Abstract
Three ethylene–octene copolymers (EOC) with a wide range of octene content (17, 30, and 38 wt%) and with the same melt flow index of 1 g/10 min were cross-linked by e-beam radiation (in range 30–120 kGy). The testing methods comprised of rheology, a high-temperature creep test, an analysis of the gel content, and a dynamic mechanical analysis (DMA) test. It was discovered that copolymers with a high octene content attain a higher level of cross-linking density. Cross-linking influenced properties below T m only marginally as seen from the DMA results. However, the properties above T m were highly influenced as detected by high-temperature creep and rheology. Above T m, without the presence of crystals, only the chemical bonds holding the amorphous chains together manifested a gradually decreasing creep at 150 °C with an increasing irradiation level. The loss factor (tanδ at 0.1 Hz) at 150 °C revealed a decreasing trend (or higher cross-linking level) with an increasing octene content. High-temperature results were supported by an increasing gel content with increasing octene content. Samples irradiated to 30 kGy dissolved completely in xylene but showed significantly changed rheological characteristics indicating only an increase in the molecular weight and branching. Analysis according to the Charlesby–Pinner equation revealed increased cross-linking to the scission ratio G(X)/G(S) for EOCs with a higher octene content. While the q 0 value which relates to cross-linking changed only slightly, a significant decrease in the p 0 value which relates to chain scission was discovered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of Dow’s INSITE® metallocene catalysts led to the launch of many new polyolefin products which had previously been unattainable from the conventional Ziegler–Natta catalysis [1]. Copolymers of ethylene and alpha-olefins, e.g., linear low-density polyethylene (LLDPE), synthesized using the conventional multisite Ziegler–Natta catalysts are known to have broad molecular weight distributions (MWD) and also broad comonomer distributions [2]. This new class of copolymers has a narrow MWD, a very uniform narrow comonomer distribution and a controlled level of long chain branching (LCB) which leads to an unusually good processability (shear thinning). The incorporation of an alpha-olefin comonomer in the ethylene chains disrupts the chain regularity which then leads to a very special spot-like crystalline structure and smaller crystallinity. This unique crystalline structure then influences the mechanical properties. During the tensile test, the yield point is not present. In addition, the elastic behaviour is also greatly improved, which is manifested by a hugely reduced permanent set after repeated elongation to 100 % [2].
The unique microstructure of constrained geometry catalyst technology (CGCT) copolymers introduces an opportunity to study structure–property relationships solely as a function of one variable while keeping other variables constant. For example, Alamo et al. investigated the influence of comonomer types (butene, hexene, and octene) and molecular weight [3], Bensason et al. studied the influence of the comonomer content [4], and Wood-Adams et al. focused on the influence of LCB [5].
These copolymers have good tear resistance and a long shelf life which make them ideal packaging materials [6]. They may also be used as high-performance elastic fibers for apparel [7] (to replace Elastane), soft foams [8, 9], and in biomedical applications, including catheters and blood bags [6].
Commercially successful olefinic copolymers include ethylene–propylene, ethylene–butene, and ethylene–octene. Side groups differ only by the length or by the number of CH2 groups (1, 2, or 6) that are attached to the ethylene main chain. Interestingly, a similar rubber product, ethylene–styrene copolymer [1, 10] was produced for several years and used, e.g., in the foaming industry; however, after several years, its production stopped due to its low profitability. In contrast, ethylene–octene copolymer (EOC) has found its place in high-volume production, and now, after more than 15 years, it is being produced and sold.
The above-mentioned disruption of the chain regularity leads not only to improved elastic properties, but at the same time to a decreased melting temperature (T m) [3, 11, 12]. The “spot-like” crystals act as tie points for amorphous chains. However, at temperatures above T m, the polymer starts to flow freely. This could be a serious problem for many articles made from these copolymers, since T m for the most elastic articles is in the range of 45–55 °C [11]. Fortunately, this shortcoming can be overcome by cross-linking, as shown for Dow XLA™ elastic fiber [7]. Then, after the disappearance of physical cross-linking above T m (melting of crystalline tie points), chemical cross-linking (covalent bonds) ensures the elastic recovery after stretching.
Commercially successful methods for cross-linking of saturated polyolefins include peroxide cross-linking at elevated temperatures (such as dicumyl peroxide) [8, 13, 14]; grafting by vinyltrimethoxy silane in the presence of peroxide followed by the addition of a catalyst and exposure to water or humidity at elevated temperatures [15–17] and finally cross-linking by gamma [18–20] or electron beam [21–23] radiation. All these cross-linking methods have advantages and disadvantages. Nevertheless, all of them are being used commercially.
In spite of the fact that some researchers have attempted to compare the cross-linking behaviour of these copolymers under radiation, regrettably they varied several parameters at the same time (e.g., octene content and also molecular weight) which have led to questionable conclusions. At present, there is no solid understanding of the effect of octene content on cross-linkability by irradiation. To get a scientifically sound conclusion, in this study, there was only one variable (octene content) while keeping all other parameters constant.
This paper focuses only on electron beam irradiation cross-linking in connection with ethylene–octene copolymers. More specifically, the influence of the octene content (or branching density) on cross-linkability with the help of the measurement of rheology and creep behaviour at 150 °C and cross-link density by the gel content after the extraction of the soluble portion of the non-cross-linked polymer chains was examined. The data were evaluated by the Charlesby–Pinner equation.
Experiment
Materials
Three different ethylene–octene copolymers with the trade name ENGAGE® were used; they were supplied by The Dow Chemical Company (Midland, Michigan, USA). The octene contents of these copolymers were 17, 30, and 38 wt%. For a better understanding, the abbreviations EOC-17, EOC-30, and EOC-38 were chosen according to the octene content in wt%. The detailed compositions and densities are listed in Table 1. The initial melt flow indexes (MFI) were purposely the same for all three copolymers, being 1.0 g/10 min at 190 °C.
Low-density polyethylene (LDPE) which has been used for a comparison of some properties was Bralene RB 2-62, manufactured by Slovnaft Petrochemicals, Bratislava, Slovak Republic. MFI and the density of Bralene RB 2-62 were 2 g 10 min−1 and 0.918 g cm−3, respectively.
Compression moulding of the sheets
Sheets with a thickness of 0.5 mm (stainless steel frame size was 12 × 6 cm) were prepared by compression moulding. Pellets were pre-heated at 130 °C under minimal pressure for 5 min and compressed at 10 MPa for 3 min in a laboratory press and then quenched in another cold press under pressure.
Electron beam irradiation
Electron irradiation was performed in normal air at room temperature in BGS Beta-Gamma-Service GmbH, Germany. The temperature was controlled not to exceed 50 °C. The source of radiation was toroid electron accelerator Rhodotron (10 MeV, 200 kW). The irradiation was carried out in a tunnel on a continuously moving conveyer with the irradiation dosage ranging from 30 to 120 kGy in steps of 30 kGy per pass. Samples were arranged in one layer sealed between PET sheets. Other important parameters were: 10 mA, conveyer belt speed 3 m/min, distance from scanner to sample 78 cm, and irradiation time 2 s.
Dynamic mechanical analysis (DMA)
Dynamic mechanical analysis was carried out in an IT Keisoku-seigyo (DVA-200S) machine (Osaka, Japan). Specimens were tested in the dynamic tensile mode with a frequency of 10 Hz and a strain of 0.1 % from −100 to +200 °C with a heating rate of 5 °C min−1 and a grip-to-grip distance of 13.0 mm. Sample dimensions were 40 × 5 × 0.5 mm.
Rheology
Advanced Rheometric Expansion System ARES 2000 (Rheometric Scientific, Inc., Piscataway, NJ, USA) equipped with 25 mm parallel plates geometry was used to determine storage modulus G′, loss modulus G″, and complex viscosity η* in the frequency range 0.1–100 rad s−1 at constant temperature (150 °C) and strain (1 %). The loss factor was calculated as tanδ = G″/G′.
High-temperature creep test
Tensile samples were cut out of the cross-linked sheets and were used for the tensile creep experiments according to ISO 899. Creep testing was carried out in a Memmert UFE 400 oven with digital temperature control. Creep was recorded through the glass window using a SONY SLT-A33 camera, capable of HD 1920 × 1080 video (25 frames/s). This video was later analysed at regular time intervals. The effects of octene content, MFI, and the radiation dose on the creep behaviour of e-beam cross-linked EOC were studied at a fixed stress level of 0.1 MPa and 150 °C.
Gel content
The gel contents of the e-beam cross-linked EOC and LDPE samples were determined by an evaluation of the content of insoluble fraction of cross-linked material after solvent extraction according to ASTM D2765-01. About 0.3 g of cross-linked sample was wrapped in a 120 mesh stainless steel cage and extracted in refluxing xylene containing 1 wt% of antioxidant (Irganox 1010) for 6 h. The sample was then dried in a vacuum at 55 °C and weighed. The gel content was calculated as a ratio of the final weight to the initial weight of the sample multiplied by one hundred. Three samples were always averaged.
Size-exclusion chromatography
The molecular weight measurements were performed at 160 °C on a Polymer Laboratories PL 220 high-temperature chromatograph (Polymer Laboratories, Varian Inc., Church Stretton, Shropshire, England) equipped with three 300 mm × 7.5 mm PLgel Olexis columns and a differential refractive index detector. 1,2,4-trichlorobenzene (TCB) was used as an eluent, stabilised with butylhydroxytoluene (BHT) (Ciba, Basel, Switzerland) as an antioxidant. A mobile phase flow rate of 1 mL min−1 was used, and 200 μL was injected in all the cases. All samples were prepared to a concentration of 0.5 mg mL−1 in TCB. Narrowly distributed polyethylene standards (Polymer Standards Service GmbH, Mainz, Germany) were used for calibration purposes.
Theoretical background
When radiation from a γ-ray, electron beam, or X-ray source interacts with a polymer material, its energy is absorbed by the polymer material and active species, such as radicals are produced, thereby initiating various chemical reactions. The fundamental processes that are the results of these reactions include [24]:
-
Cross-linking, where polymer chains are joined and a network is formed.
-
Chain scission, where the molecular weight of the polymer is reduced through chain scission.
-
Oxidation, where the polymer molecules react with oxygen via peroxide radicals (oxidation and chain scission often occurs simultaneously).
-
Long-chain branching, where polymer chains are joined, but a three-dimensional network is not yet formed.
-
Grafting, where a new monomer is polymerized and grafted onto the base polymer chain.
Different polymers have different responses to radiation, especially when it comes to cross-linking vs. chain scission. A parameter called the G value is widely used by radiation chemists to quantify the chemical yield resulting from the radiation. The G value is defined as the chemical yield of radiation in a number of molecules reacted per 100 eV of absorbed energy. Materials with G(S):G(X) ratios <1.00 are favoured for cross-linking. Materials with G(S):G(X) ratios >1.00 tend to undergo degradation more. Materials, whose G(X) and G(S) values of both are low, are more resistant toward radiation. The different responses to radiation for different polymers are intrinsically related to the chemical structures of the polymers.
Cross-linking and chain scission are two competing processes that always co-exist under radiation. The overall effect depends on which of the two is pre-dominant at a certain time. Whenever G(X) is larger than G(S), the overall result is cross-linking, and whenever G(S) is larger than G(X), the overall result is degradation. It should also be kept in mind that for a given polymer, both G(X) and G(S) change with radiation conditions, such as the absorbed dose and the temperature. For the relationship with the radiation dose, both G(X) and G(S) increase with the increase in the dose. However, G(S) for a polymer generally increases more than G(X) does with an increasing dose.
The different reactions that radiation incurs on a polymer bring about different effects on the physical properties of the polymer. Cross-linking normally enhances the mechanical properties and thermal stability of the polymer while reducing the melt flow and increasing the viscosity of polymer solution. Chain scission, on the contrary, deteriorates the mechanical integrity and thermal resistance. It increases the melt flow and decreases the viscosity of polymer solution. Oxidation may give rise to discolouration and brittleness and introduces carbonyl-containing functional groups to the polymer. Long-chain branching brings the modification of rheology and, hence, the processability of the polymer.
Estimation of G values of cross-linking by the Charlesby-Pinner method
In 1959, Charlesby and Pinner published research results concerning the solubility of irradiated polyethylene. They analysed the data with the help of the following equation [25]:
where \(s\) is the sol fraction (\(s + g = 1\)), g is the gel fraction, r is the radiation dose, \(p_{0}\) is the fracture density per unit dose, \(q_{0}\) is the density of cross-linked units per unit dose, and \(u_{1}\) is the number-average degree of polymerization.
These days, the same equation is frequently expressed as [26]:
where \(P_{n}\) is the initial number-averaged degree of polymerization, and D is the absorbed radiation dose in kGy [27].
Many radiation-induced chemical changes are measured quantitatively in terms of a G value, this being the number of such changes produced per 100 eV absorbed in the system [25]. G values for cross-linking and chain scission are expressed as G(X) and G(S), respectively. Cross-linking and chain scission are two competing processes that always co-exist under radiation. The overall effect depends on which of the two is pre-dominant at a certain time. Whenever G(X) is larger than G(S), the overall result is cross-linking, and whenever G(S) is larger than G(X), the overall result is degradation. Charlesby and Pinner equation containing G values is expressed as [24]:
where \(M_{n}\) is the number-averaged molecular weight.
Turgis et al. used a modified version of this equation for copolymers AB [28]:
where \(m_{A}\) and \(m_{B}\) are molecular weights of comonomer units A and B, respectively. \(x_{B}\) is the molar fraction of comonomer B.
Results and discussion
In general, rubber studies have involved only the complex dynamic shear modulus, \(G^{*}\), rather than its two components, \(G^{\prime }\), the storage or elastic shear modulus, and \(G^{\prime \prime }\), the loss or viscous modulus [29],
The cross-linking system can be assumed to be an ideal rubber from the gel point to somewhere near the rubber to glass transition. From the rubber elasticity theory, we can relate the modulus determined at small deformations directly to the cross-link density, \(X\) [29]:
In general, G″ ≪ G′ after the gel point. Thus, one can focus on the development of only G′ value [29].
Initially, the rheology at 150 °C was measured for all three ethylene–octene copolymers exposed to various levels of irradiation. The three EOC samples before irradiation had slightly different modulus G′ values. Therefore, rather than plotting the absolute G′ values, it seemed more appropriate to show the relative G′ increase after irradiation. This is shown in Fig. 1a. For the EOC-17, the relative increase of G′ was in the range 15–42. For the EOC-30, the relative increase of G′ was higher (in the range 34–101). The highest relative increase of G′ was found for EOC-38 (in the range 53–126).
For the irradiations 60, 90, and 120 kGy, the G′ values for EOC-17 were 27.9, 32.0, and 43.4 kPa, and for EOC-38, they were 29.7, 38.9, and 53.7 kPa. These increases in G′ have slope values 259.5 and 400.2, for EOC-17 and EOC-38, respectively. The slope value for EOC-38 is about 54 % higher than that for EOC-17. From the steeper increase in G′ for EOC-38, one can assume a steeper increase in cross-link density X as a function of irradiation.
Figure 1b shows the influence of the octene content on relative G′ increase for various radiation levels (30, 60, 90, and 120 kGy). This measurement suggests that an EOC with a higher octene content cross-links more efficiently than the one with a lower octene content. A rheology measurement was analysed further with the focus on a loss factor tanδ (tanδ = G″/G′) which is the richest source of information regarding the molecular structure. In general, it is accepted that lower tanδ relates to better cross-linking. The tanδ results from ARES rheology (at 150 °C) are shown in Fig. 2.
First, Fig. 2a shows tanδ as a function of frequency for all three EOCs before cross-linking. Note that the tanδ values at 0.1 rad/s are in the range of 2.7–5.5. After irradiation to 120 kGy level, the tanδ values at 0.1 rad/s dropped in all EOCs to 0.17–0.35 range. At a higher frequency, the dangling chain behaves like the cross-linked one and the entanglement behaves like cross-linking. At a lower frequency, the chain shows characteristic viscoelastic behaviour like the linear chain. Therefore, the lower frequency (0.1 rad/s) was chosen to illustrate the differences in cross-linkability, as shown in Fig. 2c, d.
The influence of radiation dose on tanδ decrease is best visible in Fig. 2c. Note that tanδ axis is in a logarithmic scale; that large was the decrease caused by radiation cross-linking. The influence of octene content is best visible in Fig. 2d. EOCs with a high octene content exhibit lower values of tanδ which corresponds to better cross-linking.
The rheology measurements rendered not only the G′ and tanδ values (shown in Figs. 1, 2) but also the viscosity values. Viscosity is very sensitive to changes in chain length and is routinely used to characterise polymer molecular weight. Power law can be used to describe the relation of viscosity and molecular weight for entangled polymers [30]:
From the increase of viscosity caused by radiation, one can estimate the increase in molecular weight. Since radiation dose 60–120 kGy led to insoluble gel, only the 30 kGy level could be used for this calculation. The following equation was used to estimate the molecular weight increase:
The increase in viscosity \(\left( {\eta_{{ 3 0 {\text{kGy}}}} /\eta_{{ 0 {\text{kGy}}}} } \right)\) due to the irradiation 30 kGy for EOC-17, 30, and 38 was about 6.1, 8.8, and 10.7, respectively. Consequently, the calculated molecular weight increase \(\left( {M_{{ 3 0 {\text{kGy}}}} /M_{{ 0 {\text{kGy}}}} } \right)\) for EOC-17, 30, and 38 was about 1.7, 1.9, and 2.0, respectively. Copolymers with a higher octene content manifested the higher increase in viscosity caused by 30 kGy irradiation which can be interpreted by a higher increase in the molecular weight.
Visibility of the of cross-linking level is better at elevated temperatures (above melting point) when the molecules are only held together by chemical covalent bonds and not by physical cross-linking (when the crystals hold together the amorphous chains). Therefore, the samples were tested for creep behaviour at 150 °C. Figure 3a shows the creep development over time. Within 100 s, the creep values were small and similar for all three EOCs. In the range 100–1000 s, the creep curves grew substantially and differently for different EOC samples. The influence of EOC content is best visible in Fig. 3b. For all listed times [log(time/s) values], the creep values are smaller for EOCs with a higher octene content. High-temperature creep was also evaluated quantitatively as a slope of the line in the time range 4–25 min. The slope values for EOC-17, 30, and 38 were 35.3, 23.3, and 14.1, respectively. There is a huge influence of the octene content on a slope of the creep compliance. EOC with higher octene content exhibits significantly lower slope values.
The gel content study (see Fig. 4) reveals the amount of an insoluble cross-linked portion of polymer chains. After 6 h boiling in 138 °C xylene in a cage formed from stainless wire net with a gap between the wires of 0.2 mm, all the soluble molecules dissolve into the xylene. In the case of original samples without radiation exposure (see Fig. 4a), all of them completely (100 %) dissolved in xylene. Interestingly, also all 30 kGy radiated samples completely dissolved. If we looked only at these results, one could conclude that 30 kGy irradiation did not cause any visible change. However, we have to look also at the results from other analyses (previously shown in Figs. 2 and 3 where even 30 kGy caused a considerable increase in G′ and a decrease in tanδ. One conclusion is that 30 kGy causes only an increase in molecular weight and branching, but the full three-dimensional network has not been formed yet. A radiation dose of 60 kGy had already caused the generation of an insoluble 3D network ranging according to the sample from 52 to 74 %. In the radiation range 60–120 kGy, the gel content is increasing only moderately (compared to a large jump in the range 30–60 kGy). Figure 4b shows the influence of octene content on the gel content for radiation doses 60, 90, and 120 kGy. In this gel content study, we also tested a low-density polyethylene (LDPE) to which the 0 octene content was assigned. Interestingly, the LDPE fits almost perfectly to the linear regression lines, as shown in Fig. 4b. With increasing octene content, the gel content also increased.
The solubility of the three ethylene–octene copolymers and LDPE was initially analysed with the help of Eq. (2) (see Fig. 4c). This led to intercept values \(p_{0} /q_{0}\) (scission to cross-linking ratio) and slope values being \(1/q_{0} P_{n}\). More detailed analysis shown in “Appendix 2” then led also to other values: \(q_{0}\), \(p_{0}\), \(G\left( X \right)/G\left( S \right)\), \(G\left( X \right)\), and \(G\left( S \right)\). Comparison of these values is shown in Fig. 5.
The density of cross-linked units per unit dose (\(q_{0}\)) is quite similar for all samples. However, the fracture density per unit dose (\(p_{0}\)) is quite different; it is considerably lower for EOCs with a higher octene content. Reduced scission then leads to a substantially higher cross-linking to a scission ratio \(G\left( X \right)/G\left( S \right)\) for the copolymers with a higher octene content. With a higher content of octene short branches, the probability of a radical formation on a short branch increases, and consequently, the main chain is attacked to a lesser extent. The mobility of the short branch is much higher, compared to the one on the main chain, so that the radicals on octene have a higher probability for recombination with other free radicals which leads to cross-linking. The situation is schematically shown in Fig. 7. A free radical can lead to cross-linking or scission. A copolymer with a higher octene content has more short-chain branches and thus the probability of radical formation on a branch is higher (options c and d). While scission on a main chain (options a and b) leads to a decreased molecular weight, scission on an octene short branch influences the molecular weight only minimally.
Another significant fact is that a copolymer with a higher octene content includes a higher number of tertiary carbon atoms. After being exposed to e-beam radiation, the hydrogen atoms are extracted from carbon atoms. The radical on a tertiary carbon atom can be created more easily, even though the radicals on the main chain or on an octene branch are formed as well (LDPE was also cross-linked, however, to a lower extent). The polymer radicals can then form a cross-link by a recombination with another polymer radical or they can extract a hydrogen atom from the neighbouring chain. Cross-linking options in Fig. 6b–b, b–d, a–b, and b–c play an important role during electron beam cross-linking of ethylene–octene copolymers. As it was shown, LDPE also cross-links (a–a option) but to a lesser extent. Solubility results agreed well with previously shown rheology and creep results.
The presented results are in good accord with other researchers [8, 9, 31, 32]. For instance, Vachon et al. extensively examined the foaming behaviour of gamma-irradiated ethylene–octene copolymers. Cross-linking not only stabilises bubble growth during expansion but also enhances the resistance of cellular material to thermal collapse. While insufficient cross-linking may lead to collapse, excessive cross-linking may restrict the foam expansion. The EOCs had the same melt flow index (MFI = 1 g/10 min) while increasing octene content EOC-30 (Engage 8003) and EOC-38 (Engage 8100). After irradiation to 50 kGy in the air, the copolymer with a higher octene content (EOC-38) demonstrated 80 % gel content, while the one with the lower octene content (EOC-30) had only 68 % gel content. After irradiation to 100 kGy in a vacuum, the values were 88 and 85 % for EOC-38 and EOC-30, respectively [9].
Liu et al. investigated UV photodegradation of polyethylene compared to ethylene–octene copolymers with an increasing octene content 20, 30, and 38 wt%. After 200 h of UV exposure, the values of gel content for LDPE, EOC-20, EOC-30, and EOC-38 were 18, 22, 31, and 41 %, respectively. They evaluated the gel content also as a function of time. While LDPE exhibited a steady increase of gel, after 200, 300, 400, and 600 h, the values of gel content were 18, 31, 33, and 36 %, the EOC-38 had an initial sharp increase followed by a steady decrease, and the values were 41, 30, 18, and 7 %. These results indicate a much higher reactivity of EOC containing a tertiary carbon atom which initially leads to a higher level of cross-linking but later leads to a chain scission [32].
Sirisinha et al. investigated a silane cross-linked EOC (Engage 8003) in comparison with LDPE. These products are commonly used for a number of industrial applications, including wire and cable coating, hot-water piping insulation, and heat-shrinkable products. They found out that LDPE had a lower cross-linking rate and a lower gel content compared to EOC. The values of gel content after various water immersion times of 120, 360, and 600 h were for LDPE 52, 66, and 68 %, and for EOC-30, they were 77, 82, and 85 % [31].
Abe et al. conducted research concerning the foaming behaviour of ethylene–1-hexene copolymers that can be used as thermal insulation, floatation, automotive trim, and sports goods. He focused on two ethylene–1-hexene copolymers; LL1 had 25 branches, and LL2 had 7 branches per 1000 backbone carbon atoms with the same molecular weight of M n = 4.6 × 104 g/mol. The foams were produced directly by a moulding with 0.1–0.9 wt% of dicumylperoxide (DCP) and 8 phr of a blowing agent. The copolymer with the higher branching density (LL1) rendered a foam with a higher gel content, higher storage modulus G′, and a lower tanδ. For example, for the peroxide concentrations 0.3, 0.5, 0.7, and 0.9 %, the values of gel content were 70, 88, 91, and 94 % for LL2 with 7 branches, and 73, 94, 96, and 98 % for LL1 with 25 branches per 1000 backbone carbon atoms. For peroxide concentrations 0.3 and 0.9 %, the storage modulus G′ values (at 190 °C) were 273 and 431 kPa for LL2 with 7 branches and 319 and 611 kPa for LL1 with 25 branches per 1000 backbone carbon atoms. For peroxide concentrations 0.3 and 0.9 %, the loss factor tanδ values (at 190 °C) were 0.161 and 0.084 for LL2 with 7 branches and 0.122 and 0.053 for LL1 with 25 branches per 1000 backbone carbon atoms [8].
Nicolas et al. studied the cross-linking behaviour of EOCs by e-beam and peroxide. They selected Engage 8411 (with MFI = 18 g/10 min and 33 wt% of octene) and Engage 8400 (with MFI = 30 g/10 min and 40 wt% of octene). They concluded that increasing comonomer content reduces the cross-linking efficiency, because, for example, after irradiation with 200 kGy, the EOC with 40 wt% of octene had only 58 % gel content and EOC with 33 wt% of octene had 68 % gel content [14]. The problem with their conclusion is in the fact that these two copolymers have very different initial molecular weights. Recently, we have discovered the enormous influence of the initial molecular weight on cross-linking. For example, EOCs with approximately the same octene content (35, 38, and 39 wt%) with the initial melt flow index being 3, 1, and 0.5 g/10 min (molecular weight M w being about 93,000, 129,000, and 167,000 g/mol), respectively, exhibited increasing gel content after e-beam irradiation to 120 kGy. The gel content values were 80, 88, and 91 %, respectively. The EOC with the lowest MFI (or the highest molecular weight) showed the highest gel content (91 %). Even more pronounced difference was visible at 60 kGy, gel content values being 56, 74, and 78 %, respectively.
In the past, we have investigated the cross-linking of EOCs by peroxide at higher temperatures (150–200 °C) [33]. This research led to the conclusion that the EOCs with lower octene content cross-linked better. The difference can be explained by a different cross-linking mechanism in the solid state (with the presence of a crystalline phase) versus cross-linking in the melting phase (with only an amorphous phase and much higher mobility). It is possible that a higher reactivity of tertiary carbon atoms at elevated temperatures initially leads to higher cross-linking, but later, the chain scission destroys the formed network, as reported by Liu et al. [32].
Conclusions
The three different analyses (rheology, high-temperature creep, and gel content) independently confirm that ethylene–octene copolymers with higher octene content cross-link better than ones with low octene content.
Rheology at 150 °C highlighted the huge differences in cross-linked polymers, especially in the 0–60 kGy range and at small frequencies (0.1–1 Hz). After irradiation, tanδ decreases significantly with increasing octene content which corresponds to a better elasticity (or a higher level of cross-linking).
The high-temperature creep test showed again how a decreasing trend in creep (or better resistance to flow) with an increasing octene content confirms a higher level of cross-linking.
Analysis of insoluble gel content illustrates how higher values for EOCs with higher octene content confirm a higher level of cross-linking. Analysis according to the Charlesby–Pinner equation manifests enlarged cross-linking to the scission G(X)/G(S) ratio for higher octene EOCs. Compelling is a substantial decrease in G(S) parameters. This was interpreted by scission on an octene short-chain branch which does not cause the main chain scission (and, e.g., EOC-38 has more octene short branches than EOC-30 and still more than EOC-17). Furthermore, in EOC-38, the amount of tertiary carbon atoms with higher reactivity is greater.
References
Chum PS, Kruper WJ, Guest MJ (2000) Materials properties derived from INSITE metallocene catalysts. Adv Mater 12(23):1759–1767
Chum PS, Kao CI, Knight GW (1995) Structure-property relationships in polyolefins made by constrained geometry catalyst technology. Plast Eng 51(6):21–23
Alamo RG, Viers BD, Mandelkern L (1993) Phase-structure of random ethylene copolymers—a study of counit content and molecular-weight as independent variables. Macromolecules 26(21):5740–5747
Bensason S, Minick J, Moet A, Chum S, Hiltner A, Baer E (1996) Classification of homogeneous ethylene-octene copolymers based on comonomer content. J Polym Sci Pt B Polym Phys 34(7):1301–1315
Wood-Adams PM, Dealy JM, deGroot AW, Redwine OD (2000) Effect of molecular structure on the linear viscoelastic behavior of polyethylene. Macromolecules 33(20):7489–7499
Li JQ, Peng J, Qiao JL, Jin DB, Wei GS (2002) Effect of gamma irradiation on ethylene-octene copolymers. Radiat Phys Chem 63(3–6):501–504
Casey P, Chen HY, Poon B, Bensason S, Menning B, Liu LZ et al (2008) Polyolefin based crosslinked elastic fiber: a technical review of DOW XLA (TM) elastic fiber technology. Polym Rev 48(2):302–316
Abe S, Yamaguchi M (2001) Study on the foaming of crosslinked polyethylene. J Appl Polym Sci 79(12):2146–2155
Vachon C, Gendron R (2003) Effect of gamma-irradiation on the foaming behavior of ethylene-co-octene polymers. Radiat Phys Chem 66(6):415–425
Chen HY, Chum SP, Hiltner A, Baer E (2001) Comparison of semicrystalline ethylene-styrene and ethylene-octene copolymers based on comonomer content. J Polym Sci Pt B Polym Phys. 39(14):1578–1593
Kale LT, Plumley TA, Patel RM, Jain P, Tappi (1995) Structure-property relationships of ethylene/1-octene and ethylene/1-butene copolymers made using insite technology. 1995 Polymers, laminations & coatings conference, books 1 and 2. Tappi, Atlanta, pp 423–433
Eynde SV, Mathot VBF, Koch MHJ, Reynaers H (2000) Thermal behaviour and morphology of homogeneous ethylene-1-octene copolymers with high comonomer contents. Polymer. 41(13):4889–4900
Molloy BM, Hyslop DK, Parent JS (2014) Comparative analysis of delayed-onset peroxide crosslinking formulations. Polym Eng Sci 54(11):2645–2653
Nicolas J, Ressia JA, Valles EM, Merino JC, Pastor JM (2009) Characterization of metallocene ethylene-1-octene copolymers with high comonomer content cross-linked by dicumyl peroxide or beta-radiation. J Appl Polym Sci 112(5):2691–2700
de Melo RP, Aguiar VDO, Marques MDFV (2015) Silane crosslinked polyethylene from different commercial PE’s: influence of comonomer, catalyst type and evaluation of HLPB as crosslinking coagent. Mater Res Ibero Am J 18(2):313–319
Sirisinha K, Chuaythong P (2014) Reprocessable silane-crosslinked polyethylene: property and utilization as toughness enhancer for high-density polyethylene. J Mater Sci 49(14):5182–5189
Sirisinha K, Boonkongkaew M (2013) Improved silane grafting of high-density polyethylene in the melt by using a binary initiator and the properties of silane-crosslinked products. J Polym Res 20(4):9
Singh P, Kumar R, Singh R, Roychowdhury A, Das D (2015) The influence of cross-linking and clustering upon the nanohole free volume of the SHI and gamma-radiation induced polymeric material. Appl Surf Sci 328:482–490
Karabelli D, Lepretre JC, Dumas L, Rouif S, Portinha D, Fleury E et al (2015) Crosslinking of poly(vinylene fluoride) separators by gamma-irradiation for electrochemical high power charge applications. Electrochim Acta 169:32–36
Ghosal S, Mukhopadhyay M, Ray R, Tarafdar S (2014) Competitive scission and cross linking in a solid polymer electrolyte exposed to gamma irradiation: simulation by a fractal model. Phys A 400:139–150
Zimek Z, Przybytniak G, Nowicki A, Mirkowski K, Roman K (2014) Optimization of electron beam crosslinking for cables. Radiat Phys Chem 94:161–165
Ng HM, Bee ST, Ratnam CT, Sin LT, Phang YY, Tee TT et al (2014) Effectiveness of trimethylopropane trimethacrylate for the electron-beam-irradiation-induced cross-linking of polylactic acid. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 319:62–70
Zhu SF, Shi MW, Zhu MF (2013) Effects of electron-beam irradiation crosslinking on PA6 fibers. Fiber Polym 14(4):525–529
Makuuchi K, Cheng S (2012) Radiation processing of polymer materials and its industrial applications. Wiley, Hoboken, New Jersey, USA
Charlesby A, Pinner SH (1959) Analysis of the solubility behaviour of irradiated polyethylene and other polymers. Proc R Soc Lond Ser A 249(1258):367–386
Dubey KA, Chaudhari CV, Rao R, Bhardwaj YK, Goel NK, Sabharwal S (2010) Radiation processing and characterization of poly(vinyl alcohol) nano-composites, part 1: nano-particulate filler tuned crosslinking behavior. J Appl Polym Sci 118(6):3490–3498
Mondal M, Gohs U, Wagenknecht U, Heinrich G (2013) Efficiency of high energy electrons to produce polypropylene/natural rubber-based thermoplastic elastomer. Polym Eng Sci 53(8):1696–1705
Turgis JD, Coqueret X (1999) Electron beam sensitivity of butyl acrylate copolymers: effects of composition on reactivity. Macromol Chem Phys 200(3):652–660
Mussatti FG, Macosko CW (1973) Rheology of network forming systems. Polym Eng Sci 13(3):236–240
Colby RH, Fetters LJ, Graessley WW (1987) Melt viscosity molecular-weight relationship for linear-polymers. Macromolecules 20(9):2226–2237
Sirisinha K, Chimdist S (2008) Silane-crosslinked ethylene-octene copolymer blends: thermal aging and crystallization study. J Appl Polym Sci 109(4):2522–2528
Liu ZY, Chen SJ, Zhang J (2011) Photodegradation of ethylene-octene copolymers with different octene contents. Polym Degrad Stab 96(11):1961–1972
Svoboda P, Poongavalappil S, Theravalappil R, Svobodova D, Mokrejs P (2013) Effect of octene content on peroxide crosslinking of ethylene-octene copolymers. Polym Int 62(2):184–189
Gupta J, Nunes C, Vyas S, Jonnalagadda S (2011) Prediction of solubility parameters and miscibility of pharmaceutical compounds by molecular dynamics simulations. J Phys Chem B 115(9):2014–2023
Acknowledgments
This work has been supported by the Internal Grant Agency of the Tomas Bata University in Zlin number IGA/FT/2016/009.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
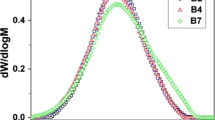
Furthermore, the irradiated samples were also analysed by dynamic mechanical analysis (DMA). The results are shown in Figs. 7 and 8. The curves of storage modules for the three samples (see Fig. 7) are quite different. Initially, there is a glassy plateau down to about −50 °C which is very similar for all three samples. Then, from −50 °C, the values of storage modulus differ to a large extent. EOC-17 decreases with increasing temperatures the least. On the contrary, EOC-38 decreases with increasing temperature quite significantly. EOC-30 is placed in between. The first drop is connected with glass transition temperature (T g), and the second drop is connected with melting temperature (T m). Figure 7b shows the tanδ dependence on temperature when T g can be easily read as a peak position. The tanδ peak increases and moves towards a lower temperature with an increasing octene content.
The presented DMA results of the cross-linked samples resemble results for pure ethylene–octene copolymers presented by Bensason et al. [4]. He discovered that β and α relaxations strongly depend on octene content, while γ relaxation was the least sensitive to comonomer content. With increasing comonomer content, the α relaxation shifts to lower temperatures and starts to overlap with increasingly intense β relaxation. β relaxation (the largest peak is approximately −40 °C) is being interpreted as the glass transition temperature (T g).
Figure 7a, b are shown for samples irradiated to 120 kGy. Is there any influence of the radiation on T g? The answer to this question can be seen in Fig. 8a.
There is a clear trend in a T g decrease with increasing octene content. However, as for the different levels of radiation, the T g values are almost unchanged. Clearly, the radiation cross-linking influences the polymer behaviour more significantly above the melting point, at elevated temperatures as presented above. At lower temperatures, almost no difference was observed.
In contrast to almost no effect of radiation on T g, there is a significant decrease in a storage modulus with increasing octene content; the polymer is becoming softer and rubbery (see Fig. 8b). A slightly smaller difference was observed at lower temperatures (−40 and −50 °C), but quite a large difference at higher temperatures (−20, 0, 20, 40 °C). Note that the storage modulus axis is in logarithmic scale, so the softness of the EOC samples is to a large extent different.
Tensile strength and modulus of semicrystalline polymers at temperatures below melting point come mainly from lamellar crystals that hold together amorphous chains. Tie molecules connect different crystals through amorphous phase. It is called “physical cross-linking”. Such system has cohesive energy density (CED) of several orders of magnitude higher than lightly cross-linked semicrystalline polymer above melting point. Therefore, the effect of radiation on solid-state mechanical properties is very small. The cohesive energy represents the total attractive forces within a condensed state resulting from intermolecular interactions and consists of electrostatic interactions, van der Waals interactions, and hydrogen bonds interactions [34]. It is equivalent to the amount of energy required to separate the constituent atoms/molecules to an infinite distance, where it approaches zero potential energy. Cohesive energy density (CED) refers to the energy required to vaporise a mole of liquid per unit volume and is mathematically defined by:
where \(\Delta E_{V} =\) internal energy change of vaporisation, \(\Delta H_{v} =\) enthalpy of vaporisation, \(V_{m} =\) molar volume of the liquid at the temperature of vaporisation, \(R =\) gas constant, and \(T =\) absolute temperature.
At higher temperatures (e.g., at 150 °C), above the melting point of EOC, there are no crystals to hold the amorphous chains together. The strength of the material comes only from the chemical bonds between chains (chemical cross-linking), and therefore, such high-temperature tests are very sensitive to the level of cross-linking.
Appendix 2
Example of calculation for EOC-38.
38 wt% of octene, M n = 61,469 g/mol
Charlesby–Pinner equation:
Calculation of G parameters according to Charlesby-Pinner equation (see Table 2):
Appendix 3
During my study of the literature concerning ethylene–octene copolymers, I found many interesting papers, but quite a large number of them did not have correct values of octene content or melt flow index. This could be caused by the fact that the manufacturer at the present time does not list comonomer content in their materials data sheets. These numbers appeared briefly on the DuPont-Dow Elastomers’ web page in the year 2002, but then, this information disappeared. Fortunately, I have this table, and I would like to share it with the wider scientific community (see Table 3, columns 1–4). In addition, I have added comonomer content in mol% in column 5 and ethylene/octene molar ratio (see column 6). The molar fraction of octene was calculated according to Eq. 10.
Rights and permissions
About this article
Cite this article
Svoboda, P. High-temperature study of radiation cross-linked ethylene–octene copolymers. Polym. Bull. 74, 121–144 (2017). https://doi.org/10.1007/s00289-016-1703-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1703-6