Abstract
These days, biopolymers are utilized significantly more often than other synthetic polymers because of the benefits they offer, which include non-toxicity, biodegradability, and renewability. The primary objective of the current work is to fabricate pectin-based biopolymer electrolytes containing magnesium triflate salt (MgTf2) for use in electrochemical devices. Biopolymer electrolytes of pectin with MgTf2 were synthesized by solution-casting method and are analyzed by XRD, DSC, TGA, SEM, AC impedance, FTIR, LSV, and CV techniques. The ionic conductivities of the samples were determined by AC impedance analysis, and the highest conductivity has been obtained as 4.511 × 10−3 S cm−1 for 50 M wt% pectin: 50 M wt% MgTf2. The total ionic transference number and Mg2+ transference number were found to be 0.99 and 0.315, respectively, for the highest-conducting sample. The electrochemical stability of 2.3 V has been obtained by linear sweep voltammetry (LSV) analysis for the highest-conducting electrolyte. A primary magnesium battery has been constructed with the highest conducting sample, and the open circuit voltage of the battery is 2.05 V.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid polymer electrolyte, a novel ionic material used in electrochemical device applications, has attracted tremendous technological interest in the last couple of years, owing to its advantages like (i) flexibility, (ii) being able to be made into the desired shape, (iii) having good mechanical and chemical strength, (iv) leak-proof, (v) having high ionic conductivity, and (vi) offering good electrode–electrolyte contact. Despite all these advantages, it is non-biodegradable. With the intention of obtaining eco-friendly products, the researchers are trying their level best to replace the synthetic polymers with biodegradable materials. Biopolymers, one of the green materials, find a lot of applications in electrochemical devices due to their special features, like (i) being found in abundance; (ii) sustainability; (iii) low-cost as they are easily available from natural sources; (iv) non-toxic; and (v) eco-friendly due to their biodegradable nature [1]. Mobarak et al. [2] prepared biodegradable carboxymethyl kappa carrageenan (sulphate per disaccharide) and carboxymethyl iota carrageenan (two sulphates per disaccharide) with different compositions of LiNO3, by solution casting technique. Raphael et al. [3] synthesized biopolymer electrolytes based on agar and acetic acid. Polymer electrolytes were synthesized by blending methylcellulose-potato starch, incorporating lithium perchlorate, and then plasticizing with glycerol [4]. Shukur et al. [5] developed chitosan-based biopolymer electrolytes doped with NH4Br and plasticized with glycerol. Sampathkumar et al. [6] synthesized the biopolymer electrolytes consisting of tamarind seed polysaccharide incorporated with LiClO₄ salt and plasticized with EC (ethylene carbonate). Jenova et al. [7] synthesized solid polymer electrolytes based on gum tragacanth (GT) and lithium nitrate (LN) by solution casting method and reported 8.28 × 10−3 S cm−1 as the maximum conductivity for 1 g GT: 0.4 g LN. The maximum ionic conductivity of 5.4 × 10−4 S cm−1 has been acquired for 40 wt% of LiCF3SO3 [8]. Chitra et al. [9] investigated iota-carrageenan-based-biopolymer electrolytes doped with LiCF3SO3 and reported 1.27 × 10−3 S cm−1 as the highest ionic conductivity for 1.0 g i-carrageenan: 0.4 wt% LiCF3SO3. Naachiyar et al. [10] synthesized the biopolymer electrolytes utilizing gellan gum biopolymer doped with NH4SCN and reported 1.41 × 10−2 S cm−1 as the highest conductivity of 1 g gellan gum doped with 1.1 M wt% of NH4SCN. Maithilee et al. [11] developed the biopolymer membrane using tamarind seed polysaccharide (TSP) incorporated with NaClO4 and reported 1 g TSP with 0.8 g of NaClO4 has the highest ionic conductivity of 1.70 × 10−3 S cm−1. Chitra et al. [12] synthesized moringa gum (MG) solid proton electrolytes by incorporating NH4NO3, and 2.66 × 10−3 S cm−1 was obtained as the maximum conductivity for 1 g moringa gum with 0.5 wt% NH4NO3. Arockia Mary et al. [13] reported 1 g k-carrageenan: 0.65 wt% of LiNO3 offered a maximum conductivity of 1.89 × 10−3 S cm−1 at room temperature.
Pectin belongs to a group of water-soluble colloidal carbohydrates with a high molecular weight and is a natural polymer. It consists of a polysaccharide backbone with α(1–4) linked D-galacturonic acid. Pectin is in two categories: high-methoxy pectin and low-methoxy pectin. Eating a diet rich in pectin stabilizes the blood glucose level and may prevent intestinal tumors. Pectin is also used in cancer treatment and it is non-toxic substance.
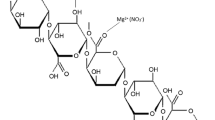
Bioploymers or polymers (host matrix) when used as electrolytes should contain a large number of polar groups so that the more number of cations of the salt can be attached to the polar groups, and by hopping mechanism, they can jump from one site to other site leading to ionic conductivity. Pectin contains a large number of polar groups particularly –OH and = O. So, a large number of cations can attach themselves to these polar groups causing high ionic conductivity. This is the unique characteristics of pectin that makes it suitable for electrochemical applications. Figure 1 shows the interaction of polymer pectin with MgTf2 salt.
Table 1 provides the number of OH groups available in the monomer of few biopolymers. It is observed that pectin has got more number of OH groups than other biopolymers mentioned in the table.
Vahini et al. [14] synthesized pectin-based proton-conducting biopolymer electrolytes with NH4SCN and reported 4.05 × 10−6 S/cm as the highest ionic conductivity of 0.91 g pectin: 0.08 g NH4SCN. Kiruthika et al. [15] reported 7.70 × 10−4 S cm−1 as the maximum ionic conductivity of 50 mol% pectin: 50 mol% Mg(NO3)2. Muthukrishnan et al. [16] reported 1.5 × 10−3 S cm−1 as the highest ionic conductivity of 40 mol% pectin: 60 mol% NH4SCN system. Perumal et al. [17] developed pectin-based solid biopolymer membranes with LiClO4 and reported the highest ionic conductivity of 60 M wt% pectin:40 M wt% LiClO4 as 5.15 × 10−5 S cm−1. Vijaya et al. [18] reported the maximum conductivity as 4.52 × 10−4 S cm−1 and 1.07 × 10−3 S cm−1 at 303 K for 70 M wt% pectin-30 M wt% NH4Cl and 60 M wt% pectin-40 M wt% NH4Br, respectively. Kiruthika et al. [19] observed a highest conductivity of 1.14 × 10−3 S cm−1 for 30 M wt% pectin: 70 M wt% MgCl2. Eswaragomathy et al. [20] obtained 4.49 × 10−3 S cm−1 as the maximum ionic conductivity for 40 M wt% of pectin: 60 M wt% of ZnCl2 system. Anandha Jothi et al. [21] studied the optical and dielectric properties of pectin-based polymer electrolytes with NaCl and reported that 3 wt% of NaCl doped polymer-salt complex system showed better performance.
Recently, lithium-based batteries have been replaced by magnesium-based batteries owing to their advantages: high energy density, being easily available, being safer, being less expensive, and being easy to handle. Perumal et al. [22] reported 5.66 × 10−4 S cm−1 as the maximum ionic conductivity for tamarind seed polysaccharide with 0.25 (mm%) of magnesium perchlorate. Mahalakshmi et al. [23] identified 4.05 × 10−4 S cm−1 as the highest ionic conductivity of 40% CA: 60% Mg (ClO4)2. Manjuladevi et al. [24] observed a highest ionic conductivity of 1.71 × 10−3 S cm−1 for 92.5PVA:7.5PAN:0.3 mm% Mg(NO3)2 blend polymer electrolyte. Shanmuga Priya et al. [25] developed biopolymer membranes based on Iota-carrageenan doped with Mg(NO3)2 and found that 1 gm I-carrageenan with 0.4 wt% of Mg(NO3)2 offered a maximum ionic conductivity of 6.1 × 10−4 S cm−1. Ponraj et al. [26] reported 70 M wt% poly(VdCl-co-AN-co-MMA): 30 M wt% Mg(NO3)2 offered the maximum conductivity of 1.6 × 10−4 S cm−1. Manjuladevi et al. [27] observed the highest ionic conductivity of 2.96 × 10−4 S cm−1 for 92.5PVA:7.5PAN:0.25 mm% Mg(ClO4)2. Ponraj et al. [28] found that 70 wt% poly(VdCl-co-AN-co-MMA):30 wt% MgCl2 offered a maximum ionic conductivity of 1.89 × 10−5 S cm−1. Sangeetha et al. [29] reported that 1 g of k-carrageenan: 0.6 M wt% of MgCl2 exhibits the highest Mg-ionic conductivity of 4.76 × 10−3 S cm−1. Sarangika et al. [30] developed solid polymer electrolytes based on polyethylene oxide (PEO) with Mg(Tf)2 by incorporating the ionic liquid, 1-butyl-1-methylpyrrolidinium bis(trifluoromethan esulfonyl)imide and found that the ionic conductivity was increased to 3.66 × 10−4 S cm−1 from 1.19 × 10−4 S cm−1 for PEO/Mg(Tf)2 (15:1 molar ratio) electrolyte. Sangeetha et al. [31] observed 7.05 × 10−4 S cm−1 as the maximum ionic conductivity of 1 g kappa-carrageenan: 0.5 mol% Mg(NO3)2.6H2O. The author also reported that the ionic conductivity has increased to 7.34 × 10−3 S cm−1 owing to the addition of 0.5 M wt% ethylene carbonate to the highest conducting electrolyte. Rani et al. [32] reported the maximum conductivity as 1.83 × 10−3 S cm−1 for 80 wt% of carboxymethyl cellulose (CMC): 20 wt% of Mg(CH3COO)2). Buvaneshwari et al. [33] observed 1.063 × 10–2 S cm−1 as the highest ionic conductivity of 1 g gellan gum: 0.5 wt% of Mg (ClO4)2.
The above literature clearly shows that only a few works have been reported using pectin with magnesium salts. The purpose of this study is to make novel biopolymer electrolytes with pectin and MgTf2 salt by solution casting method. The synthesized biopolymer electrolytes were subjected to a variety of characterization methods, including XRD, FTIR, DSC, TGA, SEM, AC impedance analysis, linear sweep voltammetry (LSV), and cyclic voltammetry (CV). Finally, a primary magnesium battery has been constructed using the highest-conducting biopolymer electrolyte, and its performance has been studied.
Experimental technique and characterization
Materials
Pectin with a molecular weight of 194.1 g/mol purchased from SRL Pvt. Ltd., magnesium triflate with a molecular weight of 322.44 g/mol purchased from SRL Pvt. Ltd., and double-distilled water are used as host polymer, salt, and solvent, respectively.
Sample preparation
This study involves the synthesis of the biopolymer electrolytes using the solution casting method using pectin, and various weight ratios of X M wt% of pectin to Y M wt% of MgTf2 (X values, 70, 60, 50, 40; Y values, 30, 40, 50, 60). The appropriate weight of pectin and MgTf2 was dissolved separately in distilled water for 2 h by a magnetic stirrer to obtain a clear solution. These two solutions were then combined and constantly agitated for about 48 h until the uniform mixture was obtained. The mixture was poured into the Petri dishes and heated to 60 °C in a hot air oven. In the span of 24 h, films ranging in thickness from 0.043 to 0.011 cm are obtained in the free-standing form. Figure 2 illustrates the process involved in preparing the biopolymer electrolyte.
Sample characterization
To examine the amorphous/crystalline behavior of the synthesized electrolytes, XRD analysis has been done at room temperature using an X′ Pert PRO diffractometer with CuKα (λ = 1.541 Å) radiation at 2θ ranging from 10 to 90° at a scan rate of 2° per minute. A Fourier-transform infrared (FTIR) analysis was conducted in the transmittance mode at ambient temperature, utilizing a SHIMAZDU IR Affinity-1 spectrometer with a resolution of 1 cm−1 in the wavenumber range of 4000–400 cm−1. Differential scanning calorimetry (DSC) measurements were carried out using a DSCQ20 V4.10 build 122 instrument in a nitrogen atmosphere. The heating rate used was 5 K/min, covering a temperature range from 273 to 413 K. Thermal analysis has been conducted using SDT Q600 V20.9 Build 20 to study the thermal stability of the prepared biopolymer electrolytes in a nitrogen atmosphere at a flow rate of 200 ml/min, operating between 30 and 700 °C and at a heating rate of 10 °C/min. A scanning electron microscope (SEM) is used to study the surface morphology of the biopolymer electrolytes. AC impedance studies of the samples have been conducted using a HIOKI 3532 LCR meter at room temperature, covering a frequency range of 42 Hz to 1 MHz. Wagner polarization method and Evan polarization method were adopted to find the total ionic transport number and the transference number of Mg2+ ions of the highest-conducting sample. Linear sweep voltammetry study was performed using Biologic Science Instruments VSP-300, France, to find the electrochemical stability of the highest conducting sample with a scanning speed of 100 m Vs−1 in the range of 0 V to 5 V. Cyclic voltammogram was obtained using a potentiostat/galvanostat (EG & G PARC Model VersaStat) with a scan rate of 100 m Vs−1, spanning a potential range from − 3 to + 3 V.
Fabrication of primary magnesium battery
A circular specimen of magnesium with a diameter of 12 mm was sectioned out of a sheet of Mg AZ21 alloy, polished using emery paper, cleaned with acetone, and used as an anode. MnO2 and graphite were taken in a 3:1 ratio, to which a small pinch of biopolymer electrolyte was added and grounded thoroughly for about 2 h in a motor until a fine powder was obtained. The mixture was then made into a thin pellet with a thickness ≤ 1 mm by applying a pressure of 5 tons cm−2. This was used as a cathode. The highest ion conducting membrane 50 M wt% pectin: 50 M wt% MgTf2 was used as an electrolyte. A primary magnesium battery has been constructed by placing this electrolyte between the electrodes. The battery performance has been studied in terms of open circuit voltage and closed circuit voltage when the cell was connected to a load of 100 KΩ.
Results and discussion
XRD analysis
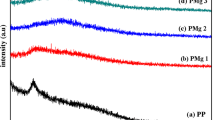
X-ray diffraction (XRD) studies were performed to investigate the amorphous/ crystalline behavior of the polymer matrices. The XRD pattern of pure MgTf2, pure pectin, and different compositions of pectin with different concentrations of MgTf2 are depicted in Fig. 3.
The broad peak of pure pectin at 2θ = 21° indicates that the biopolymer electrolyte is amorphous. This is in good agreement with the previous reports [15, 34, 35]. As the concentration of the salt increases, the intensity of the peak at 2θ = 21° reduces, and the peak broadness increases for the biopolymer electrolytes 70 M wt% pectin: 30 Mwt% MgTf2 and 60 Mwt% pectin: 40 Mwt% MgTf2. However, a very small peak corresponding to MgTf2 salt appears at 45° for 70 M wt% pectin: 30 M wt% MgTf2. This might be due to a very small percentage of the salt did not dissolve completely in the polymer matrix. The intensity of the peak at 21° further decreases and its broadness increases for the composition 50 M wt% pectin: 50 M wt% MgTf2. This indicates that the amorphous behavior of the biopolymer electrolyte increases as the salt concentration increases. This is in good agreement with Hodge et al. [36] criterion that determines the relationship between peak intensity and degree of crystallinity. Generally, when the salt is added to a polymer, the cation attaches themselves to the polar groups of the polymer. This modifies the polymer network, resulting in the increase of the amorphous nature. When MgTf2 concentration increases, a large number of Mg2+ ions get attached to the polar groups of pectin, modifying the network of pectin, leading to the increase in amorphous nature. When the amorphous nature increases, the broadness of the peak also increases. When the salt concentration is further increased for 40 M wt% pectin: 60 M wt% MgTf2, the intensity of the peak increases and its broadness decreases. At high concentration of magnesium salt (40 M wt% pectin: 60 M wt% MgTf), as the polymer network could not accommodate all the salts that were added, a few of them could not dissolve, they appear as salt peaks. The observed salt peaks are over the pectin peak and hence the peaks appear as broad peak with moderate intensity. From the above observations, it is concluded that 50 M wt% pectin: 50 M wt% MgTf2 composition has got high amorphous nature.
The deconvoluted XRD pattern for pure pectin and different compositions of pectin with different concentrations of MgTf2 are shown in Fig. 4. Using this graph, the crystallinity percentage has been calculated using the formula.
Crystallinity (%) = [Area under the crystalline region / Total area of the peaks] × 100 –––––– (1).
The crystallinity percentage for pure pectin and the various compositions of pectin with various compositions of MgTf2 salt is tabulated in Table 2. The percentage of crystallinity is observed to be high for pure pectin. The addition of salt to pure pectin decreases the percentage of crystallinity, as the salt disrupts the biopolymer network, increasing its amorphous nature. Generally, the decrease in crystallinity percentage is due to the increased molecular weight concentration of the salt in pure biopolymer. The 50 M wt% pectin: 50 M wt% MgTf2 composition has a lower crystallinity percentage, indicating a more amorphous nature than the other compositions. A further increase in salt concentration leads to a rise in crystallinity due to incomplete dissociation of the salt.
FTIR analysis
Fourier-transform infrared (FTIR) spectroscopic analysis has been performed to confirm the complex formation between the polymer pectin and MgTf2 salt. The interaction among atoms or ions in an electrolyte system makes changes in the vibrational modes of molecules. Figures 5 and 6 show the FTIR spectrum of the prepared biopolymer electrolytes and MgTf2 salt, respectively. Table 3 lists the corresponding peak assignment values.
O–H stretching is the cause of the vibration peak observed at 3286 cm−1 and it corresponds to pure pectin [27]. This band was shifted to 3357 cm−1 for 70 M wt% pectin: 30 M wt% MgTf2, 3372 cm−1 for 60 M wt% pectin: 40 M wt% MgTf2, 3355 cm−1 for 50 M wt% pectin: 50 M wt% MgTf2, and 3360 cm−1 for 40 M wt% pectin: 60 M wt% MgTf2 biopolymer electrolytes. The change in the frequency of O–H stretching is due to the addition of MgTf2 salt. The peak at 1742 cm−1 observed in pure pectin, ascribed to C = O stretching of the methyl-esterified carboxylic group (–COOCH3) [16] has been moved to 1740 cm−1, 1747 cm−1 and 1748 cm−1 for 70 M wt% pectin: 30 M wt% MgTf2, 60 M wt% pectin: 40 M wt% MgTf2, and 50 M wt% pectin: 50 M wt% MgTf2, respectively. The shift in frequency is because of the interaction of the MgTf2 salt with C = O. The vibration band at 1604 cm−1 identified in pectin is associated with the asymmetric stretching of the carbonyl group of COO− [37] and was shifted to 1635 cm−1 for 70 M wt% pectin: 30 M wt% MgTf2, 1631 cm−1 for 60 M wt% pectin: 40 M wt% MgTf2, 1630 cm−1 for 50 M wt% pectin: 50 M wt% MgTf2, and to 1632 cm−1 for 40 M wt% pectin: 60 M wt% MgTf2 systems. The shifting of the peak and the increase in peak intensity occur due to interactions between the carbonyl group of the carboxylate ion and the Mg2+ ion of MgTf2. New peaks have been observed at 1226 cm−1 for 70 M wt% pectin: 30 M wt% MgTf2, and at 1225 cm−1 for 60 M wt% pectin: 40 M wt% MgTf2, 50 M wt% pectin: 50 M wt% MgTf2, and 40 M wt% pectin: 60 M wt% MgTf2 systems. This peak corresponds to MgTf2 salt. This indicates the complex formation between the salt and the polymer. The peak located at 1095 cm−1 due to C–C asymmetric stretching of pure pectin [38] has been found to shift to 1101 cm−1 for 70 M wt% pectin: 30 M wt% MgTf2 and 60 M wt% pectin: 40 M wt% MgTf2 biopolymer electrolytes, and to 1103 cm−1 for 50 M wt% pectin: 50 M wt% MgTf2 and 40 M wt% pectin: 60 M wt% MgTf2 systems. The vibrational band of pure pectin at 1014 cm−1 ascribed to –CH–O–CH stretching [18] has been displaced to 1027 cm−1 for 70 M wt% pectin: 30 M wt% MgTf2, 60 M wt% pectin: 40 M wt% MgTf2 and 40 M wt% pectin: 60 M wt% MgTf2, and to 1028 cm−1 for 50 M wt% pectin: 50 M wt% MgTf2. The shift of the peak may be due to the interaction of pectin and MgTf2, confirming the complexation of pectin with magnesium salt.
DSC analysis
The glass transition temperature (Tg) of the samples is determined by DSC analysis. Figures 7 and 8 show the DSC curves for the prepared samples. It has been found that the Tg value increases to 86 °C for 70 M wt% pectin: 30 M wt% MgTf2 when compared to pure pectin (Tg = 43 °C). Generally, when a salt is added to a polymer network, cations of the salt attach themselves to the oxygen atoms of the polar group of the polymer by weak van der Waals force (transient cross-linkage). It is observed from the measurement that this transient cross-linkage strength between Mg2+ and the oxygen atom of the polar group of pectin is high leading to an increase in Tg value compared to pure pectin. When the salt concentration is further increased, the Tg value reduces to 84 °C for 60 M wt% pectin: 40 M wt% MgTf2 and to 72 °C for 50 M wt% pectin: 50 M wt% MgTf2. This reduction in the Tg value is due to the decrease in the strength of the transient cross linkage up to 50 M wt% pectin: 50 M wt% MgTf2. When the salt concentration is further increased, the Tg value increases to 74 °C for 40 M wt% pectin: 60 M wt% MgTf2, owing to the existence of undissociated magnesium salt in the polymer system. The presence of the undissociated magnesium salt could be observed in the XRD pattern as the salt peak for the membrane 40 M wt% pectin: 60 M wt% MgTf2. The salt peak due to MgTf2 is seen with good intensity for this membrane. Due to the undissociated salt, the segmental motion is reduced leading to an increase in the Tg value. The Tg values of all the samples are tabulated in Table 4.
TGA analysis
Figure 9 depicts the TGA thermogram of pure pectin and 50 M wt% pectin: 50 M wt% MgTf2. TGA thermogram of pure pectin and 50 M wt% pectin: 50 M wt% MgTf2 shows that both the samples have three stages of weight loss along with the initial weight loss. The various stages of weight loss are tabulated in Table 5.
Initial weight loss in both pure pectin and 50 M wt% pectin: 50 M wt% MgTf2 is due to the evaporation of moisture/occluded water in the membrane. For pure pectin, the first stage of weight loss is due to the moisture evaporation and residual solvent in the polymer matrix. For 50 M wt% pectin: 50 M wt% MgTf2 membrane, the weight loss in the first stage is due to the evaporation of the residual solvent and decomposition of the salt. For pure pectin and 50 M wt% pectin: 50 M wt% MgTf2 membrane, the second stage of weight loss is due to pyrolitic decomposition, a result of primary and secondary decarboxylation involving the acid side group and a carbon in the anhydroglucose ring [35]. The third stage of weight loss of pure pectin and 50 M wt% pectin: 50 M wt% MgTf2 is due to oxidation reaction, carbonization, and ash formation [35, 39].
SEM analysis
Figure 10 shows the SEM images of pure pectin, 50 pectin: 50 M wt% MgTf2, and 40 pectin: 60 M wt% MgTf2. SEM images of pure pectin show that it is moderately amorphous in nature. SEM images of the highest conducting membrane 50 pectin: 50 M wt% MgTf2 show that the diameter of the pores has increased much due to the addition of salt. SEM image of 40 M wt% pectin: 60 M wt% MgTf2 shows the presence of undissociated salt and aggregation of salt over the amorphous pectin network.
AC impedance studies
Cole–Cole plot
AC impedance measurements have been carried out to find the electrical conductivity of the synthesized biopolymer electrolytes. During the impedance measurement, an AC voltage is applied across the electrodes connected to the sample at a wide range of frequencies, and the current is measured. The applied voltage causes the ions to move back and forth in phase. In the impedance plot, two well-defined regions have been observed. The semicircle observed in the high-frequency range is attributed to the bulk effect of the electrolyte, while the inclined spike in the low-frequency range is due to the blocking effect at the electrode/electrolyte interface [40]. The high-frequency semicircle represents a parallel combination of a resistor and a capacitor [24], and the low-frequency region represents the constant-phase element (CPE) [19].
The cole–cole plots of the synthesized electrolytes at room temperature are shown in Figs. 11 and 12, respectively, and their corresponding ionic conductivity values are given in Table 6. Pure pectin shows a semicircle in the high-frequency region, followed by a low-frequency non-vertical spike at low frequency. All the other samples show only spikes, which prove the existence of the resistive component of the biopolymer electrolytes, whereas the capacitive nature vanishes. The bulk resistance (Rb) values of the synthesized samples have been obtained by the EQ software program [41]. The ionic conductivity σ of the prepared biopolymer electrolytes was found using the formula:
where l, Rb, and A represent the thickness (in cm), the bulk resistance (in ohms), and the surface area (in cm2) of the synthesized biopolymer electrolytes, respectively. The ionic conductivity increases with increasing salt concentration, owing to the production of more mobile charge carriers and also due to the increase of ion transport in the polymer segments. 50 M wt% pectin: 50 M wt% MgTf2 exhibits the highest ionic conductivity of 4.511 × 10−3 S cm−1 at room temperature. The DSC result also points out that this electrolyte possesses a low Tg value, indicating that this film is more flexible than the other films. On further increase in salt concentration, the ionic conductivity was found to decrease for 40 M wt% pectin: 60 M wt% MgTf2 because of the accumulation of salt. The accumulation of salt suppresses the density of free mobile ions, leading to a reduction in ionic conductivity [42].
Conductance spectra analysis
Usually, the conductance spectra show three regions: a low-frequency dispersion region, a middle-frequency plateau region, and a high-frequency dispersion region, which corresponds to space charge polarization at the blocking electrodes, the DC conductivity of the prepared samples, and the bulk relaxation phenomenon, respectively. Figure 13 depicts the conductance spectra of the prepared biopolymer electrolytes. Pure pectin contains all three zones, while the other films only display the middle-frequency plateau area and the low-frequency dispersion area. The intermediate plateau area has been extended to zero frequency in order to calculate the DC conductivity of the synthesized biopolymer electrolytes. This value is the same as the ionic conductivity values attained from the Cole–Cole plot. 50 M wt% pectin: 50 M wt% MgTf2 offers the highest DC conductivity. When the concentration of salt is increased further, ions aggregate or multiply, causing a decrease in the DC conductivity [24].
Transference number measurement
Wagner’s polarization method
The total ion transport number (tion) of the highest conducting sample was identified by Wagner’s polarization technique. This method can be employed to ascertain if the conducting entities are ions or electrons. A steady DC voltage is applied to SS/50 M wt% pectin: 50 M wt% MgTf2/SS and the polarization current variation is monitored over time. The DC polarization curve of SS/50 M wt% pectin: 50 M wt% MgTf2/SS cell at room temperature is shown in Fig. 14(a). Initially, the current has decreased at a faster rate with time, and it attains a constant value in the fully depleted situation. The decrease in the initial current is due to the depletion of ionic species in the electrolyte. At a steady state, the cell is polarized, and the current flows across the electrolyte interface due to ion transport. The total ion transport number (tion) is calculated using the formula:
where It and Ie represent the total current and the residual current, respectively. tion value of 50 M wt% pectin: 50 M wt% MgTf2 is found to be 0.99, which confirms that the charge transport is mainly due to ions when compared with that of electrons. 0.98 has been reported as the ionic transference number for 80 wt% of carboxymethyl cellulose (CMC): 20 wt% of magnesium acetate at ambient temperature [32]. Buvaneshwari et al. [33] reported 0.99 as the total ionic transference number of 1.0-g gellan gum with 0.5 wt% of Mg(ClO4)2.
Evan’s polarization method
The transference number of Mg2+ ions has been determined using Evan’s polarization method. In this case, a constant DC voltage of 1.5 V is supplied across the cell, Mg /50 M wt% pectin: 50 M wt% MgTf2/ Mg, and Fig. 14(b) shows the associated DC polarization curve. Figures 15 and 16 depict the AC impedance plot of the cell, Mg /50 M wt% pectin: 50 M wt% MgTf2/ Mg before and after polarization, respectively. The cell resistance before polarization (Ro) and the cell resistance after polarization (Rs) are attained by impedance measurements. The t+ ion value can be obtained from the formula:
where Io is the initial current and Is is the final current (noted from Fig. 14(b)), Ro is the cell resistance before polarization and Rs is the cell resistance after polarization, and ΔV is the battery voltage (1.5 V). The t+ ion value of Mg2+ ion for Mg/ 50 M wt% pectin: 50 M wt% MgTf2/ Mg is found to be 0.315. The transference number of Mg2+ ions for K-carrageenan (1 g) with 0.6 M wt% of MgCl2 has been obtained as 0.26 [29]. The transport number of Mg2+ ions has been reported as 0.313 for 40% CA: 60% Mg(ClO4)2 biopolymer electrolyte [23]. A biopolymer electrolyte 1.0 g I-carrageenan with 0.6 wt% of Mg(ClO4)2 has been developed, and 0.313 was reported as the transference number of Mg2+ ions [43]. The t+ value of Mg2+ ions for the blend polymer membranes 92.5 PVA: 7.5 PAN: 0.5 mm% MgCl2 and 92.5PVA:7.5PAN:0.3 mm% Mg(NO3) films has been found to be 0.38 and 0.30, respectively [24, 38]. 0.33 was reported as the cationic transference number for PMMA-based gel polymer electrolyte with 0.5 g of Mg(CF3SO3)2 [44]. 0.33 has been reported as the cationic transference number of Mg2+ ions for 1.0-g gellan gum with 0.5 wt% of Mg(ClO4)2 [33].
Electrochemical stability analysis
The electrochemical stability of the highest-conducting sample has been analyzed via linear sweep voltammetry analysis. The specimen has been placed between two stainless steel electrodes and subjected to a potential of 0 V –5 V with a scan rate of 100 mVs−1. Figure 17 shows the linear sweep voltammogram of 50 M wt% pectin: 50 M wt% MgTf2. The sample was stable up to 2.3 V and above that voltage, it decomposes.
Cyclic voltammogram studies
The cycling stability of the highest conducting sample was found by placing the sample between two stainless steel electrodes with a potential range from − 3 to + 3 V and a scan of 100 mVs−1. The sample exhibits good cycling stability, maintaining an unchanged cyclic voltammetry pattern over 5 cycles. This CV pattern remains consistent across each cycle, without much variation in the sweep area, confirming the electrolyte’s reversibility. The CV plot of 50 M wt% pectin: 50 M wt% MgTf2 is shown in Fig. 18.
Fabrication and characterization of primary magnesium battery
In the present work, a magnesium primary battery has been fabricated by placing 50 M wt% pectin: 50 M wt% MgTf2 between the anode and cathode, as shown in Fig. 19. The fabricated battery has the following configuration: Mg/50 M wt% pectin: 50 M wt% MgTf2/ MnO2 + Graphite.
The following chemical reactions take place at the anode and cathode.
Anode reaction:
Cathode reaction:
Overall reaction:
The hydroxyl group of the polymer pectin and the presence of occluded moisture/water in the polymer act as a source of hydroxide ions for the Mg-MnO2 battery [24]. The occluded moisture/water is unwanted water retained by the physical forces within the microscopic pores, which are unevenly distributed throughout the polymer pectin.
Figure 20 displays the open circuit voltage of the constructed magnesium primary battery with 50 M wt% pectin: 50 M wt% MgTf2. A constant potential of 2.05 V is observed for 250 h. Figure 21 depicts the discharge characteristics of the designed magnesium primary cell when connected to a 100 kΩ load. Initially, the potential decreases from 2.05 to 1.82 V due to the electrode–electrolyte interface potential [24]. It has been noticed that this voltage has remained constant for 11 days. After connecting the load of 100 kΩ, the current measured was 19 µA. The cell parameters of the designed primary magnesium battery with 50 M wt% pectin: 50 M wt% MgTf2 electrolyte are shown in Table 7.
Conclusion
The literature survey points out that a very few studies were carried out on pectin with magnesium salt. Pectin-based biopolymer electrolytes were prepared with MgTf2 salt via the solution casting method. The amorphous behavior of the electrolytes has been confirmed by XRD studies. FTIR investigation confirms the complex formation between pectin and MgTf2. The DSC analysis shows that the sample, 50 M wt% pectin: 50 M wt% MgTf2, has a low Tg value and matches the AC impedance result. Thermal stability of the membranes has been observed from TGA analysis. The surface morphology of the biopolymer electrolytes has also been studied. AC impedance analysis shows that the maximum ionic conductivity was obtained as 4.511 × 1 0−3 S cm−1 for 50 M wt% pectin: 50 M wt% MgTf2 at ambient temperature. The total ion transport number (tion) and the contribution of Mg2+ ions to the total conductivity of the highest-conducting sample were found to be 0.99 and 0.315, respectively. The electrochemical stability of the highest-conducting biopolymer electrolyte, measured by linear sweep voltammetry, points out that the film is stable up to 2.3 V. The highest-conducting sample showed excellent cycling stability, maintaining a consistent CV pattern over three cycles. A primary magnesium battery was fabricated with 50 M wt% pectin: 50 M wt% MgTf2, and the open-circuit voltage was found to be 2.05 V. The cell parameters were also reported. These results confirm the suitability of 50 M wt% pectin: 50 M wt% MgTf2 electrolyte for battery applications.
Data Availability
No datasets were generated or analysed during the current study.
References
Xu JJ, Ye H (2005) Polymer gel electrolytes based on oligomericpolyether/cross-linked PMMA blends prepared via in situ polymerization. Electrochem Commun 7(8):829–835
Mobarak NN, Jumaah FN, Ghani MA, Abdullah MP, Ahmad A (2015) Carboxymethyl carrageenan based biopolymer electrolytes. Electrochim Acta 175:224–231
Raphael E, Avellaneda CO, Manzolli B, Pawlicka A (2010) Agar-based films for application as polymer electrolytes. Electrochim Acta 55(4):1455–1459
Yusof YM, Kadir MFZ (2016) Electrochemical characterizations and the effect of glycerol in biopolymer electrolytes based on methylcellulose-potato starch blend. Mol Cryst Liq Cryst 627(1):220–233
Shukur MF, Azmi MS, Zawawi SMM, Majid NA, Illias HA, Kadir MFZ (2013) Conductivity studies of biopolymer electrolytes based on chitosan incorporated with NH4Br. Phys Scr T 157:014049. https://doi.org/10.1088/0031-8949/2013/t157/014049
Sampathkumar L, Christopher Selvin P, Selvasekarapandian S, Perumal P, Chitra R, Muthukrishnan M (2019) Synthesis and characterization of biopolymer electrolyte based on tamarind seed polysaccharide, lithium perchlorate and ethylene carbonate for electrochemical applications. Ionics 25(3):1067–1082
Jenova I, Venkatesh K, Karthikeyan S, Madeswaran S, Arivanandhan M, Joice Sheeba D (2021) Characterization of solid polymer electrolyte based on gum tragacanth and lithium nitrate. Polymer-Plastic Technology and Materials 60(17):1898–1912
Noor ISM, Majid SR, Arof AK, Djurado D, Claro Neto S, Pawlicka A (2012) Characteristics of gellan gum–LiCF3SO3 polymer electrolytes. Solid State Ionics 225(4):649–653
Chitra R, Sathya P, Selvasekarapandian S, Meyvel S (2019) Investigation of seaweed derivative iota-carrageenan based biopolymer electrolytes with lithium trifluoromethanesulfonate. Materials Research Express 7(1):015309. https://doi.org/10.1088/2053-1591/ab5d79
Naachiyar RM, Ragam M, Selvasekarapandian S, Krishna MV, Buvaneshwari P (2021) Development of biopolymer electrolyte membrane using Gellan gum biopolymer incorporated with NH4SCN for electro-chemical application. Ionics 27(8):3415–3429
Maithilee K, Sathya P, Selvasekarapandian S, Chitra R, Vengadesh Krishna M, Meyvel S (2022) Na-ion conducting biopolymer electrolyte based on tamarind seed polysaccharide incorporated with sodium perchlorate for primary sodium-ion batteries. Ionics 28(2):1783–1790
Chitra R, Vengadesh Krishna M, Selvasekarapandian S (2022) Study on novel biopolymer electrolyte Moringa oleifera gum with ammonium nitrate. Polym Bull 79(6):3555–3572
Arockia Mary I, Selvanayagam S, Selvasekarapandian S, Chitra R, Leena Chandra MV, Ponraj T (2020) Lithium ion conducting biopolymer membrane based on K-carrageenan with LiNO3. Ionics 26(9):4311–4326
Vahini M, Muthuvinayagam M (2018) AC impedance studies on proton conducting biopolymer electrolytes based on pectin. Mater Lett 218:197–200
Kiruthika S, Malathi M, Selvasekarapandian S, Tamilarasan K, Moniha V, Manjuladevi R (2019) Eco-friendly biopolymer electrolyte, pectin with magnesium nitrate salt, for application in electrochemical devices. J Solid State Electrochem 23(12):1–13
Muthukrishnan M, Shanthi C, Selvasekarapandian S, Manjuladevi R, Perumal P, Christopher Selvin P (2019) Synthesis and characterization of pectin-based biopolymer electrolyte for electrochemical applications. Ionics 25(1):203–214
Perumal P, Christopher Selvin P, Selvasekarapandian S, Sivaraj P (2019) Structural and electrical properties of bio-polymer pectin with LiClO4 solid electrolytes for lithium ion polymer batteries. Materials Today: Proceedings 8:196–202. https://doi.org/10.1016/j.matpr.2019.02.100
Vijaya N, Selvasekarapandian S, Sornalatha M, Sujithra KS, Monisha S (2016) Proton-conducting biopolymer electrolytes based on pectin doped with NH4X (X=Cl, Br). Ionics 23(10):2799–2808
Kiruthika S, Malathi M, Selvasekarapandian S, Tamilarasan K, Maheshwari T (2019) Conducting biopolymer electrolyte based on pectin with magnesium chloride salt for magnesium battery application. Polym Bull 77(1):6299–6317
Eswaragomathy S, Selvanayagam S, Selvasekarapandian S, Muniraj Vignesh N, Aafrin Hazaana S, Meera Naachiyar R (2023) Preparation of pectin biopolymer electrolyte for zinc-ion battery application. Ionics 29:2329–2340
Anandha Jothi M, Vanitha D, Nallamuthu N, Sundaramahalingam K (2022) Optical and dielectric characterization of biopolymer pectin based electrolytes with NaCl. J Elastomers Plast 54(5):800–814
Perumal P, Abhilash KP, Sivaraj P, Selvin PC (2019) Study on Mg-ion conducting solid biopolymer electrolytes based on tamarind seed polysaccharide for magnesium ion batteries. Mater Res Bull 18:110490. https://doi.org/10.1016/j.materresbull.2019.05.015
Mahalakshmi M, Selvanayagam S, Selvasekarapandian S, Moniha V, Manjuladevi R, Sangeetha P (2019) Characterization of biopolymer electrolytes based on cellulose acetates with magnesium perchlorate (Mg(ClO4)2) for energy storage devices. Journal of Science: Advanced Materials and Devices 4(2):276–284
Manjuladevi R, Selvasekarapandian S, Thamilselvan M, Mangalam R, Monisha S, Selvin PC (2018) A study on blend polymer electrolyte based on poly(vinyl alcohol)-poly(acrylonitrile) with magnesium nitrate for magnesium battery. Ionics 24(11):3493–3506
Shanmuga Priya S, Karthika M, Selvasekarapandian S, Manjuladevi R (2018) Preparation and characterization of polymer electrolyte based on biopolymer I-Carrageenan with magnesium nitrate. Solid State Ionics 327:136–149
Ponraj T, Ramalingam A, Selvasekarapandian S, Srikumar SR, Manjuladevi R (2019) Mg-ion conducting triblock copolymer electrolyte based on poly(VdCl-co-AN-co-MMA) with magnesium nitrate. Ionics 26:789–800. https://doi.org/10.1007/s11581-019-03244-6
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Mangalam R, Premalatha M, S. Monisha S, (2017) Mg-ion conducting blend polymer electrolyte based on poly(vinyl alcohol)-poly (acrylonitrile) with magnesium perchlorate. Solid State Ionics 308:90–100
Ponraj T, Ramalingam A, Selvasekarapandian S, Srikumar SR, Manjuladevi R (2020) Plasticized solid polymer electrolyte based on triblock copolymer poly(vinylidene chloride-co-acrylonitrile-co-methyl methacrylate) for magnesium ion batteries. Polym Bull 78:35–57. https://doi.org/10.1007/s00289-019-03091-5
Sangeetha P, Selvakumari TM, Selvasekarapandian S, Srikumar SR, Manjuladevi R, Mahalakshmi M (2019) Preparation and characterization of biopolymer K-carrageenan with MgCl2 and its application to electrochemical devices. Ionics 26:233–244. https://doi.org/10.1007/s11581-019-03193-0
Sarangika HNM, Dissanayake MAKL, Senadeera GKR, Rathnayake RRDV, Pitawala HMJC (2016) Polyethylene oxide and ionic liquid-based solid polymer electrolyte for rechargeable magnesium batteries. Ionics 23(10):2829–2835
Sangeetha P, Selvakumari TM, Selvasekarapandian S, Mahalakshmi M (2021) Characterization of solid biopolymer electrolytes based on kappa-carrageenan with magnesium nitrate hexahydrate and its application to electrochemical devices. Polymer- Plastics Technology and Materials 60(12):1317–1330
Rani MSA, Isa NS, Sainorudin MH, Abdullah NA, Mohammad M, Asim N, Razali H, Ibrahim MA (2021) Magnesium ion-conducting biopolymer electrolytes based on carboxymethyl cellulose derived from palm oil empty fruit bunch fibre. Int J Electrochem Sci 16(3):210354
Bhuvaneshwari P, Mathavan T, Selvasekarapandian S, Vengadesh Krishna M, Meera Naachiyar R (2022) Preparation and characterization of biopolymer electrolyte based on gellan gum with magnesium perchlorate for magnesium battery. Ionics 28(23):3843–3854
Andrade JR, Raphael E, Pawlicka A (2009) Plasticized pectin based gel electrolytes. Electrochim Acta 54(26):6479–6483
Leones R, Botelho MBS, Sentanin F, Cesarino I, Pawlicka A, Camargo ASS, Silva MM (2014) Pectin-based polymer electrolytes with Ir(III) complexes. Mol Cryst Liq Cryst 604(1):117–125
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 37(8):1371–1376
Maciel VBV, Yoshida CMP, Franco TT (2015) Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr Polym 132(5):537–545
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Christopher Selvin P, Mangalam R, Monisha S (2017) Preparation and characterization of blend polymer electrolyte film based on poly(vinyl alcohol)-poly(acrylonitrile)/MgCl2 for energy storage devices. Ionics 24(4):1083–1095
Vanitha N, Shanmugapriya C, Selvasekarapandian S, Vengadesh Krishna M , Nandhini K (2022) Investigation of N–S-based graphene quantum dot on sodium alginate with ammonium thiocyanate (NH 4 SCN) biopolymer electrolyte for the application of electrochemical devices. J Mater Sci: Mater Electron 33(18):14847–14867
Premalatha M, Vijaya N, Selvasekarapandian S, Selvalakshmi S (2016) Characterization of blend polymer PVA-PVP complexed with ammonium thiocyanate. Ionics 22(8):1299–1310
Boukamp BA (1986) A non-linear lease square fit procedure for analysis of immittance data of electrochemical systems. Solid State Ionics 20(1):31–44
Karthikeyan S, Selvasekarapandian S, Premalatha M, Monisha S, Boopathi G, Aristatil G, Arun A, Madeswaran S (2016) Proton conducting I-carrageenan-based biopolymer electrolyte for fuel cell application. Ionics 23(10):2775–2780
Shanmuga Priya S, Karthika M, Selvasekarapandian S, Manjuladevi R, Monisha S (2018) Study of biopolymer I-carrageenan with magnesium perchlorate. Ionics 24(12):3861–3875
Kumar GG, Munichandraiah N (2002) Poly (methylmethacrylate)—magnesium triflate gel polymer electrolyte for solid state magnesium battery application. Electrochim Acta 47(7):1013–1022
Author information
Authors and Affiliations
Contributions
S.K- Contributed to experimental work and writing of the initial draft. M.M- Contributed to the review and editing of the final manuscript.S.S- conceived the work, supervision and data curation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kiruthika, S., Malathi, M. & Selvasekarapandian, S. Study of pectin-based biopolymer electrolytes with magnesium triflate (MgTf2) for applications in magnesium batteries. Ionics 30, 5237–5251 (2024). https://doi.org/10.1007/s11581-024-05690-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05690-3