Abstract
Nowadays, hydrogels are widely used in many fields, for instance as biosensors, in tissue engineering and in agriculture due to their high swelling behavior in water and their softness. The objective of this work was to study the preparation and physical properties of maleated poly (vinyl alcohol) (PVAM)-graft-butanediol (BDO) (PVAM-graft-BDO) using K2S2O8 as an initiator. The highest grafting efficiency of 92% was found at 7:3 PVAM-graft-BDO. The swelling behavior of the PVAM-graft-BDO increased with an increasing BDO content. The highest swelling ratio of the PVAM-graft-BDO of 250% was found at 7:3 PVAM:BDO. Moreover, the maximum tensile strength of the PVAM-graft-BDO was observed at 9:1 PVAM:BDO. The elongation at break of the PVAM-graft-BDO was improved by the addition of natural rubber-graft-cassava starch (NS). The PVAM-graft-BDO was used as a polymer matrix for the encapsulation of capsaicin. The results showed that the rate of capsaicin release from the polymer matrix in a water medium was controlled by the BDO portion and the NS content. After use, it was easily degraded in natural soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(vinyl alcohol) (PVA) is a water soluble polymer with interesting properties such as toughness, adhesiveness, biocompatibility, high swelling behavior, and lack of toxicity [1, 2]. The excellent chemical and physical properties of PVA make it attractive for use in areas such as papermaking [3], textiles [4], coatings [5], adhesives [6], packaging [7], pharmaceuticals [8] and biomedicine [9]. Grafting is an interesting method for modifying the properties of PVA [10]. The grafting reaction can be conducted simply and with a variety of monomers or copolymers to obtain a modified PVA with the desired properties. In previous work, PVA has been grafted with poly(N,N-diethylacrylamide) [11], butadiene (PB) [12], starch [13], olyanionic [14], acrylic acid (AAc) monomer [15], graphene oxide [16] and poly(3-hexylthiophene) [17]. For example, PVA was successfully grafted on PB latex particles via an emulsion grafting polymerization induced by 60Co γ-ray pre-irradiation. The PB-g-PVA possessed a core–shell structure with the PB as the core, and the grafted PVA as the shell, which was revealed by both positive and negative staining [12]. The elongation at break of the PVA was improved after grafting with the PB due to H-bond association of the grafted PVA, enhanced by the interaction of the PB main chains.
Graft copolymers of PVA with thiophene side-groups and pyrroles were characterized many years ago. These copolymers were prepared in two-steps; the thiophene-side-group-containing PVA was first synthesized by esterification at room temperature, after which it was oxidatively and chemically polymerized in order to graft the polypyrrole onto the surface of the thiophene-side-group of the PVA [11]. However, as reported, this method is unsuitable for grafting polythiophene onto the surface of PVA bearing thiophene side groups [11]. In this article, the effect of the BDO content on the grafting efficiency of PVAM-graft-BDO and its physical properties including the swelling ratio, tensile strength and elongation at break are reported. In addition, the chemical structure, morphology, and biodegradation of the PVAM-graft-BDO were evaluated. The influence of natural rubber-graft-cassava starch (NS) on the swelling, tensile strength, elongation at break and biodegradation of the PVAM-graft-BDO was studied. The use of the PVAM-graft-BDO and PVAM-graft-BDO/NS blend as a matrix for the encapsulation of capsaicin was evaluated.
Experimental
Materials
Commercial PVA with a degree of saponification of 97 to 100 mol % was purchased from Thai Chemical Industries (TCI) (Bangkok, Thailand). It was kept and stored in a UNICO UN 650 F mode box under an atmosphere of argon, as it is hygroscopic. 3-Hexylthiophene (3HT) and anhydrous FeCl3 were obtained from TCI and used without any further purification. The analytical grade solvents used, chloroform, acetonitrile, hexane and methanol were purchased from Wako Pure Chemical Industry Ltd and were used as received. Maleic anhydride (MA) used as a grafting agent for preparing the PVAM and potassium persulphate (KPS) for use as a water-soluble initiator were purchased from Fluka. BDO (99%) from Alfa Aesar (Karlsruhe, Germany) was used with no further purification. The NS was synthesized in our laboratory and was prepared according to our previous work [18, 19]. Gelatinized cassava starch (CS) was mixed with K2S2O8 solution (KPS), and was stirred at 60 °C for 45 min to obtain modified CS. A quantity of 17 g of NR latex was blended with modified CS in the presence of Triton X100 and stirred at 60 °C for 3 h to obtain the NS solution used in the preparation of the PVAM-graft-BDO/NS hydrogels.
Capsaicin, which is produced from chili (local market, Pathumthani, Thailand), was prepared by the extraction method. The chili was mashed in a grinder then immersed in a 50:50 water–ethanol solution for 14 days. Next, the mixed chili was filtered to obtain a solution which was then evaporated to obtain a 1000 ppm capsaicin extraction as evaluated by UV spectroscopy.
Preparation of the PVAM-graft-BDO
The PVAM was synthesized according to our previous work [10]. It was prepared by adding 3 g MA in 20 ml distilled water to 70 g of 10% PVA solution. The mixture was stirred at 70 °C for 2 h, to obtain 90 g of the 11% w/w PVAM (7:3 PVA: MA) solution, which was cooled at room temperature.
After that, the KPS was added into the PVAM solution at 70 °C and then stirred for 5 min. BDO (different ratios, 9.5/0.5−5.0/5.0) into the mixture at 70 °C to form the PVAM-graft-BDO solution which was stirred at 70 °C for 1 h then cooled at room temperature. The PVAM-graft-BDO solution was poured into a mold and left for 7 days at room temperature. After 7 days, the PVAM-graft-BDO sheet was immersed in distilled water for 3 days at room temperature to remove any remaining reactants and impurities, and dried at room temperature for 3–4 days, then dried at 50 °C in an oven until its weight was constant.
Preparation of PVAM-graft-BDO/NS Blend Hydrogels
The 7:3 PVAM-graft-BDO was blended with NS at ratios of 2:8, 3:7, 4:6 and 5:5 and stirred for 30 min at room temperature to obtain PVAM-graft-BDO/NS hydrogels, which were poured into molds and left to cure at room temperature. After 7 days, the hydrogel sheets were immersed in distilled water for 3 days at room temperature to remove impurities then dried at room temperature for 2 days.
Grafting Efficiency
The degree of grafting in the copolymer was determined by the extraction of samples in boiling water for 6 h using a Soxhlet apparatus. The extracted samples were dried in an oven at 40 °C until a constant weight was achieved. The degree of grafting efficiency was calculated by the following Eq. (1):
where W1 and W0 are the weights of the dried sample after and before extraction, respectively.
FTIR
The dried PVAM-graft-BDO samples were analyzed by attenuated total reflection Fourier transform infrared (ATR-FTIR) using a Bruker EQUINOX 55 (Bruker, USA) in the range of 4000–500 cm−1.
1H-NMR
The PVA and 5:5 PVAM-graft-BDO were also analyzed by 1H-NMR (Ascend TM 600 Bruker, Switzerland) operating at a spin rate of 6000 Hz, a temperature of 298 K, and a recycle delay time of 4 s. The hydrogel was swollen in D2O, and 50 μl aliquots were used for analysis.
SEM Studied
A scanning electron microscope (SEM) was used to obtain a topographical characterization of the samples. A PVAM-graft-BDO sample was deposited on a brass hold and sputtered with gold. SEM photographs were taken with a JSM 6400 Scanning Microscope (Japan) at the required magnification at room temperature.
Hydrophilic Testing and Mechanical Properties
The PVAM-graft-BDO samples used to determine the swelling ratio in distilled water were 2 × 2 cm pieces cut from the hydrogel sheets. The samples were weighed before swelling in distilled water. For the swelling ratio, the samples were immersed in 25 ml of distilled water at room temperature for 7 h. After that, they were reweighed after removing excess water from the hydrogel surface with tissue paper. The swelling ratio was calculated by Eq. (2):
where W2 is the initial dry weight of the sample (g) and W3 is the weight of the swollen sample (g).
Tensile testing was performed using an Instron (IX3366, Japan) machine at a crosshead speed of 100 mm/min according to ISO 37. Dumbbell-shaped sample pieces were cut from the dried sheets for the test and the tensile strength and elongation at break were measured.
Encapsulation of Capsaicin and Capsaicin Release from the Polymer Hydrogel
The 7:3 PVAM-graft-BDO blend hydrogel was selected to be used as a polymer membrane for the encapsulation of capsaicin which was added to 100 g of the 7:3 PVAM-graft-BDO blend hydrogel solution and stirred, then poured into a mold and allowed to solidify for 7 days at ambient temperature. The efficiency of the entrapment of the capsaicin in the hydrogel matrix was calculated from the ratio between the initial mass of capsaicin to be encapsulated, and its mass in the final product. For that purpose, a known amount of capsaicin (ca. 12 mg) was dispersed in a distilled water medium and stirred for 2 days at room temperature. Subsequently, the suspension was filtered, and the content of dissolved capsaicin in the distilled water medium solution was determined using a UV spectrometer (Shimadzu UV-1601) at 360 nm (λmax). At definite intervals of time, an aliquot (5 ml) was taken for analysis of the capsaicin in the hydrogel. The experiment was performed three times. The release results were evaluated using the following equation to estimate the value of the release exponent (Eq. (3)):
where Mt/M∞ is the fraction released at time t, n is the release exponent, and K is the release factor. From the slope and intercept of the plot of log (Mt/M∞) against log (t), the kinetic parameter (n) was calculated.
%Biodegradation
To examine the biodegradation of the dried PVAM-graft-BDO samples (3 cm × 3 cm) of a known weight were buried 5 cm deep in 100 g of top soil collected in Klong-Luang, Pathumthani, Thailand, and water was added every week for 30 days. Each week, the samples were carefully removed and rinsed with distilled water to remove the soil, and dried in a desiccator at 40 °C for 24 h before being reweighed. The biodegradation of the samples in soil was obtained according to Eq. (4):
where W4 is the initial dry weight of the samples (g) while W5 is the dry residual weight of the samples after biodegradation in soil (g).
Enzymatic Degradation
The PVAM-graft-BDO samples were cut into 1 cm × 1 cm pieces with a weight of ± 4.5 mg and immersed in 1 ml phosphate buffer saline (PBS) at pH 7.4 containing 0.08 g/l lipase (Sigma-Aldrich, type XIII, ≥ 20 units/mg solid) with enzyme activity of approximately 100 U/mg, 3 U/ml. The samples were agitated and incubated in vitro at 37 °C and 100 rpm. The enzymatic degradation solution was changed every 5 days. The samples were removed from the enzymatic solution after 5, 10 and 15 days, and washed with distilled water 2 or 3 times, dried with filter paper then placed in a vacuum for 2 days. The weight loss of the samples by enzymatic degradation was calculated using Eq. 5:
where W7 and W6 are the weight of samples after enzymatic biodegradation and the initial weight before degradation, respectively.
Results and Discussion
Plausible Chemical Reaction of the PVAM-graft-BDO and Grafting Efficiency
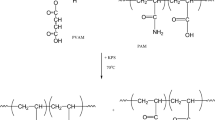
The plausible chemical reaction of the PVAM-graft-BDO is represented in Fig. 1. The OH groups of the BDO reacted with the CH2 free radical of the PVAM to form the graft copolymer as shown in Fig. 1. When the K2S2O8 was added and heated it changed into a K2S2O8 free radical which then reacted with a BDO molecule. The BDO radical attached to the methylene carbon of the PVAM, which was activated by the K2S2O8 leading to grafting with the BDO. These results agree with our previous works [4, 6].
The effect of the PVAM:BDO ratio on the degree of grafting of the PVAM-graft-BDO is illustrated in Fig. 2. It can be seen that the percentage of graft copolymer slightly increased to values between 80 and 92% with an increased BDO content. The highest grafting efficiency of 92% was found at 7:3 PVAM-graft-BDO. When the proportion of BDO exceeded 7:3 PVAM-graft-BDO (i.e., 6:4 and 5:5), the percentage of graft copolymer decreased. This result corresponded to that of previous work [2], where a PVA grafted with gelatin (GT) was prepared using potassium peroxodisulfate as an initiator. It was found that the weight fraction of the GT in the graft copolymer was enhanced with increases in the GT content due to the higher proportion of GT. Moreover, the degree of grafting efficiency decreased with an increasing GT content. Therefore, the excess GT content that did not react with the PVAM was removed by Soxhlet extraction in water. Moreover, these results are in agreement with our previous work conducted by the author [20], in which the grafting efficiency of a maleated poly(vinyl alcohol) (PVAM) grafted copolymer with anionic polyacrylamide (PAM) (PVAM-graft-PAM) was studied using K2S2O8 as an initiator in an eco-friendly process. The results showed that the percentage of graft copolymer slightly increased with an increased PAM content due to the higher proportion of PAM monomer.
FTIR and 1H-NMR
In the FTIR spectrum, the PVA showed bands at about 3330, 1425, 1375 cm−1 corresponding to OH stretching, CH2 bending, and CH2 deformation, respectively, as shown in Fig. 3.
After the PVAM was grated with the BDO, FTIR bands of the PVAM-graft-BDO appeared at 1713 and 1223 cm−1 assigned to C=O and C–O stretching of the PVAM, respectively, as shown in Fig. 4. Moreover, the intensity of the FTIR peaks at 3340 cm−1 of the hydroxyl group of the PVAM-graft-BDO increased with an increasing BDO content. The FTIR peaks of the BDO observed in the PVAM-graft-BDO at 2880 and 2995 cm−1 were both assigned to CH2 while those at 1440 and 1418 cm−1 were assigned to C–C stretching. Those at 973 and 915 cm−1 were assigned to CH2, that at 1783 cm−1 was assigned to C=O stretching, and those at 1230 and 1080 cm−1 were assigned to C–O stretching. The intensity of the carboxyl group in the PVAM-graft-BDO increased with increasing BDO. The 600 MHz 1H NMR spectra of PVA dissolved in D2O (Fig. 4) showed the three separate signals at 3.9 ppm which were assigned to the proton in the (OH) group of PVA. The peak which appeared at 3.7 ppm was assigned to CH. A large line assignable to H2O protons appeared at 3.5 ppm. The two weak peaks were attributed to side band peaks, observed within the H2O signal. The sharp singlet peak observed at 4.7 ppm was assigned to the D2O solvent. The signal located at 2.0 ppm was attributed to CH3 in the residual acetate, and the peak situated at 1.4 and 1.8 ppm was assigned to CH2. The 1H-NMR of the PVAM-graft-BDO is presented in Fig. 4. The –CH=CH– proton of the PVAM-graft-BDO appears at 6.30 ppm and the hydroxyl group from the BDO is located at 4.8 ppm.
Swelling Ratio
The effect of the BDO portion on the swelling ratio of the PVAM-graft-BDO hydrogel is presented in Fig. 5. The swelling ratio of the PVAM-graft-BDO hydrogel increased with increases in the BDO content due to the hydrophilicity of the BDO but then decreased when using over 7:3 PVAM:BDO as shown in Fig. 5. The BDO consists of a hydrophilic hydroxyl group. Therefore the BDO brings a hydroxyl group to the graft copolymer so that there is hydrogen bridging between the H2O and hydroxyl groups, resulting in the higher swelling ratio in water. The 9.5:0.5 PVAM:BDO showed the lowest swelling ratio compared to the other samples. After the incorporation of the BDO into the PVAM, the swelling ratio improved; for example, the swelling ratios of the PVAM-graft-BDO at 9.5:0.5, 9:1 and 8:2 were 120, 130 and 180%, respectively.
The highest swelling ratio of 250% was observed in the 7:3 BDO:PVAM. However, when the amount of BDO in the PVAM-graft-BDO hydrogel increased from 7:3 to 6:4 PVAM:BDO, the swelling ratio dropped, owing to excessive BDO dissolution in the PVAM-graft-BDO. These results are in agreement with previous work in which a polymer hydrogel from PVAM and GT using K2S2O8 as an initiator was studied [2]. The results showed that the swelling of the PVAM-graft-BDO was enhanced with increases in the BDO content due to an increasing number of hydrophilic groups on the chemical structure of the graft copolymer and then decreased owing to excess BDO. Moreover, the results are in agreement with previous work, in which the swelling ratio of a hydrogel made from PVAM and CS was studied [10]. When the CS in the polymer hydrogel increased, the swelling ratio of the sample also increased.
Mechanical Properties
The strength and elongation at break of the samples before and after immersion in water is presented in Fig. 6a and b, respectively. The tensile strength of the 9.5:0.5 PVAM-graft-BDO was 14 MPa. When the BDO increased from 9.5:0.5 PVAM-graft-BDO to 9:1 PVAM-graft-BDO, the tensile strength significantly increased to 26 MPa. However, when the proportion of PAM in the graft copolymer increased, the tensile strength was almost constant. This might have been due to an imbalance between the BDO and PVAM or the different chemical structure of the PVAM and BDO. When the PVAM-graft-BDO samples were immersed in water for 30 min, the tensile strength slightly decreased. In addition, the tensile strength of the PVAM also decreased to 10 MPa. The tensile strength after immersion correlates with the tensile strength before immersion in water and the highest tensile strength of 27 MPa was observed in the 6:4 PVAM-graft-BDO.
The elongation at break of the 9.5:0.5 PVAM-graft-BDO was 180% as shown in Fig. 6b. When the amount of BDO was increased from 9.5:0.5 PVAM-graft-BDO to 9:1 PVAM-graft-BDO, the elongation at break of the sample slightly increased.
The maximum elongation at break was 330% which was observed in the 6:4 PVAM-graft-BDO as shown in Fig. 6. After the sample was immersed in water, the elongation at break of all the samples increased and the values for the 9:1, 8:2, 7:3 and 6:4 PVAM-graft-BDO samples were 200, 230, 240 and 370%, respectively. However, when the proportion of BDO increased from 6:4 to 5:5 PVAM/BDO, the elongation at break decreased due to phase separation. These results are opposite to those for tensile strength and might be due to the strong hydrogel bond between water and the polymer matrix [10], leading to stiffness.
SEM Morphology
The morphology of the PVAM-graft-BDO samples with different BDO contents was confirmed by SEM and the results are shown in Fig. 7. The morphology of the PVAM presents good structural integrity, smoothness and flatness with no cracking. After the addition of BDO into the matrix, the BDO shows an even distribution in the PVAM matrices in the films, demonstrating the high compatibility of the two polymers and a compact structure lacking phase separation. No air bubbles, pores, cracks, and droplets were observed, further confirming the high compatibility of the two polymers (PVAM and BDO). However, when the BDO content was increased, visibly rough areas appeared. The rough areas of the PVAM:BDO films containing higher proportions of BDO showed scaly structures and many droplets. The roughness is because the addition of the BDO into the PVAM matrix allows the BDO molecules to disrupt the compact structure of the PVAM matrix.
These results are not in agreement with previous work, which studied the morphology of a PVA/chitosan blend [21]. The results showed that the morphology of the PVA/chitosan blend was smooth and homogeneous owing to high miscibility and blend homogeneity between the chitosan and PVA.
Effect of NS on the Swelling Ratio and Mechanical Properties of the PVAM-graft-BDO
The influence of the NS on the swelling ratio of the samples is presented in Fig. 8. The %swelling ratio of the 7:3 PVAM-graft-BDO was 350%. After the incorporation of the NS into the PVAM-graft-BDO, the swelling ratio of the sample continued to decrease as a function of the BDO content. For example, the swelling ratio of the PVAM-graft-BDO in the presence of 1, 2, 4 and 6 g was 240, 200, 180 and 160%, respectively. These results are in accord with previous work [21, 22], which reported the swelling ratio of a pH–temperature-sensitive elastomer derived from maleate poly(vinyl alcohol)-graft-isopropylacrylamide (PVAM-graft-PNIPAM) (PVNI) and deproteinized natural rubber (DPNR). The swelling ratio in water of the PVNI/DPNR hydrogel decreased as a function of the DPNR portion in hydrogel due to increasing hydrophobic groups from the DPNR in the hydrogel. Surprisingly, the percentage of swelling in water of the 7:3 PVNI/DPNR hydrogel was 24 times that of pristine DPNR.
Figure 9a shows the tensile strength curves of the PVAM films using the averaged statistical data generated from the five specimens analyzed. The ultimate stress of the 7:3 PVAM-graft-BDO film containing 1 g NS was 12 MPa. When the amount of NS increased from 1 g to over 1 g, the tensile strength of all the samples decreased continually with increasing NS contents. This might be due to the increasing portion of NR causing the film to have low tensile strength.
In regard to the deformation tension of the PVAM-graft-BDO, after samples of the 7:3 PVAM-graft-BDO were prepared with different NS contents, their tensile strength decreased dramatically owing to water absorption. The addition of the NS into the PVAM-graft-BDO matrix induced an enhancement of the elongation at break values for the graft copolymer films relative to those for neat PVAM-graft-BDO as shown in Fig. 9b. Therefore, the mechanical properties of the PVAM-graft-BDO films may suggest that the NS content has a greater influence on the elongation at break. The elongation at break of the 7:3 PVAM-graft-BDO increased as a function of the NS content. These results are in accord with previous work [22] in which it was found that after the addition of DPNR and NR into a PVAM-graft-PNIPAM, the tensile strength of the sample decreased with an increasing rubber content and the elongation at break of the sample increased as a function of the NR content. Moreover, the maximum elongation at break of the PVAM-graft-PNIPAM/DPNR hydrogel was found at 2:8 PVAM-graft-PNIPAM:DPNR.
Encapsulation of Capsaicin
The capsaicin release behavior of the 9:1 and 7:3 PVAM-graft-BDO hydrogel films was studied in a water medium with the pH value at 2 as shown in Fig. 10a. It can be seen that the capsaicin release by the 9:1 PVAM-graft-BDO hydrogel film at pH 2 was lower than that of the 7:3 PVAM-graft-BDO sample. The initial capsaicin release burst was found within 10 h. This might be because in the pH 2 medium, the H+ ions of the medium attracted the COO– ions of the 9:1 and 7:3 PVAM-graft-BDO hydrogel film during the collapse of their matrix as shown in Fig. 10a. This occurred due to osmotic pressure inside the polymer matrix and resulted in an increase in capsaicin release in the pH 2 medium. This repulsive force might have caused the expansion of the PVAM-graft-BDO, resulting in increasing capsaicin release in the 7:3 PVAM-graft-BDO due to its high swelling capacity. The possible model of capsaicin release in the 9:1 and 7:3 PVAM-graft-BDO hydrogel films at pH 2 is shown in Fig. 10b.
The mechanism of capsaicin release from the 9:1 and 7:3 PVAM-graft-BDO hydrogel films was confirmed to be by Fickian diffusion and the results are shown in Fig. 10b with the plots of log (Mt/M∞) against log (t). The influence of the ratios between the PVAM and BDO on the release exponent (n) values was calculated and the results are shown in Fig. 10b. When the 9:1 PVAM-graft-BDO hydrogel sample was immersed in water with a pH of 2, n was equal to 0.1558. This result indicates that the release pattern was a controlled Fickian diffusion. An n of 0.5 or lower indicates that the release is controlled by Fickian diffusion [22,23,24] whereas an n of 1 represents a zero-order release. A value between 0.5 and 1 indicates a potential reactive agent release mechanism, a non-Fickian diffusion, or a chain. When the ratio between the PVAM and BDO of the medium was altered from 9:1 to a ratio of 7:3 PVAM-graft-BDO, the value of n was 0.2159, which indicates that the capsaicin release from the sample was controlled by Fickian diffusion in the case of both those ratios of PVAM to BDO.
The influence of the NS on the capsaicin release behavior of the 7:3 PVAM-graft-BDO/NS blend hydrogel film was studied in a water medium with a pH of 2 as shown in Fig. 11a. It can be seen that the capsaicin release from the hydrogel film with 1 g NS was higher than that at 2 g NS. This might be because the 6:4 PVAM-graft-BDO/2 g NS blend hydrogel was found to have a strong matrix, leading to a reduction of water penetration into the sample as shown in Fig. 11a. For example, the % capsaicin release from the hydrogel film at 1 g NS over 8 h was 50%, while the % capsaicin release in the sample containing 2 g NS was 55%. These results are in accord with our previous work [22]. A repulsive force might have caused the expansion of the polymer film resulting in a decrease in capsaicin release in the higher NR content sample. The possible model of the capsaicin release in the 6:4 PVAM-graft-BDO/2 g NS blend hydrogel film at pH 2 is given in Fig. 11b as plots of the log (Mt/M∞) against log (t).
The influence of the NS on the release exponent (n) values was calculated and the results are also shown in Fig. 11b. When the 9:1 PVAM-graft-BDO/1 NS blend hydrogel sample was immersed in water with a pH of 2, the value of n was 0.5186. This result indicates that the release pattern was not controlled by a Fickian diffusion which would be indicated by an n of 0.5 or lower. When the NS of the sample was altered from 1 g NS to 2 g NS, the value of n was 0.4117, indicating that the capsaicin release from the hydrogel samples was controlled by Fickian diffusion in either the case of blending 1 g and 2 g NS into the hydrogel.
Biodegradation
The changes in weight of the PVAM-graft-BDO and PVAM-graft-BDO/NS blends were evaluated after immersion in natural soil for 3 months and the results are summarized in Fig. 12, and the influence of the BDO on the % weight loss of the PVAM-graft-BDO can also be seen in Fig. 12.
The results show that the biodegradation of the PVAM-graft-BDO increased as a function of the BDO portion in the graft copolymer. This might be due to the higher BDO portion in the blend. The BDO is able to be easily degraded by bacteria and fungi in soil which are activated by moisture and heat. The rate of the biodegradation of the graft copolymer was enhanced by the addition of the NS. In previous work, the author studied the influence of CS on the biodegradation of NS. The rate of biodegradation of the NS increased as a function of the CS content. The higher molecular weight of the NR of around 106 g/mole results in it being more difficult to biodegrade. However, the NR can be slowly degraded in nature by specific microorganisms [25]. The biodegradation of the PVAM-graft-BDO containing 1 g was 75%. When the amount of the NS increased from 1 to 2 g, the biodegradation of this sample was 80%. The maximum biodegradation was observed when using 3 g NS. However the biodegradation of the sample slightly decreased due to the higher NR portion in the polymer matrix.
The lowest biodegradation was observed when using 6 g NS. There were many holes found in the PVAM-graft-BDO after burial in natural soil for 3 months due to the action of fungi and bacteria as shown in Fig. 13
Enzymatic Degradation
In this research, the enzyme degradation of the PVAM-graft-BDO with or without NS was performed in lipase solution at 37 °C for 15 days. Table 1 shows the weight loss due to enzymatic degradation of the PVAM-graft-BDO samples with/without NS. The results show that the PVAM-graft-BDO initially suffered a low rate of weight loss and after degradation for 5, 10 and 15 days, the weight loss for the 9.5/0.5 PVAM-graft-BDO was 8, 12 and 15%, respectively. Therefore, the degradation was faster in the higher BDO content sample than that with a low BDO content owing to the higher hydrophilic portion in the sample. Further, after the addition of the NS, the % degradation of the PVAM-graft-BDO was lower than that without the addition of the NS. This might be due to the higher molecular weight and chemical structure of the cassava starch in the NS. The weight losses in the samples containing 1, 2, 4, 5 and 6 g NS were 29, 30, 32, 33 and 32%, respectively, with a treatment time of 15 days. These results are in agreement with those for biodegradation in natural soil.
Conclusions
A maleate poly(vinyl alcohol)-graft-BDO was successfully made from the PVAM and BDO using KPS as an initiator. The highest grafting efficiency of 92% was found at 7:3 PVAM-graft-BDO. The maximum swelling ratio of the PVAM-graft-BDO of 250% was found at a ratio of the 7:3 PVAM:BDO. However, the maximum tensile strength of the PVAM-graft-BDO was observed at 9:1 PVAM:BDO. The elongation at break was improved after the addition of NS. The most likely application of the PVAM-graft-BDO/NS blend would be in medical material and the PVAM-graft-BDO was used as a polymer matrix for the encapsulation of capsaicin. The rate of capsaicin release from the polymer matrix was controlled by the BDO and NS content. After its use, it was easily degraded in natural soil.
References
Sukhlaaied W, Riyajan SA, Palmese GR (2016) Polym Test 56:387
Sukhlaaied W, Riyajan SA (2016) Polym Bull 73:2303
Merayo N, Balea A, de la Fuente E, Blanco A, Negro C (2017) Cellulose 24:2987
Grande R, Pessan LA, Carvalho AJF (2018) Carbohydr Polym 191:44
Drumond N, Stegemann S (2018) Colloid Surf B 166:17
Mohanapriya S, Raj V (2018) Mater Sci Eng C 86:70
Shao P, Yan Z, Chen H, Xiao J (2018) J Appl Polym Sci 135:46117
Ikeuchi-Takahashi Y, Ishihara C, Onishi H (2017) Drug Dev Ind Pharm 43:1489
Yang J, Fan L, Xu Y, Xia J (2017) J Nano Res 19:333
Riyajan SA, Sukhlaaied W, Keawmang W (2015) Carbohydr Polym 122:301
Işıklan N, Kazan H (2018) J Appl Polym Sci 135:45969
Bo T, Wei D, Yuguang L (2017) Radiat Phys Chem 135:81
Zhai M, Yoshii F, Kume T, Hashim K (2002) Carbohydr Polym 50:295
Li R, Wu G, Cai X, YeIn Y (2017) Radiat Phys Chem 134:27
Wang Q, Zhou X, Zeng J, Wang J (2016) Nucl Instrum Method B 368:90
Zhang S, Liu P, Zhao X, Xu J (2017) Appl Surf Sci 396 :1098
Phung Hai TA, Sugimoto R (2018) Synth Met 240:37
Riyajan SA (2017) Polym Test 58:300
Riyajan SA (2015) Carbohydr Polym 134:267
Riyajan S (2019) Polym Bull 76:4611
Zheng H, Du V, Yu J, Ronghua H, Zhang L (1999) Fiber Biomater 20:1479
Riyajan SA (2017) KGK Kauts Gummi Kunstst 70:49–53
Riyajan SA, Sakdapipanich JT (2009) Polym Bull 63:609
Riyajan SA, Sasithornsonti Y, Phinyocheep P (2012) Carbohydr Polym 89:251
Sukhlaaied W, Riyajan SA (2017) Polym Bull 74:803–821
Acknowledgements
The author gratefully acknowledges the financial support provided by Faculty of Science and Technology, Thammasat University Contract No 2019, the Center of Scientific Equipment for Advanced Research, Thammasat University and the Center of Scientific Equipment, Faculty of Science and Technology, Thammasat University. This study was financially supported by The Thailand Research Fund/Prince of Songkla University/Thammasat University (RSA5780018) and by Thammasat University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Riyajan, SA. Environmentally Friendly Novel Maleated Poly(vinyl alcohol) Grafted 1, 4-Butanediol Modified with Biopolymer for Encapsulation of Capsaicin. J Polym Environ 27, 2637–2649 (2019). https://doi.org/10.1007/s10924-019-01542-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01542-8