Abstract
Natural rubber (NR) grafted by methyl methacrylate (MMA) was used to produce bio-based wood adhesive. The effect of total solid content (%TSC) at 55, 57, and 60%, represented as 55NR-g-MMA, 57NR-g-MMA, and 60NR-g-MMA, respectively, and the effect of storage time on lap shear strength were investigated. It was found that contact angle sharply decreased from 95° for NR to approximately 65° for all of NR-g-MMAs. Because the MMA groups were incorporated into various NR-g-MMA samples and the highest relative amount of grafted MMA was obtained by 57-NR-g-MMA which is determined by peaks intensities ratio between wave number at 1725 (C=O stretching) and 1450 (CH2 stretching), investigated by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR). These influences resulted in an increase in storage modulus (E′), whereas tanδ was decreased as compared to that of NR, characterized by dynamic mechanical analyzer (DMA). In addition, the apparent viscosity performed by plate-and-plate rheometer trended to increase with total solid content and storage time. However, the highest lap shear strength was achieved by 57NR-g-MMA. It means that the lap shear strength was not only governed by viscosity as well as total solid content, but the amount of grafted MMA also plays an important role.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Presently, there has been an increase in the development of environmentally friendly wood adhesive from bio-based material for the substitution of petroleum-based adhesive. Bio-based wood adhesive has been developed from biomass resource such as cassava starch [1], soy bean protein [2] and lignin [3] in order to substitute for synthetic thermosetting resins as adhesives because of environmental issues worldwide. Moreover, natural rubber (NR) is abundance and alternative material to produce this adhesive due to good mechanical properties and ability to crystallize under applied loading. According to these advantages, it has been used to be material for producing both household and engineering products such as tires, rubber gloves, bridge bearing, adhesive, etc. However, it is difficult to bond with high surface energy materials such as wood and polar polymers. To address this problem, the chemical modification of NR including epoxidation [4], chlorination [5], and grafting with polar monomer such as maleic anhydride [6] and methacrylate monomer [7] was investigated. Therefore, it can blend and adhere with polar materials such as acrylonitrile butadiene rubber (NBR) [8], chloroprene rubber [9], and wood [10]. The modification of NR has been performed in both latex and dry rubber stage in the mixing process. In addition, the modification in the latex stage was easily accomplished with high grafting yield and practical to apply for adhesive manner. The application of NR latex-based wood adhesive was investigated by several techniques such as epoxidized NR used to be adhesive for making particleboard from para rubber wood which was obtained high internal strength [10]. In addition, NR latex-based wood adhesives were prepared by mixing prevulcanized NR latex with various amounts of ammonium caseinate, carboxymethyl cellulose, cooked starch, and phenol formaldehyde resin acting as adhesion promoter [11]. According to these techniques, the adhesion between NR and wood which is mainly composed of cellulose can be improved [12]. Viscosity as well as storage time of latex adhesive was necessarily considered to produce wood adhesive. The viscosity of latex wood adhesive influences the ability to wet or spread on wood surface, whereas the stability due to long-distance transportation of adhesive or relatively long-term storage was represented by storage time [13]. The effect of structure change of urea–formaldehyde resins during storage has been studied [14], and the effect of viscosity of acrylic and protein of wood adhesive on adhesive strength was also investigated [13, 15].
They claimed that the appropriate and stabilized viscosity during storage of synthetic adhesive in the latex form was important because of them directly influenced to adhesive strength. In the case of NR latex, it was stable emulsion in water medium and the viscosity also varied with total solid content and storage time [16, 17].
The effect of viscosity and storage time of NR-based wood adhesive on adhesive strength has not been reported. In this study, the influence of total solid content (%TSC) on viscosity of NR-g-MMA latex adhesive resulted in lap shear strength between wood and latex adhesive which was investigated. Finally, the stability and adhesive properties of latex wood adhesive during storage were also investigated and discussed in detail.
Experimental procedure
Materials
High ammonium natural rubber (HANR) latex, 60% dry rubber content (DRC), was purchased from Chemical and Material Co. Ltd. Methyl methacrylate monomer (MMA) was purchased from Carlo Erba Reagents. Cumene hydroperoxide (CHP) and tetraethylene pentamine (TEPA) acting as redox initiators were purchased from Sigma-Aldrich. Potassium laurate (K-laurate), potassium hydroxide (KOH), and isopropyl alcohol were supplied by Sigma-Aldrich, and all solvents used were of analytical grades.
Adhesive preparation
Graft copolymerization of MMA onto NR backbone was performed by emulsion polymerization technique. Redox bipolar initiating system of CHP/TEPA was used by a ratio as shown in Table 1. HANR, diluted to obtain desire total solid content (%TSC), was poured into glass reactor equipped with condenser, and then, K-laurate acting as surfactant and KOH acting buffer were added under continuous stirring and nitrogen atmosphere at room temperature for 30 min. And MMA monomer was then charged to glass reactor before heating to 50 °C for 1 h to allow the latex particles to absorb the monomer. After that, CHP/TEPA was added to the glass reactor and the reaction was allowed to proceed for 4 h of total reaction time under nitrogen atmosphere. After the completion of reaction, the small amount of sample was coagulated for sample analysis by using 10% CaCl2 before leaching by distilled water. It was then extracted by petroleum ether and acetone to remove free NR and free MMA and dried at 50 °C for 24 h, respectively. The grafted samples were obtained at different %TSCs at 55, 57, and 60 identified as 55NR-g-MMA, 57NR-g-MMA, and 60NR-g-MMA, respectively.
Characterization
Lap shear strength
The adhesive performance was evaluated by lap shear test following ASTM D2339-98. Para rubber wood was selected as adherend in this research. Sample preparation with a dimension of 2.5 × 10 × 1 cm3 was allowed at room temperature for 48 h. Two hundred milligrams of NR-g-MMAs adhesive was performed to wood specimen which was 2.5 × 2.5 cm2 of applied adhesive area and was then dried for 15 min before assembly. After that, assembly samples were kept at room temperature for 24 h before lap shear test. The bonding strength of the assembly was investigated by a shear strength test using a crosshead speed of 50 mm/min, with ten replicate assemblies per treatment. The adhesive shear strength presented was averaged across the replicates.
Contact angle measurement
The wettability of NR-g-MMAs was determined by contact angle measurement, model Data physic OCA 40. Deionized water was used as test liquid, and the measurement was done five times in different locations and average values.
Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR)
The chemical compositions of NR-g-MMA were determined by FTIR 2000 PerkinElmer with ATR-FTIR mode. The spectra were recorded with the resolution of 4 cm−1 in the range of 4000–400 cm−1, and a diamond crystal was used.
Dynamic mechanical analyzer (DMA)
Dynamic mechanical properties of samples were investigated by DMA GABO model EPLEXOR 100. The sample size was 1 × 3 × 0.2 cm3 and was determined by tension mode at a frequency of 1 Hz and temperature from − 100 to 100 °C at heating rate 2 °C/min.
Rheological analysis
Two grams of NR-g-MMA of different total solid contents was placed onto rheometer. Plate-and-plate configuration of Thermo Fisher Scientific HAAKE MARS III was used in this study.
Results and discussion
Preparation of NR-g-MMAs wood adhesive of various total solid contents
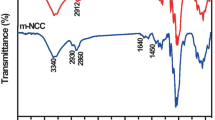
HANR was diluted by distilled water to obtain 55, 57, and 60 total solid content (%TSC) before grafted by MMA to obtain NR-g-MMAs (55NR-g-MMA, 57NR-g-MMA, and 60NR-g-MMA). NR-g-MMAs latex adhesive was extracted by acetone soxhlet extraction before characterization by water contact angle measurement as shown in Table 2. The water contact angle of NR-g-MMAs is sharply decreased from 95° to approximately 65° after modification, whereas the water contact angle of wood is approximately 60°. It could be explained that wettability and hydrophilicity are achieved by grafted MMA, and they can increase interaction between NR-g-MMAs and wood which mainly composes of cellulose and hemi-cellulose. In addition, the chemical composition of NR-g-MMAs was characterized by ATR-FTIR and is displayed in Fig. 1a. The absorption band of NR is found at 833 cm−1 which is assigned to H–C=C stretching and the absorption bands at 1450 and 1375 cm−1 correspond to CH2 and CH3 stretching, respectively. The broad absorption band around 1665 cm−1 is attributed to C=C stretching. The new absorption band of MMA at 1153 cm−1 and 1725 cm−1 is detected after modification at various samples which correspond to C–O and C=O stretching. According to contact angle and ATR-FTIR results of various samples, the wettability of NR-g-MMAs latex adhesive could be improved by the introduction of those functional groups of MMA onto NR backbone. Moreover, the mechanical properties of NR-g-MMA were characterized by dynamic mechanical analyzer (DMA) as shown in Fig. 1b. The dynamic mechanical properties of 57NR-g-MMA are chosen to represent. 57NR-g-MMA demonstrates relatively high storage modulus (E′) and low tanδ as compared to that of NR. It implied that the increase in E′ may be associated with an achievement of MMA grafted onto NR backbone which results in the improvement of elastic modulus of NR-g-GMA.
The influence of NR-g-MMA on lap shear strength
The influence of latex adhesive of NR-g-MMA on lap shear strength is compared with NR as shown in Fig. 2. Lap shear strength of NR-g-MMA is higher than that of NR. The failure mode was observed by visualization, and mixed modes (adhesive and cohesive failure) were obtained. This result could claim that the modification improves adhesive strength of NR-g-MMA adhesive latex because the ability to wet or spread onto wood surface and hydrophilicity of NR-g-MMA are better than those of NR as stated in the previous section.
According to ATR-FTIR and DMA results, the incorporation of the new chemical functional groups into NR-g-MMA backbone such as carbonyl and ester groups can possibly promote the interaction with cellulose in wood surface such as multiple hydrogen bonding, obtaining good bonding resulting in difficulty to rupture under applied loading, and the cohesive strength under shear force of this adhesive could be improved due to higher stiffness as compared with NR. The obtained result could be compared with the similar adhesive latex criterion. And the lap shear strength was higher than that of grafting diacetone acrylamide (DAAM) onto NR latex approximately 12% because the introduction between MMA and DAAM onto NR was difference. The grafting efficiency of NR-g-MMA was 97%, whereas NR-g-DAAM was approximately 50–62% which depended on monomer concentration. The higher DAAM monomer concentration tended to form homopolymer, resulting in a reduction in grafting efficiency [18].
The influence of total solid content of NR-g-MMAs latex adhesive on lap shear strength
The lap shear test was used to determine adhesive strength between various total solid contents of NR-g-MMAs latex adhesives and wood as shown in Fig. 3. Figure 3 shows that the lap shear strength increases until maximum value at 1025 kPa of 57NR-g-MMA, and thereafter, it decreases. It does not vary with apparent viscosity which is correlated well with total solid content. In addition, it does not also vary with the amount of grafted MMA which is determined by the intensities ratio between I1725 and I1450. The intensities ratio was sharply reduced in the initial percentage of total solid content; thereafter, it slightly decreased. These effects mean that lap shear strength is not governed by both apparent viscosity and percentage total solid content, but the amount of MMA onto NR backbone also plays important roles in the adhesion. Sun and Cheng [13] suggest that the lower viscosity produced over penetration and the higher viscosity influenced shallower penetration. Both of them resulted in dry-out at the interface, and the concentration of functional groups reduced significantly which resulted in the reduction of the interaction between wood and adhesive. The appropriate adhesive viscosity results in the best wetting performance; after that, the chemical interaction and physical interaction such as hydrogen bonding could be developed and accompanied with good adhesive strength.
The influence of storage time of NR-g-MMAs latex adhesive on lap shear strength
The effect of storage time on lap shear strength of 57NR-g-MMA is displayed in Fig. 4a. This latex adhesive was kept at room temperature before determining lap shear strength at a given storage time. The result shows that the apparent viscosity trends to increase during storage from 1 to 4 weeks and this effect results in the reduction in lap shear strength as displayed in Fig. 4a. Our result is in good agreement with Christjanson [14]. Urea formaldehyde adhesive was kept at room temperature, and the viscosity sharply increased with an extended period because of the reaction between free terminal amino groups and hydroxymethyl groups. The reason to explain how to increase in viscosity of 57NR-g-MMA latex adhesive during storage could be explained by ATR-IR results as shown in Fig. 4b. The absorption band around 3600–3100 cm−1 and 1729 cm−1 appears for 4 weeks of storage time, which can be assigned to the OH and C=O stretching due to the hydrolysis or aging process of ester groups of methacrylate. Because the 57NR-g-MMA latex adhesive is exposed to basic solution of pH around 7–8 and temperature around 30–35 °C. This reaction influenced the ester groups as shown in Fig. 5 and then formed multiple hydrogen bonding which was in good agreement with Bettencourt [19]. Therefore, the increase in viscosity above optimum value could reduce the wettability of latex adhesive due to an insufficient spreading of adhesive on the surface according to the report by Plaz [15].
Conclusion
NR latex wood adhesive of various total solid contents could be achieved by grafted copolymerization between MMA monomer and NR using redox initiation system. Contact angle was decreased from 95° for NR to about 65° for NR-g-MMAs of various total solid contents due to the introduction of hydrophilic groups to NR-g-MMAs such as carboxyl and ester groups. These effects result in an increase in storage modulus, whereas tan delta was decreased as compared to that of NR. In addition, the apparent viscosity was increased with an increase in percentages of total solid content as well as storage time; conversely, the amount of grafted MMA trended to decrease with increasing percentages total solid content. The highest lap shear strength was accomplished for 57NR-g-MMA, and it trended to decrease with increasing viscosity.
References
Wang Z, Li Z, Gu Z, Hong Y, Cheng L (2012) Preparation, characterization and properties of starch-based wood adhesive. Carbohydr Polym 88:699–706
Lei H, Du G, Wu Z, Dong Z (2014) Cross-linked soy-based wood adhesives for plywood. Int J Adhes Adhes 50:199–203
Liu Y, Li K (2006) Preparation and characterization of demethylated lignin-polyethylenimine adhesives. J Adhes 82:593–605
Nakason C, Wannavilai P, Kaesaman A (2006) Effect of vulcanization system on properties of thermoplastic vulcanizates based on epoxidized natural rubber/polypropylene blends. Polym Test 25:34–41
Ho CC, Khew MC (1999) Surface characterisation of chlorinated unvulcanised natural rubber latex film. Int J Adhes Adhes 19:387–398
Nakason C, Supasanthitikul P, Kaesaman A (2004) The grafting of maleic anhydride onto natural rubber. Polym. Test 23:35–41
Kochthongrasamee T, Prasassarakich P, Kiatkamjornwong S (2006) Effects of redox initiator on graft copolymerization of methyl methacrylate onto natural rubber. J Appl Polym Sci 101:2587–2601
Radabutra S, Thanawan S, Amornsakchai T (2009) Chlorination and characterization of natural rubber and its adhesion to nitrile rubber. Eur Polym J 45:2017–2022
Radabutra S, Amornsakchai T, Thanawan S (2012) Epoxidation of vulcanized natural rubber for bonding to chloroprene rubber. J Adhes Sci Technol 26:783–792
Thongnuanchan B, Nokkaew K, Kaesaman A, Nakason C (2007) Epoxidized natural rubber-bonded para rubber wood particleboard. Polym Eng Sci 47:421–428
John N, Joseph R (1997) Studies on wood-to-wood bonding adhesives based on natural rubber latex. J Adhes Sci Technol 11:225–232
Kalkornsurapranee E, Kaewsakul W, Daengli P (2015) Particle board from para rubber wood bonded with natural rubber-g-methyl (methacrylate). Adv Environ Biol 9:20–24
Cheng E, Sune X (2006) Effects of wood-surface roughness, adhesive viscosity and processing pressure on adhesion strength of protein adhesive. J Adhes Sci Technol 20:997–1017
Christjanson P, Siimer K, Pehk T, Lasn I (2002) Structural changes in urea-formaldehyde resins during storage. Eur J Wood Wood Prod 60:379–384
Paz E, Narbon JJ (2016) Influence of acrylic adhesive viscosity and surface roughness on the properties of adhesive joint. J Adhes Sci Technol 92:877–891
Sridee J (2006) Rheological properties of natural rubber latex. Dissertation, Suranaree University of Technology
Gan SN (1996) Storage hardening of natural rubber. J Macromol Sci Pure Appl Chem 33(12):1939–1948
Thongnuanchan B, Rattanawadee N, Kaesaman A, Nakason C (2015) A novel method to crosslink natural rubber latex adhesive at ambient temperature. Polym Bull 72:135–155
Bettencourta A, Caladoab A, Amara J (2015) Surface studies on acrylic bone cement. Int J Pharm 28:181–186
Acknowledgements
This work was support by Center of Excellence for Innovation in Chemistry (PERCH-CIC) Thailand and National Science and Technology Development Agency (NSTDA) Thailand, Young Scientist and Technologist Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Radabutra, S., Khemthong, P., Saengsuwan, S. et al. Preparation and characterization of natural rubber bio-based wood adhesive: effect of total solid content, viscosity, and storage time. Polym. Bull. 77, 2737–2747 (2020). https://doi.org/10.1007/s00289-019-02881-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02881-1