic, renewable, water solubility and biodegradable property of pectin made the researchers to use pectin as the polymer electrolyte for electrochemical application. Pectin structure has more hydroxyl groups, and it has oxygen atom which aids the formation of coordination bond with cations. The possible interaction of pectin with MgCl2 salt is shown in Scheme 1.
Perumal et al. [18] prepared two different solid biopolymer electrolytes based on pectin by solution casting technique and reported that 50 (m.m.%) pectin: 50 (m.m.%) LiCl membrane offered the highest ionic conductivity of 1.96 × 10−3 S cm−1 which is greater than LiClO4 incorporated electrolyte which showed the ionic conductivity of 5.38 × 10−5 S cm−1 for 60 (m.m.%) pectin: 40 (m.m.%) LiClO4 membrane. A conductivity value of 1.5 × 10−3 S cm−1 for 40 mol% pectin: 60 mol% NH4SCN has been reported by Muthukrishnan et al. [19]. Vijaya et al. [20] reported the highest ionic conductivity value of 4.52 × 10−4 S cm−1 for 30 mol% NH4Cl-doped pectin and 1.07 × 10−3 S cm−1 for 40 mol% NH4Br-doped pectin. Kavitha et al. [21] reported 6.64 × 10−5 S cm−1 as the maximum conductivity value for 70 mol% pectin: 30 mol% NH4NO3 at ambient temperature. Mendes et al. [22] reported the conductivity as 1.43 × 10−6 S cm−1 at 25 °C for pectin 0.43[N1112(OH)] [NTf2] composition and 6.03 × 10−5 S cm−1 at 90 °C for pectin 0.43[N1112(OH)] [NTf2] composition.
Today, magnesium-based batteries are considered superior to lithium-based batteries due to the following reasons: high energy density, more abundant in nature, less expensive, easy to handle and much safer. Mahalakshmi et al. [23] prepared the magnesium ion conducting biopolymer electrolytes using cellulose acetates and different wt% of magnesium perchlorate with DMF as a solvent by the solution casting technique and reported that 40% CA:60% Mg(ClO4)2 membrane offers the maximum ionic conductivity of 4.05 × 10−4 S cm−1 at room temperature. Mg-ion conducting solid electrolytes based on tamarind seed polysaccharide (TSP) with magnesium perchlorate (Mg(ClO4)2) salt have been prepared by Perumal et al. [24] and reported that the TSP with 0.25 (m.m.%) of Mg(ClO4)2 offers a highest Mg-ion conductivity of 5.66 × 10−4 S cm−1 at ambient temperature. Priya et al. [25, 26] synthesized two different biopolymer electrolytes based on the iota-carrageenan incorporated with Mg(ClO4)2 and Mg(NO3)2 salts and reported the maximum conductivity of 2.18 × 10−3 S cm−1 for 0.6 wt% of Mg(ClO4)2 salt and 6.1 × 10−4 S cm−1 for 0.4 wt% of Mg(NO3)2. Two different polymer electrolytes based on poly(vinyl alcohol)-poly(acrylonitrile) incorporated with MgCl2 and Mg(NO3)2 salts were prepared by Manjuladevi et al. [27, 28] and reported that 92.5PVA:7.5PAN with 0.3 m.m.% Mg(NO3)2 and 92.5PVA:7.5PAN with 0.5 m.m.% of MgCl2 offer a maximum conductivity of 1.71 × 10−3 S cm−1 and 1.01 × 10−3 S cm−1 at room temperature, respectively.
The above literature survey reveals that no work has been made on the biopolymer pectin with magnesium salt. The present work aims to prepare the biopolymer electrolytes based on different compositions of pectin with different concentrations of MgCl2 salt by solution casting technique, and the prepared biopolymer electrolytes were characterized by X-ray diffraction analysis (XRD), Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetric analysis (DSC), AC impedance analysis, linear sweep voltammetry (LSV) and cyclic voltammetry (CV).
Experimental methods
Biopolymer electrolytes of pure pectin and different compositions of pectin with different concentrations of MgCl2 salt (70 M wt% pectin: 30 M wt% MgCl2, 60 M wt% pectin: 40 M wt% MgCl2, 40 M wt% pectin: 60 M wt% MgCl2, 30 M wt% pectin: 70 M wt% MgCl2) were prepared by solution casting technique using pectin of molecular weight 194.1 g/mol from SRL Pvt. Ltd and MgCl2 salt of molecular weight 203.31 g/mol from SRL Chem.Com as the raw materials, and their codes are given in Table 1. Double-distilled water has been used as a solvent. The appropriate weight of pectin and MgCl2 salt was dissolved separately in double-distilled water at room temperature, and these solutions were mixed together and stirred for several hours using magnetic stirrer to obtain homogenous solutions. The obtained homogeneous solutions were poured into petri dishes and dried in hot air oven at 40 °C for 24 h. Free standing films with thickness ranging from 0.0104 cm to 0.025 cm have been obtained. These films are then subjected to various characterization studies like XRD, FTIR, DSC, AC impedance analysis, LSV and CV.
Characterization
The amorphous/crystalline nature of the prepared biopolymer electrolytes has been investigated from XRD analysis using XPERT-PRO diffractometer system with Cukα radiation (λ = 1.541 Å) at room temperature in the range of 2θ = 10° to 90°. The nature of chemical bonding and the complex formation between the polymer and salt have been investigated by FTIR analysis using SHIMAZDU IR Affinity-1 spectrometer at room temperature in the region from 400 to 4000 cm−1 with a resolution of 1 cm−1 in the transmittance mode. The glass transition temperature of the prepared biopolymer electrolytes was identified from DSC measurements taken by DSCQ20 V4.10 build 122 system at a heating rate of 5 K/min under nitrogen atmosphere in the temperature range of 273 K to 413 K. The ionic conductivity of the biopolymer electrolytes has been studied at room temperature using HIOKI 3532 LCR meter by placing the biopolymer electrolytes between two aluminum blocking electrodes, and a frequency of 42 Hz to 1 MHz has been applied. The total ionic transport number tion of the highest conducting sample was obtained by the Wagner’s polarization technique by placing the sample between two stainless steel electrodes and by passing a DC voltage of 1.5 V. The transport number of Mg2+ ions was obtained by Evan’s polarization method which is a combination of AC impedance spectroscopy and DC polarization studies, by placing the highest conducting sample between the magnesium electrodes and passing a DC voltage of 1.5 V. The electrochemical stability of the biopolymer electrolyte PMg 4 was evaluated by linear sweep voltammetry (LSV) using Biologic Science Instruments VSP-300, France, by placing the sample between two stainless steel electrodes in the potential range of 0–4 V at the scanning rate of 100 mV s−1. Cyclic voltammetry study has been made for the highest conducting membrane using potentiostat/galvanostat (EG&G PARC Model VersaStat) by placing the sample between two stainless steel electrodes at a scan rate of 100 mV s−1 in the potential range of − 2.5 V to + 2.5 V.
Results and discussions
XRD analysis
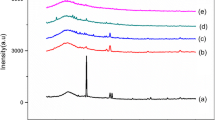
XRD analysis has been carried out to confirm the increase in the amorphous nature of the biopolymer electrolyte. XRD patterns of the biopolymer electrolyte PP and different compositions of pectin with different concentrations of MgCl2 salt are shown in Fig. 1. A broad peak observed at 2θ = 21° for the biopolymer electrolyte PP (Fig. 1a) reveals the amorphous nature of pure pectin. This agrees well with the previous reports [29,30,31]. From Fig. 1b–e, it has been noted that as MgCl2 salt concentration increases, the peak intensity decreases and the peak broadening increases. This shows that the amorphous nature increases as the salt concentration increases which has good agreement with Hodge et al. [32] criterion, which states the relationship between the intensity of the peak and the degree of crystallinity. From Fig. 1, it has been observed that the biopolymer electrolyte PMg 4 exhibits high amorphous nature. The absence of peak corresponding to MgCl2 salt clearly points out the complete dissociation of the salt in the polymer pectin. We could not obtain free standing membrane for the concentration 20 M wt% pectin: 80 M wt% MgCl2.
FTIR analysis
In the present study, Fourier transform infrared (FTIR) spectroscopy has been used as a powerful tool to analyze the complex formation and interactions between the biopolymer pectin and MgCl2 salt by monitoring the changes in the vibrational modes of the molecule. Figure 2 shows the FTIR spectra for the biopolymer electrolyte PP and different compositions of pectin with different concentrations of MgCl2 salt. The FTIR spectral assignments for pectin/MgCl2 biopolymer electrolytes are shown in Table 2. The vibrational peak of pure pectin at 3286 cm−1 assigned to O–H stretching [28] has shifted to 3442 cm−1, 3422 cm−1, 3422 cm−1, 3451 cm−1 for the biopolymer electrolytes PMg 1, PMg 2, PMg 3, PMg 4, respectively. The frequency shift is due to the interaction of MgCl2 with O–H as shown in Fig. 2. The vibrational band at 1742 cm−1 is assigned to the vibrations of the C=O stretching of the biopolymer electrolyte PP [19]. This peak gets suppressed/submerged for the biopolymer electrolytes PMg 1, PMg 2 and PMg 4 but appears as a small hump at 1746 cm−1 for the biopolymer electrolyte PMg 3. The change in the frequency of C=O stretching is due to the addition of MgCl2 salt.
The vibration peak at 1604 cm−1 assigned to carbonyl group of carboxylate ion (COO−) [33] for the biopolymer electrolyte PP has shifted to 1637 cm−1 for all compositions of pectin with different compositions of MgCl2 salt. The shifting of the peak is due to the interaction between the carbonyl group of carboxylate ion and Mg2+ ion of MgCl2 salt. The –OH bending observed at 1368 cm−1 [34] for the biopolymer electrolyte PP has shifted to 1384 cm−1 for the biopolymer electrolytes PMg 1 and PMg 2. The peaks observed at 1095 cm−1 and 1014 cm−1 for the biopolymer electrolyte PP assigned to C–C asymmetric stretching [28] and –CH–O–CH stretching [35], respectively, get submerged for the biopolymer electrolyte PMg 1 and becomes a broad peak for PMg 2. The peaks at 1095 cm−1 and 1014 cm−1 observed for the biopolymer electrolyte PP get shifted to 1105 cm−1 and 1016 cm−1, respectively, for the biopolymer electrolyte PMg 3 and to 1105 cm−1 and 1012 cm−1, respectively, for the biopolymer electrolyte PMg 4. The shift in the position of the peak owing to the interaction between the polymer and the salt confirms the complex formation between biopolymer pectin and MgCl2 salt.
DSC analysis
DSC analysis has been done to find the glass transition temperature (Tg) of the prepared biopolymer electrolytes. The DSC curve for the biopolymer electrolyte PP and different compositions of pectin with different concentrations of MgCl2 salt are shown in Fig. 3A and B, respectively.
From Fig. 3B, it has been observed that Tg value of the biopolymer electrolyte PMg 1 increases from the value of 43 °C (Tg value of the biopolymer electrolyte PP) to 147 °C. This increase in Tg value may be due to the reduction in the segmental motion caused by the increase in the intermolecular coordination between oxygen and magnesium cations in the polymer chain owing to the addition of the salt [35]. As the concentration of MgCl2 salt increases, the Tg value decreases to 96 °C for the biopolymer electrolyte PMg 4. This may be due to the plasticization of the electrolyte with addition of MgCl2 salt which weakens the dipole–dipole interactions between the polymer chains leading to the softening of the polymer. Thus, the decrease in Tg indicates an increase in the flexibility of the polymer chains causing the high segmental motion of the polymer electrolyte which supports the easy flow of ions through the polymer network in the presence of the applied electric field.
AC impedance analysis
Impedance spectroscopic technique is used to study the electrical characteristics of the prepared biopolymer electrolytes. Cole–Cole plot of the biopolymer electrolyte PP and different compositions of pectin with different concentrations of MgCl2 salt at room temperature along with the equivalent circuit are shown in Fig. 4A and B, respectively, and their corresponding ionic conductivities were tabulated in Table 3. A semicircle present in the impedance plot at the high-frequency region (depicted by a parallel combination of the bulk resistance (Rb) and the bulk capacitance (Cp)) is due to the bulk effect of the electrolyte, and an inclined spike present at the low-frequency region (depicted by a constant phase element (CPE)) is due to the blocking effect at the electrode/electrode interface [36]. In our study, a high-frequency semicircle and a low-frequency non-vertical spike have been observed for the biopolymer electrolyte PP and for all other compositions of pectin with different concentrations of MgCl2 salt, only spikes have been observed which proves that the resistive component of the biopolymer electrolyte exists and the capacitive nature disappears. EQ software program developed by Boukamp [37] has been used to find the value of the bulk resistance (Rb) of the biopolymer electrolytes. The ionic conductivities of the biopolymer electrolytes are calculated using Eq. (1):
where l represents the thickness of the biopolymer electrolyte (in cm), A represents the surface area of the biopolymer electrolyte (in cm2), and Rb represents the bulk resistance of the biopolymer electrolyte (in ohms). As the salt concentration increases, the ionic conductivity also increases. This is due to the production of large amount of mobile charge carriers and due to the increase in the ionic transportation in the polymer segments. From Table 3, it has been observed that the biopolymer electrolyte PMg 4 exhibits the highest ionic conductivity of 1.14 × 10−3 S cm−1. This biopolymer electrolyte, having low Tg value (from DSC) owing to the softening of the polymer, supports the segmental motion and increases the ionic transportation of the system. Further, the biopolymer electrolyte PMg 4 has highest amorphous nature (from XRD), which confirms the highest ionic conductivity. As the amorphous nature increases, the flexibility of the polymer chain also increases and this enhances the segmental motion in the polymer matrix which in turn increases the ionic conductivity of the biopolymer electrolyte. [38].
Conductance spectra analysis
Figure 5 shows the conductance spectra of the biopolymer electrolyte PP and different compositions of pectin with different concentrations of MgCl2 salt. Normally, the conduction spectra consist of three well-defined regions, namely low-frequency dispersion region which indicates the space charge polarization at the blocking electrodes, the middle-frequency independent plateau region which indicates the DC conductivity of the prepared biopolymer electrolytes due to the migration of the ions to the neighboring sites and the high-frequency region which indicates the bulk relaxation process. The conduction spectra of the biopolymer electrolyte PP exhibit all the three regions, whereas the other biopolymer electrolytes exhibit only two regions: low-frequency dispersion region and the middle-frequency independent plateau region. DC conductivity value is obtained from the conduction spectra by extrapolating the middle-frequency independent plateau to log σ axis. There is a good agreement with the DC conductivity value obtained from the conduction spectra and Cole–Cole plot.
Transference number analysis
Wagner’s polarization method
To calculate the total ion transport number in the biopolymer electrolyte, Wagner’s polarization technique proposed by Wagner and Wagner [39] has been carried out, where the cell SS/PMg 4/SS was polarized by applying a DC voltage of 1.5 V. Figure 6 shows the DC polarization curve of (i) SS/PMg 4/SS cell and (ii) Mg/PMg 4/Mg cell at room temperature. The initial total current has been found to decrease with time (Fig. 6(i)) owing to the depletion of ionic species in the biopolymer electrolyte, and it attains a constant value in the fully depleted situation. Under steady-state condition, the cell is polarized and due to the migration of ions, the current flows across the electrolyte interface. The total ion transport number (tion) for the samples has been determined using Eq. (2):
Here, It represents the total current and Ie represents the residual current. The total ion transport number of the highest conducting biopolymer electrolyte PMg 4 was found to be 0.98. This clearly shows that the charge transport is mainly due to ions and the contribution of electrons is very negligible.
Evan’s polarization method
To calculate the contribution of Mg2+ ions to the total conductivity, a combination of AC and DC technique, proposed by Evans et al. [40], has been used. In this polarization method, Mg/PMg 4/Mg cell was polarized by applying a constant voltage of ΔV = 1.5 V. The AC impedance plot for the highest conducting biopolymer electrolyte PMg 4 is shown in Fig. 7. The intercept at the high-frequency region represents the bulk resistance, Rb, of the biopolymer electrolyte. Ro, the cell resistance before polarization and, RS, cell resistance after polarization are obtained by the impedance measurements. EQ software [37] has been used to fit the impedance plot. The total impedance (RS = Rb + Ri) at low-frequency region corresponds to the diameter of the semicircle, where Ri is the resistance of the passivation layer. The value of t+ ion is obtained from Eq. (3):
Here, Io and IS represent the initial current and the final current, respectively, and are noted from Fig. 6(ii). Ro and RS are the cell resistance before polarization and after polarization, respectively. The transport number of Mg2+ ion for the cell, Mg/PMg 4/Mg, is found to be 0.301. Similar result has been obtained by Mahalakshmi et al. [23] where the transference number of Mg2+ ions for 40% CA: 60% Mg(ClO4)2 biopolymer membrane is 0.313. Manjuladevi et al. [27, 28] reported the transference number of Mg2+ ions as 0.30 and 0.38 for the blend polymer membranes, 92.5PVA:7.5PAN:0.3 m.m.% Mg(NO3)2 at room temperature and 92.5 PVA: 7.5 PAN: 0.5 m.m.% MgCl2 film, respectively. Priya et al. [25] reported 0.313 as the transference number of Mg2+ ions for the electrolyte 1.0 g I-carrageenan with 0.6 wt% of Mg(ClO4)2. Kumar et al. [41] developed a PMMA-based GPE using 0.5 g of Mg(CF3SO3)2 salt and reported 0.33 as the transference number of Mg2+ ions.
Electrochemical stability analysis
The electrochemical stability is an important factor which decides the biopolymer electrolyte to be used for the electrochemical application. The electrochemical stability for the biopolymer electrolyte PMg 4 has been measured by linear sweep voltammeter by placing the sample between two stainless steel blocking electrodes, and a potential range of 0–4 V has been applied at a scan rate of 100 mV s−1. Figure 8 shows the linear sweep voltammogram of the biopolymer electrolyte PMg 4. It has been observed that the biopolymer electrolyte is stable up to 2.05 V, confirming that this electrolyte can be used for electrochemical application. Muthukrishnan et al. [19] reported 3.69 V as the electrochemical stability for the highest conducting sample, 40 mol% pectin: 60 mol% NH4SCN.
Cyclic voltammogram studies
The CV measurement has been carried out using SS/PMg 4/SS between − 2.5 V and 2.5 V. The CV plot of SS/PMg 4/SS cell taken at a scan rate of 100 mV s−1 is shown in Fig. 9. Two distinct cathodic and anodic current peaks observed from the voltammogram point out the occurrence of the anodic oxidation and cathodic reduction at the electrode–electrode interface.
Primary magnesium battery fabrication
The highest conducting biopolymer electrolyte PMg 4 has been used for the fabrication of Magnesium primary battery. Mg metal in pellet form has been used as the anode. The preferred proportion of manganese dioxide and graphite are grinded together in powder form, pressed with 5-torr pressure to form a pellet and acts as the cathode. The biopolymer electrolyte is placed between the anode and cathode material as shown in Fig. 10a. The chemical reaction in the cell is as follows:Anode reaction
Cathode reaction
Overall reaction
The hydroxyl group in the biopolymer pectin acts as the source of hydroxide ion in Mg–MnO2 battery. Occluded moisture/water will be present in any polymer which is nonessential water retained by the physical forces in the microscopic pores distributed irregularly throughout pectin. This acts as another source of hydroxyl ion [28].
Figure 10b shows the open-circuit voltage of the fabricated magnesium primary battery. The stable potential of 2.16 V has been observed for 280 h. A load of 100 kΩ has been connected to the fabricated battery, and the discharge characteristics has been observed (Fig. 10c). The initial potential decreases from 2.16 to 2.12 V owing to the polarization at the electrode–electrolyte interface [28]. This voltage is maintained constant for 11 days, and this constant region is called plateau region. A current of 153 mA has been observed for a load of 100 kΩ. The cell parameters of the magnesium primary battery constructed using the biopolymer electrolyte PMg 4 are shown in Table 4.
A white LED connected across two magnesium primary batteries which are in series, glows for 6 days without any interruption (Fig. 11).
Conclusion
The literature survey reveals that no work has been done on the biopolymer pectin with magnesium salt. The biopolymer electrolytes PP and different compositions of pectin with different concentrations of MgCl2 salt have been prepared by solution casting technique. The XRD analysis revealed that the amorphous nature of the prepared biopolymer electrolytes increases with the increase in the salt concentration. The complex formation between the biopolymer pectin and the salt, MgCl2, has been confirmed by FTIR analysis. DSC analysis revealed that the highest conducting sample has low glass transition temperature which agrees well with impedance analysis. AC impedance analysis showed that as the salt concentration increases, the ionic conductivity increases and the maximum ionic conductivity was obtained as 1.14 × 10−3 S cm−1 for the biopolymer electrolyte PMg 4. The total ion transport number (tion) of the highest conducting sample has been found to be 0.98 by Wagner’s polarization method. The contribution of Mg2+ ions to the total conductivity of highest conducting biopolymer electrolyte has been found to be 0.301 by Evan’s polarization method. The electrochemical stability analysis showed that the biopolymer electrolyte PMg 4 is stable up to 2.05 V. A primary battery has been constructed using the biopolymer electrolyte PMg 4. The open-circuit voltage and the current drawn are measured to be 2.16 V and 153 mA, respectively.
References
Malathi M, Tamilarasan K, Ganesan V (2014) Role of ceramic reinforcement in composite polymer electrolyte. Polym Compos 36(1):42–46. https://doi.org/10.1002/pc.22910
Wang SH, Kuo PL, Hsieh CT, Teng H (2014) Design of poly(acrylonitrile)-based gel electrolytes for high-performance lithium ion batteries. ACS Appl Mater Interfaces 6(21):19360–19370. https://doi.org/10.1021/am505448a
Wang J, Song S, Muchakayala R, Hu X, Liu R (2017) Structural, electrical, and electrochemical properties of PVA-based biodegradable gel polymer electrolyte membranes for Mg-ion battery applications. Ionics 23(7):1759–1769. https://doi.org/10.1007/s11581-017-1988-y
Basha SKS, Sundari GS, Kumar KV, Rao MC (2017) Preparation and dielectric properties of PVP-based polymer electrolyte films for solid-state battery application. Polym Bull 75(3):925–945. https://doi.org/10.1007/s00289-017-2072-5
Wang F, Li L, Yang X, You J, Xu Y, Wang H, Ma Y, Gao G (2018) Influence of additives in a PVDF-based solid polymer electrolyte on conductivity and Li-ion battery performance. Sustain Energy Fuels 2(2):492–498. https://doi.org/10.1039/c7se00441a
Yulianti E, Karo AK, Susita L, Sudaryanto (2012) Synthesis of electrolyte polymer based on natural polymer chitosan by ion implantation technique. Proc Chem 4:202–207. https://doi.org/10.1016/j.proche.2012.06.028
Zulkefli FN, Navaratnam S, Ahmad AH (2015) Proton conducting biopolymer electrolytes based on starch incorporated with ammonium thiocyanate. Adv Mater Res 1112:275–278. https://doi.org/10.4028/www.scientific.net/AMR.1112.275
Khiar ASA, Arof AK (2009) Conductivity studies of starch-based polymer electrolytes. Ionics 16(2):123–129. https://doi.org/10.1007/s11581-009-0356-y
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Hemalatha R, Boopathi G (2018) Synthesis and characterization of bio-polymer electrolyte based on iota-carrageenan with ammonium thiocyanate and its applications. J Solid State Electrochem. https://doi.org/10.1007/s10008-018-4028-6
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Boopathi G (2018) Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J Non-Cryst Solids 481:424–434. https://doi.org/10.1016/j.jnoncrysol.2017.11.027
Chitra R, Sathya P, Selvasekarapandian S, Monisha S, Moniha V, Meyvel S (2018) Synthesis and characterization of iota-carrageenan solid biopolymer electrolytes for electrochemical applications. Ionics 25(5):2147–2157. https://doi.org/10.1007/s11581-018-2687-z
Christopher Selvin P, Perumal P, Selvasekarapandian S, Monisha S, Boopathi G, Leena Chandra MV (2018) Study of proton-conducting polymer electrolyte based on K-carrageenan and NH4SCN for electrochemical devices. Ionics 24:3535–3542. https://doi.org/10.1007/s11581-018-2521-7
Isa MIN, Samsudin AS (2016) Potential study of biopolymer-based carboxymethylcellulose electrolytes system for solid-state battery application. Int J Polym Mater Polym Biomater 65(11):561–567. https://doi.org/10.1080/00914037.2016.1149844
Rani M, Rudhziah S, Ahmad A, Mohamed N (2014) Biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Polymers 6(9):2371–2385. https://doi.org/10.3390/polym6092371
Raphael E, Avellaneda CO, Manzolli B, Pawlicka A (2010) Agar-based films for application as polymer electrolytes. Electrochim Acta 55(4):1455–1459. https://doi.org/10.1016/j.electacta.2009.06.010
Kumar LS, Selvin PC, Selvasekarapandian S, Manjuladevi R, Monisha S, Perumal P (2018) Tamarind seed polysaccharide biopolymer membrane for lithium-ion conducting battery. Ionics 24(12):3793–3803. https://doi.org/10.1007/s11581-018-2541-3
Thakur BR, Singh RK, Handa AK, Rao MA (1997) Chemistry and uses of pectin—a review. Crit Rev Food Sci Nutr 37(1):47–73. https://doi.org/10.1080/10408399709527767
Perumal P, Selvasekarapandian S, Abhilash KP, Sivaraj P, Hemalatha R, Selvin PC (2019) Impact of lithium chlorate salts on structural and electrical properties of natural polymer electrolytes for all solid state lithium polymer batteries. Vacuum 159:277–281. https://doi.org/10.1016/j.vacuum.2018.10.043
Muthukrishnan M, Shanthi C, Selvasekarapandian S, Manjuladevi R, Perumal P, Christopher Selvin P (2019) Synthesis and characterization of pectin-based biopolymer electrolyte for electrochemical applications. Ionics 25(1):203–214. https://doi.org/10.1007/s11581-018-2568-5
Vijaya N, Selvasekarapandian S, Somalatha M, Sujithra KS, Monisha S (2016) Proton-conducting biopolymet electrolytes based on pectin doped with NH4X (X = Cl, Br). Ionics 23(10):2799–2808. https://doi.org/10.1007/s11581-016-1852-5
Kavitha S, Vijaya N, Pandeeswari R, Premalatha M (2016) Vibrational, electrical and optical studies on pectin-based polymer electrolyte. Int Res J Eng Technol 3(7):1385–1390
Mendes JP, Esperança JMSS, Medeiros MJ, Pawlicka A, Silva MM (2017) Structural, morphological, ionic conductivity, and thermal properties of pectin-based polymer electrolytes. Mol Cryst Liq Cryst 643(1):266–273. https://doi.org/10.1080/15421406.2016.1263111
Mahalakshmi M, Selvanayagam S, Selvasekarapandian S, Moniha V, Manjuladevi R, Sangeetha P (2019) Characterization of biopolymer electrolytes based on cellulose acetates with magnesium perchlorate (Mg(ClO4)2) for energy storage devices. J Sci Adv Mater Devices 4(2):276–284. https://doi.org/10.1016/j.jsamd.2019.04.006
Perumal P, Abhilash KP, Sivaraj P, Selvin PC (2019) Study on Mg-ion conducting solid biopolymer electrolytes based on tamarind seed polysaccharide for magnesium ion batteries. Mater Res Bull. https://doi.org/10.1016/j.materresbull.2019.05.015
Priya SS, Karthika M, Selvasekarapandian S, Manjuladevi R, Monisha S (2018) Study of biopolymer I-carrageenan with magnesium perchlorate. Ionics 24(12):3861–3875. https://doi.org/10.1007/s11581-018-2535-1
Priya SS, Karthika M, Selvasekarapandian S, Manjuladevi R (2018) Preparation and characterization of polymer electrolyte based on biopolymer I-carrageenan with magnesium nitrate. Solid State Ion 327(1):136–149. https://doi.org/10.1016/j.ssi.2018.10.031
Manjuladevi R, Selvasekarapandian S, Thamilselvan M, Mangalam R, Monisha S, Selvin PC (2018) A study on blend polymer electrolyte based on poly(vinyl alcohol)-poly(acrylonitrile) with magnesium nitrate for magnesium battery. Ionics 24(11):3493–3506. https://doi.org/10.1007/s11581-018-2500-z
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Christopher Selvin P, Mangalam R, Monisha S (2017) Preparation and characterization of blend polymer electrolyte film based on poly(vinyl alcohol)-poly(acrylonitrile)/MgCl2 for energy storage devices. Ionics 24(4):1083–1095. https://doi.org/10.1007/s11581-017-2273-9
Leones R, Botelho MBS, Sentanin F, Cesarino I, Pawlicka A, Camargo ASS, Silva MM (2014) Pectin-based polymer electrolytes with Ir(III) complexes. Mol Cryst Liq Cryst 604(1):117–125. https://doi.org/10.1080/15421406.2014.968049
Andrade JR, Raphael E, Pawlicka A (2009) Plasticized pectin based gel electrolytes. Electrochim Acta 54(26):6479–6483. https://doi.org/10.1016/j.electacta.2009.05.098
Kiruthika S, Malathi M, Selvasekarapandian S, Tamilarasan K, Moniha V, Manjuladevi R (2019) Eco-friendly biopolymer electrolyte, pectin with magnesium nitrate salt, for application in electrochemical devices. J Solid State Electrochem 23(7):2181–2193. https://doi.org/10.1007/s10008-019-04313-6
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 37(8):1371–1376. https://doi.org/10.1016/0032-3861(96)81134-7
Maciel VBV, Yoshida CMP, Franco TT (2015) Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr Polym 132(5):537–545. https://doi.org/10.1016/j.carbpol.2015.06.047
Perumal P, Christopher Selvin P, Selvasekarapandian S (2018) Characterization of biopolymer pectin with lithium chloride and its applications to electrochemical devices. Ionics 24(10):3259–3270. https://doi.org/10.1007/s11581-018-2507-5
Nirmala Devi G, Chitra S, Selvasekarapandian S, Premalatha M, Monisha S, Saranya J (2017) Synthesis and characterization of dextrin-based polymer electrolytes for potential applications in energy storage devices. Ionics 23(12):3377–3388. https://doi.org/10.1007/s11581-017-2135-5
Premalatha M, Vijaya N, Selvasekarapandian S, Selvalakshmi S (2016) Characterization of blend polymer PVA–PVP complexed with ammonium thiocyanate. Ionics 22(8):1299–1310. https://doi.org/10.1007/s11581-016-1672-7
Boukamp BA (1986) A non-linear lease square fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion 20(1):31–44. https://doi.org/10.1016/0167-2738%2886%2990031-7
Ravi M, Song S, Wang J, Wang T, Nadimicherla R (2016) Ionic liquid incorporated biodegradable gel polymer electrolyte for lithium ion battery applications. J Mater Sci: Mater Electron 27(2):1370–1377. https://doi.org/10.1007/s10854-015-3899-x
Wagner JB, Wagner C (1957) Electrical conductivity measurements on cuprous halides. J Chem Phys 26(6):1597–1601. https://doi.org/10.1063/1.1743590
Evans J, Vincent CA, Bruce PG (1987) Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28(13):2324–2328. https://doi.org/10.1016/0032-3861(87)90394-6
Kumar GG, Munichandraiah N (2002) Poly (methylmethacrylate)—magnesium triflate gel polymer electrolyte for solid state magnesium battery application. Electrochim Acta 47(7):1013–1022. https://doi.org/10.1016/S0013-4686(01)00832-5
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kiruthika, S., Malathi, M., Selvasekarapandian, S. et al. Conducting biopolymer electrolyte based on pectin with magnesium chloride salt for magnesium battery application. Polym. Bull. 77, 6299–6317 (2020). https://doi.org/10.1007/s00289-019-03071-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-03071-9