Abstract
A maleated poly(vinyl alcohol) (PVAM)-g-chitosan (CS) (PVAM-g-CS) film was prepared in solution form using potassium persulfate (K2S2O8) as an initiator. The influence of the PVAM:CS ratio, the reaction time and temperature and the concentration of K2S2O8 on the properties of the PVAM-g-CS were investigated. Ether linkage of the PVAM-g-CS was observed at 1154 and 1089 cm−1 by ATR-FTIR. The conditions for grafting CS to PVAM were developed and optimized. The highest degree of grafting was observed at a ratio of 9:1 PVAM:CS. The optimum grafting conditions were found to be 50 °C for a reaction time of 1 h, with a ratio of PVAM:CS of 9:1 and a concentration of 0.5 % K2S2O8. The maximum and minimum swelling ratios were 150 and 330 %, respectively. The tensile strength of the PVAM was enhanced after the incorporation of CS in the PVAM. The highest tensile strength of 24 MPa was found at a ratio of 5:5 PVAM:CS. The PVAM-g-CS shows a high efficiency of controlling capsaicin release in base–acid medium and was easily biodegradable in natural soil especially with high proportions of CS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(vinyl alcohol) (PVA) has been used in many fields such as packaging [1], biological separation [2] and in biomedical applications [3 ] because of its good water solubility, and low toxicity. On the other hand, the drawbacks of PVA are its low water resistance [4], low protein adsorption [5] and poor biocompatibility [6]. In previous work, modification by graft polymerization has been used to improve the properties of PVA which has attracted considerable interest because it imparts some desirable properties, such as chelation [7], ion exchange [8], biocompatibility [9] and protein adsorption [10]. PVA was grafted with acrylic acid and N-vinyl imidazole using gamma radiation [11]. The grafting yield and the pH of the feed solution were used to control the graft copolymer. More recently, PVA was successfully modified with maleic anhydride (MA) in an aqueous solution through esterification. Observations using FTIR indicated that a carboxylic group was lost due to cross-linking between the PVA and MA [12]. After the chemical modification of the PVA, it showed good pH sensitivity on environment.
In this study, maleated poly(vinyl alcohol) PVAM was grafted with chitosan (CS) through esterification. CS consists of a linear polymer of α (1→4)-linked 2-amino-2-deoxy-β-d-glucopyranose derived from the N-deacetylation of chitin, a common structural polymer found in exoskeletons [13]. The raw source of chitin and CS are crustaceans such as crabs, shrimp and lobsters, which are highly abundant biomasses derived from waste products from the food and beverage and canning industries. Due to its physical and chemical properties, CS has received much attention as a functional biopolymer and is being used in a wide range of applications ranging from biomedical engineering [14], pharmaceuticals [15] and cosmetic products [16]. However, CS has poor mechanical strength which may limit its practical applications. To improve its mechanical properties, copolymerization with other polymers or monomers has been investigated [17, 18]. For instance, CS was grafted with polyacrylamide (PAM) in its aqueous phase to form CMC-g-PAM which was used as a flocculant for the removal of various dyes from aqueous solutions [17]. The grafted polyacrylamide chains were found to contribute much to the improved bridging and sweeping flocculation effects, but caused a reduction in charge neutralization flocculation employed for charge screening.
CS was also grafted with poly(2-(furan-2-carbonyl)-acrylonitrile using a novel monomer prepared from a Baylis–Hillman reaction and employing potassium persulfate and sodium sulfite as a redox system [18]. The resulting graft copolymers were found to significantly retard the growth of bacteria and fungi in a manner directly dependent on the grafting percentage.
Further, carboxymethyl chitosan (CMCS), a derivative of the CS, was used as a grafting agent for saponified polyethylene terephthalate (PET) fabric, significantly improving the hydrophilic properties of the CMCS-grafted fabric over that of untreated PET fabric [19]. The decrease in the surface charge density indicated an improvement in the antistatic property of the CMCS-grafted fabric. Recently, water-soluble CS was modified with caffeic acid (CA) and ferulic acid (FA) employing a free radical-mediated method to produce in vitro and in vivo chitosan derivatives with antioxidant properties [20]. The results showed that the modified CS derivatives were able to significantly increase the activity of antioxidant enzymes.
The disadvantages of PVAM are poor in tensile strength and swelling ratio in water and difficulty to degrade in nature. Therefore, the PVAM was grafted with CS to solve these of problems and until now, however there have been no reports of a graft copolymer of PVAM and CS in its aqueous phase. In this study, CS was grafted with PVAM molecules in their aqueous form using potassium persulfate as an initiator. The effects of the liquid ratio, reaction time, temperature and K2S2O8 concentration on the physical properties including the swelling ratio, tensile strength and elongation at break as well as biodegradation of the PVAM-g-CS were investigated. The morphology and thermal stability of the PVAM-g-CS were also characterized by SEM and TGA, respectively. The polymer hydrogel from the graft copolymer was applied to use a polymer matrix for encapsulation of capsaicin. The controlled release of the capsaicin from polymer matrix in water medium was evaluated in this work.
The results indicate that this novel polymer hydrogel has potential for further development in medical material field.
Experimental
Materials
PVA with an average molecular weight of 8.9 × 104 g/mol and 89 % hydrolysis, (Nippon Gohsei Co., Ltd., Singapore) was used in the present study. The K2S2O8 used as a water-soluble initiator was manufactured by RFCL Ltd (India). The surfactant used, 10 % Triton X100 was manufactured by Sigma-Aldrich (Germany). The CS was prepared by a local company in Thailand and the degree of deacetylation of the CS was found to be 87 %. Capsaicin, 99.5 % purity, was provided by Yuanmu Biotechnology Co., Ltd. (Shanghai, China).
Grafting procedure
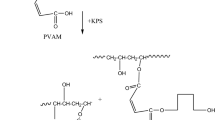
The PVAM was prepared in accordance with our previous reported work [12]. The CS sample obtained was dried in an oven at 60 °C for 48 h. The CS solution was prepared by dissolving CS in distilled water containing 2 % (v/v) of acetic acid. The blends were prepared by mixing the CS and PVAM solutions in the presence of K2S2O8 at 65 °C for 3 h to produce a homogeneous solution. The ungrafted PVAM was then water-extracted and the mixture was cast on a Petri dish at 30 °C and dried in an oven at 50 °C for 24 h then kept in desiccator before characterization.
Measurements
The chemical structure of the modified PVAM was analyzed by attenuated total reflection Fourier transform infrared (ATR-FTIR) using a Bruker Equinox 55 measuring in the range of 4000–500 cm−1. The X-ray diffractograms (XRD) of the graft copolymer samples were obtained using an X-ray diffractometer with a Cu–NF filter and Cu Ka radiation (X’Pert MPD, Philips, Netherlands). The degree of grafting of the grafted samples was determined by extraction of the samples in boiling toluene for 24 h followed by extraction in boiling water for 24 h using a Soxhlet apparatus. The PVAM-g-CS samples were investigated by extracting the samples in boiling water for 6 h using a Soxhlet apparatus. The extracted samples were dried in an oven at 40 °C until a constant weight was achieved. The degree of grafting efficiency was measured by the following equation [Eq. (1)]:
where W 1 and W 0 are the weights of the dried samples after extraction and before extraction, respectively.
To establish the swelling ratio of the PVAM-g-CS in distilled water, 2 cm × 2 cm samples were cut from the sheet. The swelling ratio was measured by immersing the samples in 25 ml of distilled water at room temperature for 0.5, 1, 2, 4 and 7 h. The swelling ratio was obtained according to the following equation [Eq. (2)]:
where W i is the initial dry weight of the sample (g) and W 2 is the weight of the swollen sample (g).
Thermogravimetric analysis (TGA) was performed using a TGA7, Perkin Elmer instrument. Samples of between 10 and 20 mg were placed in a platinum pan. Analyses were carried out under nitrogen and oxygen atmospheres, respectively (gas flow = 100 ml/min−1), at a heating rate of 10 °C/min−1, in a temperature range of 50–1300 °C.
A scanning electron microscope (JMS-5800 LV, JEOL, and SEM, Tokyo, Japan) was used to investigate the morphologies of the cross sections of both the PVAM and PVAM-g-CS at an accelerating voltage of 6 kV.
The degradation in soil of the PVAM-g-CS was measured using 2 cm × 2 cm samples. The samples were dried in a vacuum oven at 40 °C for 24 h. The water content of the soil was in the range of 35–40 %. The degradation was established by burying the samples in soil for 30 days. After 30 days, the samples were removed and rinsed with distilled water to remove the residue of the soil and were dried in an oven at 40 °C for 24 h before being weighed. The biodegradation of the resulting samples in soil was obtained according to the following equation [Eq. (3)]:
where W 3 is the initial dry weight of the sample (g) and W 4 is the weight of the sample after buried for 1 month (g).
Encapsulation
The 7:3 PVAM-g-CS solutions was selected to load with the capsaicin. In brief, an appropriate amount of 20 g of capsaicin (2000 ppm) was mixed with the 100 g of 7:3 PVAM-g-CS under stirrer with magnetic for 10 min. Subsequently, this was cast on glass plate at 29 °C and dried in an oven at 50 °C for 24 h then kept in desiccator before characterization. The efficiency for the entrapment of capsaicin in the hydrogel matrix was calculated from the ratio between the initial mass of the capsaicin to be encapsulated, and its mass in the final product. In this case, a known amount of capsaicin (ca. 12 mg) was dispersed in a 50/50 ethanol/distilled water medium and stirred for 2 days at room temperature. Subsequently, the suspension was filtered, and the content of dissolved capsaicin in the 50/50 ethanol/distilled water medium solution was determined using UV spectrometry (Shimadzu UV-1601) at 206 nm (λ max). At definite intervals of time, an aliquot (5 ml) was taken for analysis of the capsaicin in the hydrogel. Experiments were performed thrice. The release results were evaluated using an empirical equation to estimate the value of n as follows [Eq. (4)]:
where M t /M ∞ is the released fraction at time t, n is the release exponent, and K is the release factor. From the slope and intercept of the plot of log (M t /M ∞) against log (t), the kinetic parameter n was calculated.
Results and discussion
Synthesis of the graft copolymers (PVAM-g-CS) observed by ATR-FTIR
PVAM was selected as a convenient starting material to prepare the graft copolymer because of the presence of a reactive moiety in the form of a carboxylic group to link with the NH of the CS. Grafting of CS on the PVAM may bé initiated by diffèrent free radicals. CS radicals will react with PVAM molecules to initiate graft copolymerization. The grafting of the CS onto the PVAM was controlled by the rate of CS diffusion in the vicinity of PVAM macroradicals. The PVAM-g-CS was produced in its liquid phase. The possible grafting reaction between the PVAM and the CS is illustrated in Fig. 1. The synthesized copolymer from the PVAM and the CS were characterized by their functional groups using the ATR-FTIR technique as shown in Fig. 2. The major characteristic peaks of CS were found at 3450 cm−1 (O–H stretch), 2875 cm−1 (C–H stretch), 1650 cm−1 (C=O stretch of carbonyl group) and 1375 cm−1 (C–H stretch of methyl). In the case of PVAM, the absorptions at 1735 cm−1 can be attributed to the carbonyl group of the ester from the carboxylic group [3]. A characteristic transmittance peak at 1083 cm−1 was observed in the grafted PVAM spectrum but not in the pristine CS. This band from the ester group provides evidence of the grafting of the CS onto the PVAM.
XRD was used to determine the crystalline patterns of the sample films. Figure 3 shows the crystallographic structures of the pristine PVAM, CS and the PVAM-g-CS. CS, like polysaccharides such as starch and cellulose, is a partially crystalline natural polymer. Its crystallinity is mainly formed due to the accumulation of linear chains in its structure. The XRD patterns of the CS showed a characteristic peak with two theta at 20° due to its partially crystalline nature. After the CS was grafted with the PVAM, the intensity and XRD patterns of the PVAM and CS were reduced which gives an indication of the destruction of some of the crystalline chains and the involvement of the crystalline region grafting together with the amorphous region. CS exhibits two main reflection peaks with 2θ° at 111° and 201°. Changes in the peaks indicated the influence of the graft copolymer on the structure of the CS. This was reflected by a reduction in the intensity of the XRD patterns. This result was in agreement with a previous study [21]. The XRD pattern of the caffeic acid-g-CS and ferulic acid-g-CS exhibited broader and weaker peaks. This might be due to an interaction of the CS with grafted phenolic acids. Moreover, the conjugation of caffeic acid and ferulic acid with the CS reduced the crystallization of the CS to some extent, suggesting that the CS and phenolic acid chains were well mixed at a molecular level [21]. This was probably due to the inter- and intra-molecular hydrogen bonds of CS being greatly decreased after grafting.
Effect of the CS/PVAM ratio
The effect of the CS/PVAM ratio on the degree of grafting of the copolymer of the PVAM-g-CS is shown in Fig. 4a. The degree of grafting of the copolymer decreased dramatically with an increasing proportion of CS in the graft copolymer. The effect of increasing the proportion of CS on the grafting percentage after saturation could be associated with depletion of the available CS concentration as well as a reduction in the active sites on the PVAM backbone as the graft copolymerization proceeded. In addition, the increase in the CS concentration due to the amount of CS available was more helpful to copolymerization than grafting based on the concentrations determined. On the other hand, the CS had a high affinity for its copolymer substrate, which encouraged a high degree of copolymerization to occur in the polymer phase. The maximum degree of grafting of the graft copolymer was found to occur at 9:1 PVAM/CS. The tensile strength of the PVAM was 15 MPa as shown in Fig. 4b. After the PVAM was blended with the CS, the tensile strength of the sample did not alter. However, when PVAM was grafted with CS, the tensile strength of the PVAM increased from 15 to as much as 20 MPa depending on the proportion of CS in the graft copolymer. This might be due to the chemical interaction during the grafting reaction.
In regard to elongation at break, the PVAM shows the best properties compared to the other samples as shown in Fig. 4c. The elongation at break of the PVAM was 800 % while the elongation at break of the PVAM/CS and the PVAM-g-CS were 140 and 130 %, respectively. These data confirm that the PVAM was successfully grafted with the CS.
The morphology of the PVAM, the PVAM/CS blend and the PVAM-g-CS is presented in Fig. 4d. The morphology of the PVAM was dense and compact with a uniform surface. After the incorporation of CS into the PVAM, the surface of the specimen had large holes and was very rough due to incompatibility between the PVAM and the CS. However, when the CS was grafted with the PVAM, only small holes were observed due to the good adhesion between the PVAM and the CS. These phenomena agreed well with the results of the measurement of tensile strength.
The influence of the CS/PVAM ratio on the tensile strength and elongation at break of the PVAM-g-CS is shown in Fig. 5a, b, respectively. It is very obvious that the tensile strength of the PVAM-g-CS dramatically increased as a function of the proportion of CS in the graft copolymer. The highest tensile strength was found at 5:5 PVAM/CS. In contrast, the elongation at break of the PVAM-g-CS decreased as a function of the proportion of CS in the graft copolymer as shown in Fig. 5b. The minimum elongation at break of 25 % was found at 5:5 PVAM/CS while the highest elongation at break was 250 % at 9:1 PVAM/CS.
Effect of reaction time
Figure 6a shows the effect of the reaction time on the grafting percentage which increased gradually with increasing reaction time but leveled off after 60 min, when the saturated grafting value was reached. With increasing reaction time, the concentration of CS and free radicals in the system reduced resulting in the leveling off of the grafting percentage.
The influence of the reaction time on the tensile strength is shown in Fig. 6b. The maximum tensile strength of 19 MPa was found with a reaction time of 1 h. The tensile strength of the PVAM-g-CS with reaction times of 2 and 3 h was 15 and 16 MPa, respectively. On the other hand, the lowest elongation at break was observed in the sample made with a reaction time of only 30 min, while the highest elongation at break was found with a of reaction time of 2 h as shown in Fig. 6c.
Effect of reaction temperature
The influence of temperature on the degree of grafting is presented in Fig. 7a. It was clear that the degree of grafting of the copolymer dramatically decreased with increasing temperature. The highest degree of grafting at 72 % was found at 50 °C. The effect of temperature was studied by changing the reaction temperature from 50 to 80 °C and keeping other reaction conditions constant, as shown in Fig. 7a. The tensile strength reached a maximum at 50 °C as shown in Fig. 7b. With further increases in temperature above this optimum temperature, the tensile strength of the PVAM-g-CS decreased, since at a higher reaction temperature, the thermal decomposition rate of the K2S2O8 increased and the small radicals obtained caused chain scission leading to reduced tensile strength. In contrast to the tensile strength, the elongation at break of the PVAM-g-CS continually increased as shown in Fig. 7c. The maximum elongation at break of 200 % was found at 80 °C.
Effect of initiator concentration
The effect of the concentration of K2S2O8 on the degree of grafting and the tensile strength was studied and the results are shown in Fig. 8a, b, respectively. The degree of grafting between the PVAM and the CS was between 62 and 79 %, depending on the K2S2O8 content. The maximum degree of grafting of the graft copolymer was found at a concentration of 1 % K2S2O8. As shown in Fig. 8b, with increasing concentrations of the initiator, the tensile strength rapidly increased to a maximum value, and then decreased. The increase in the tensile strength may be ascribed to an increase of macroradicals. With increases in the initiator concentration, more K2S2O8 attacked the saccharide unit of the CS, more CS macroradicals were generated, and thus more active sites on the CS were able to react with the PVAM and initiate the propagation reaction. With further increases of the amount of K2S2O8 beyond the optimum level, the concentration of OSO3H and OH radicals increased and thus initiated the copolymerization of the PVAM. This resulted in a decrease of tensile strength whereas the elongation at break of the PVAM-g-CS continued to increase as shown in Fig. 8c.
Swelling ratio
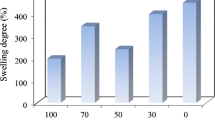
The swelling ratios of the PVAM-g-CS immersed in water for between 0.5 and 7 h are shown in Fig. 9. The influence of the ratio of PVAM to CS, the reaction time, temperature and concentration of K2S2O8 on the swelling ratio of the PVAM-g-CS are presented in Fig. 9a–d, respectively. The results show that with an increasing proportion of CS in the graft copolymer, the swelling ratio decreased dramatically except at a ratio of 7:3 PVAM:CS as shown in Fig. 9a. The swelling ratios of the PVAM/CS with ratios of 9:1, 8:2, 7:3, 6:4 and 5:5 were 190, 180, 175, 150 and 70 %, respectively, after immersion in water for 1 h. This is due to the higher chemical interaction between the PVAM and CS. These data agree well with the tensile strength results. The effect of reaction time on the swelling ratio of the PVAM-g-CS is shown in Fig. 9b. The swelling ratio of the PVAM-g-CS with a reaction time of 0.5 h was 130 %. When the reaction time was increased to 1 h, the swelling ratio of the PVAM-g-CS was 180 %. Thereafter, the swelling ratio of the PVAM-g-CS decreased when the reaction time was increased to 2 h. The optimal time for the grafting of the PVAM-g-CS was therefore 1 h. The influence of temperature on the swelling ratio of the PVAM-g-CS is shown in Fig. 9c. The swelling ratio of the PVAM-g-CS increased with increasing temperature after an immersion time of 0.5 h. These phenomena are explained by the lower degree of grafting of the copolymer resulting in a higher swelling ratio. However, when the immersion time increased from 0.5 to 7 h, the maximum swelling ratio of the graft copolymer was found at 60 °C.
The effect of the concentration of K2S2O8 on the swelling ratio of the PVAM-g-CS is presented in Fig. 9d. The highest swelling ratio of the PVAM-g-CS of 400 % was found to be at a concentration of 0.5 % K2S2O8. However, when the concentration of K2S2O8 increased from 0.5 to 1 % w/w, the swelling ratio of the graft copolymer was only around 200 %. These data are in agreement with the swelling results.
Thermal properties
The thermal properties of pure CS, pure PVAM and the different PVAM-g-CS films were analyzed using TGA. Figure 10a, b shows the thermograms and derivatograms of pristine PVAM, CS and the PVAM-g-CS, respectively. These analyses reveal that the thermal degradation of the PVAM-g-CS films was similar to that of pure CS. It can be clearly noticed that both the PVAM and CS decompose above 90 °C, and that as a result, grafted CS decomposes in a similar temperature range. The pristine CS shows a first endothermic peak in the temperature region around 90–150 °C due to the evaporation of absorbed moisture. It is important to note that the samples of different PVAM-g-CS blends had different water contents. This result agrees well with previous work [22] in which a graft copolymer between N-trimethyl chitosan (TMC) and PVA (TMC-g-PVA) was studied and was found to suffer weight loss between 25 and 125 °C.
The second step of the degradation of CS started at 250 °C and reached a maximum at around 350 °C with an observed exothermic peak at 330 °C. In the case of the PVAM, The second stage started at 170 °C and continued until 850 °C, with a weight loss of about 70 % resulting from the degradation of the PVAM. Similar results were found in our previous work [12], where three peaks were detected in a temperature range of 190–280 °C, corresponding to the glass transition temperatures and the melting point of the PVA. For the graft copolymer, the initial endothermic peak is due to the loss of bound water. Differences in the peak area and the position of the first endothermic peaks clearly show the different moisture holding capacity of these graft copolymers and their different water–polymer interaction strengths.
Contact angle measurement
The contact angle is analyzed to measure the force of attraction between liquid and the outer surface of the material to evaluate the wetting behavior of the graft copolymer film. If the liquid and the material surface have a good interaction, then the liquid drop spreads and wets the surface of the material. The smaller the water contact angle, the better is the hydrophilicity of the material and conversely a high contact angle indicates better hydrophobicity. In this work, the effect of the PVAM/CS ratio on the water contact angle was evaluated and the results are shown in Fig. 11. The water contact angle of the pristine PVAM was 81° , while the water contact angle of the CS was 79°. After the PVAM was grafted with the CS, the water contact angle of the graft copolymer increased as the quantity of the CS absorbed increased. An increasing trend in the water contact angle was observed in the CS portion of the graft copolymer, which resulted in increased hydrophobicity as discussed above.
The water contact angles of the graft copolymer at 9:1, 8:2, 7:3 and 6:4 were 91°, 106°, 116° and 122°, respectively. These results were in contrast with previous work [23] in which the carboxymethyl chitosan (CMCS) was grafted onto polyester and was found to introduce more polar groups, such as hydroxyl, amidogen and carboxyl, onto the fabric surface, which contributed to H-bond formation with water. From these phenomena, the wetting properties of the CMCS-grafted fabric were significantly improved over those of untreated PET fabric. Moreover, a reduction in the surface charge density indicated an improvement in the antistatic property of the CMCS-grafted fabric.
Release of capsaicin form PVAM-g-CS sheet capsule
The PVAM-g-CS was mixed with capsaicin to form a PVAM-g-CS sheet capsule. Then, it was immersed in water medium at two pH values, i.e., 2 and 8. The capsaicin release from PVAM-g-CS as a polymer matrix was estimated by UV spectroscopy. The profiles of capsaicin release cumulative from the PVAM-g-CS polymer matrix are depicted in Fig. 12a. Results showed that apparently, the cumulative capsaicin release from the PVAM-g-CS in pH 8 was slightly faster in comparison with that of medium at pH 2. Moreover, the cumulative release of capsaicin-loaded PVAM-g-CS as a polymer matrix at pH 2 was more than 65 % within the initial 5 h. Then, the capsaicin release occurred at a slower rate up to 3 days to reach 95 % release for the PVAM-g-CS sheet as given in Fig. 12a. After the immersion in water medium continued for 3 days, the PVAM-g-CS sheet in two mediums was stable enough not to show any apparent change. The mechanism of capsaicin release from the PVAM-g-CS as a polymer matrix was evaluated by using power law equation. In the power law fits of the diffusion data, the exponent n gave an indication of the rate-limiting mechanism. An n = 0.5 indicated the release was controlled by Fickian diffusion. Moreover, when n = 1 this represents a zero-order release. Values of n between these ideal cases indicate a potential reactive agent release mechanism, or a non-Fickian diffusion, or a chain relaxation-controlled release. From the plots of the log (M t /M ∞) against the log (t), the release exponent (n) were evaluated as shown in Fig. 12b. The influence of the medium pH on the release exponent (n) value was calculated and the results are shown in Fig. 12b. When the PVAM-g-CS sample containing capsaicin was immersed in water at a pH 2, the regression fit gave approximately n = 0.73. This data indicated that the release pattern was not a controlled Fickian diffusion. When the pH of the medium was increased from a pH 2 to a pH 8, the result of n was equal to 0.64, which indicated that the capsaicin release from the capsule was mostly similar to pH 2.
Biodegradation
CS can be easily degraded by the enzyme chitosanase produced by microorganisms present in soil. The efficacy of the biodegradation of the PVAM-g-CS (30 = mg) in natural soil was established by measuring its weight loss over time. After one month of incubation, the weight loss was analyzed and is shown in Fig. 13. The influence of the PVAM:CS ratio, the reaction time, temperature and concentration of K2S2O8 on the percentage biodegradation in soil are presented in Fig. 13a–d, respectively. The influence of the ratio of PVAM:CS on the biodegradation of the PVAM-g-CS is shown in Fig. 13a. The results show that the biodegradation of the PVAM-g-CS film was around 40–50 % after one month. The degree of biodegradation of the PVAM-g-CS did not vary as a function of the CS content in the graft copolymer. This phenomenon may be explained by the ability of the microorganisms to assimilate the CS as a carbon source. In addition, it is apparent that PVAM-g-CS biofilms are better hydrolyzed by microorganisms than those made of PVAM. Therefore, these results show that the incorporation of CS into PVAM improves the degradation of the material. These results are in agreement with those obtained in our previous work [24] which found that the degradation percentage of a graft copolymer containing carrageenan in soil increased with increasing carrageenan content. In addition, the graft copolymer in the present study was easily biodegraded in natural soil. The result of the present study is also consistent with earlier studies [24, 25]. The effect of the reaction time on the percentage biodegradation of the grafted copolymer is shown in Fig. 13b. The lowest weight loss was observed when the reaction time increased from 0.5 to 1 h since the degree of biodegradation increased considerably due to the higher proportion of CS in the graft copolymer. When the reaction time was increased from 1 to 2 h, the degree of biodegradation increased only slightly from 40 to 70 %. However, when the reaction time was increased from 2 to 3 h, the degree of biodegradation was constant.
The effect of the temperature on the biodegradation of the PVAM-g-CS is presented in Fig. 13c. It is clear that the degree of biodegradation increased as a function of temperature, except at 60 °C. This phenomenon is explained by the fact that the highest degree of grafting of the copolymer was found at 60 °C which resulted in a reduction in the degree of biodegradation. The effect of the concentration of K2S2O8 on the biodegradation of the PVAM-g-CS is illustrated in Fig. 13d. The degree of biodegradation of the PVAM-g-CS was between 55 and 68 % with the minimum biodegradation being found in the sample with a concentration of 0.5 % K2S2O8.
Conclusions
A graft copolymer of PVAM-g-CS was successfully prepared from an aqueous form of PVAM with the help of K2S2O8 as an initiator. The grafting yield between the PVAM and the CS, the reaction time, temperature and concentration of K2S2O8 were controlled by appropriate selection of the grafting conditions. The optimal condition for the PVAM-g-CS was at 50 °C with a reaction time of 1 h, a ratio of PVAM:CS at 9:1 and 0.5 % K2S2O8. The ether linkage between the PVAM and CS in the graft copolymer was observed at 1154 and 1089 cm−1 by ATR-FTIR. The crystalline portions of the CS in the graft copolymer were decreased due to a destruction of some crystalline chains and the crystalline region grafting together with the amorphous region. The water resistance of the PVAM-g-CS increased with increasing proportions of CS due to the good chemical interaction between them. The swelling ratio of the PVAM-g-CS ranged between 200 and 400 % depending on the conditions used to prepare the graft copolymer. An increasing trend in the water contact angle was observed in the CS portion of the graft copolymer, which increased its hydrophobicity. The composition of the graft copolymer was confirmed by TGA which revealed the different moisture holding capacity of the graft copolymers and the different water–polymer interaction strengths. The maximum tensile strength of the graft copolymer was found to be 27 MPa.
The cumulative capsaicin release from the PVAM-g-CS in pH 8 was slightly faster in comparison with that of medium at pH 2. After its use, the PVAM-g-CS is easily decomposed in natural soil and the degree of biodegradation was about 40–60 % after being buried in natural soil for 1 month. Further development of this novel polymer hydrogel may be expected.
References
Pereira VA Jr, Arruda INQ, Stefani R (2015) Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as time–temperature indicators for application in intelligent food packaging. Food Hydrocoll 43:180–188
Cao Y, Bai G, Chen J, Tian W, Wang S, Yang W (2006) Preparation and characterization of magnetic microspheres for the purification of interferon α-2b. J Chromatogr B 833:236–244
Jiang S, Liu S, Feng W (2011) PVA hydrogel properties for biomedical application. J Mech Behav Biomed 4:1228–1233
Kang M, Choi Y, Moon S (2002) Water-swollen cation-exchange membranes prepared using poly(vinyl alcohol) (PVA)/poly(styrene sulfonic acid-co-maleic acid) (PSSA-MA). J Membr Sci 207:157–170
Castilho LR, Deckwer W, Anspach FB (2000) Influence of matrix activation and polymer coating on the purification of human IgG with protein A affinity membranes. J Membr Sci 172:269–277
Wu J, Yu H (2007) Biosorption of 2,4-dichlorophenol by immobilized white-rot fungus Phanerochaete chrysosporium from aqueous solutions. Bioresour Technol 98:253–259
Zheng Y, Wang A (2010) Removal of heavy metals using polyvinyl alcohol semi-IPN poly(acrylic acid)/tourmaline composite optimized with response surface methodology. Chem Eng J 162:186–193
Pandey J, Mir F, Shukla A (2014) Synthesis of silica immobilized phosphotungstic acid (Si-PWA)-poly(vinyl alcohol) (PVA) composite ion-exchange membrane for direct methanol fuel cell. J Hydrogen Energy 39:9473–9481
Macias CE, Bodugoz-Senturk H, Muratoglu OK (2013) Quantification of PVA hydrogel dissolution in water and bovine serum. Polymer 54:724–729
Ye M, Mohanty P, Ghosh G (2014) Biomimetic apatite-coated porous PVA scaffolds promote the growth of breast cancer cells. Mater Sci Eng C 44:310–316
Ajji Z, Ali AM (2010) Separation of copper ions from iron ions using PVA-g-(acrylic acid/N-vinyl imidazole) membranes prepared by radiation-induced grafting. J Hazard Mater 173:71–74
Sukhlaaied W, Riyajan S (2014) Green synthesis and physical properties of poly(vinyl alcohol) maleated in an aqueous solutions. J Polym Environ 22:350–358
Mati-Baouche N, Elchinger P, Baynast HD, Pierre G, Delattre C, Michaud P (2014) Chitosan as an adhesive. Eur Polym J 60:198–212
Zhou H, Jiang L, Cao P, Li J, Chen X (2015) Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr Polym 117:524–536
DeMerlis CC, Schoneker DR (2003) Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem Toxicol 41:319–326
Camerlo A, Vebert-Nardin C, Rossi RM, Popa AM (2013) Fragrance encapsulation in polymeric matrices by emulsion electrospinning. Eur Polym J 49:3806–3813
Yang Z, Yang H, Jiang Z, Cai T, Li H, Li H, Li A, Cheng R (2013) Flocculation of both anionic and cationic dyes in aqueous solutions by the amphoteric grafting flocculant carboxymethyl chitosan-graft-polyacrylamide. J Hazard Mater 254–255:36–45
Al Sagheer FA, Khalil KD, Ibrahim EI (2013) Synthesis and characterization of chitosan-g-poly(2-(furan-2-carbonyl)-acrylonitrile): grafting of chitosan using a novel monomer prepared by a Baylis-Hillman reaction. Eur Polym J 49:1662–1672
Lv J, Zhou Q, Liu G, Gao D, Wang C (2014) Preparation and properties of polyester fabrics grafted with O-carboxymethyl chitosan. Carbohydr Polym 113:344–352
Liu J, Wen X, Lu J, Kan J, Jin C (2014) Free radical mediated grafting of chitosan with caffeic and ferulic acids: structures and antioxidant activity. Int J Biol Macromol 65:97–106
Arana LP, Guerrero P, Caban K (2014) Structure–moisture sorption relation in chitosan thin films. Mater Lett 128:125–127
Martins AF, Bueno PVA, Follmann HDM, Nocchi SR, Nakamura CV, Rubira AF, Muniz EC (2013) Synthesis, characterization, and cytotoxicity of TMC-graft-poly(vinyl alcohol) copolymers. Carbohydr Res 381:153–160
Lv J, Zhou Q, Liu G, Gao D, Wang C (2014) Preparation and properties of polyester fabrics grafted with O-carboxymethyl chitosan. Carbohydr Polym 113:344–352
Sukhlaaied W, Riyajan S (2013) Synthesis and properties of carrageenan grafted copolymer with poly(vinyl alcohol). Carbohydr Polym 98:677–685
Riyajan S, Sasithornsonti Y, Phinyocheep P (2012) Green natural rubber-g-modified starch for controlling urea release. Carbohydr Polym 89:251–258
Acknowledgments
This study was supported by The Thailand Research Fund (TRF)/the Prince of Songkla University/Thammasat University (RSA5780018) and Prince of Songkla University (SCI 580193S) as well as The Royal Golden Jubilee Ph.D. Program (2.L.PS/53/C.1.N.XX).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sukhlaaied, W., Riyajan, SA. Preparation and properties of a novel environmentally friendly film from maleated poly(vinyl alcohol) grafted with chitosan in solution form: the effect of different production factors. Polym. Bull. 73, 791–813 (2016). https://doi.org/10.1007/s00289-015-1520-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1520-3