Abstract
The energetic thermoplastic elastomers (ETPE) of glycidyl azide polymer with bonding functions were synthesized by using mixture of chain extenders including 1,4-butanediol and N-(2-cyanoethyl) diethanolamine. From FTIR results, the ability of ETPEs to form hydrogen bond weakened with the percentage of N-(2-cyanoethyl) diethanolamine increasing, which further lead to the decrease of maximum stress of ETPEs and increase of elongation at break. On the other hand, the interfacial interactions between solid ingredients and ETPEs were enhanced owing to the more –CN content. With the percentage of N-(2-cyanoethyl) diethanolamine increasing, two factors affect the adhesion between binder and fillers at the same time. The RDX/ETPE propellants were synthesized and the mechanical properties of them showed that the ETPE-50 with bonding functions can effectively prevent the dewetting, and then improve the mechanical properties of propellants. That is, when the percentage of N-(2-cyanoethyl) diethanolamine was 50 %, the synthesized ETPEs had not only binding function but also bonding interaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid propellants comprise solid ingredients like oxidizers, metallic fuels, and other minor additives as processing aids dispersed throughout a binder matrix [1]. By using the binders, the solid ingredients are to be bound together to form a composite grain with satisfactory mechanical properties.
Usually, these binders are obtained by mixing the energetic or non-energetic pre-polymers with the other ingredients followed by a curing reaction involving isocyanates. These binders are chemically crosslinked and therefore not recyclable. The disadvantages of this technology are that the mixing period is limited by the pot-life due to the increased viscosity, and that long curing times are needed to crosslink the material leading to an expensive process. A better way to solve these problems is to use thermoplastic elastomers (TPEs) [2]. Mass use of inert TPEs makes propellants low sensitivity, but also low energy. The use of energetic thermoplastic elastomers (ETPEs) will reduce energy loss. Among them, the energetic thermoplastic elastomer based on GAP is found to be thermally stable and their solid propellants are insensitive to mechanical stimuli [3, 4].
Because of the considerable amount of solid energetic materials, the poor mechanical properties and dewetting behavior have become serious problems in TPE propellants. It is generally known that the dewetting occurs rather suddenly when the adhesion between solid ingredients and TPE is not particularly strong [5]. One efficient way to solve this problem is to use small molecules bonding agent in the propellant. In the polar binding system, small molecules bonding agent is easily soluble in the polar submix which will further lead to the failure of bonding agent [6, 7]. So it’s useful and necessary to prepare the binders with bonding functions themselves.
In 1990, Kim [8] pointed out neutral polymeric bonding agents with a number-average molecular weight in the range from about 3000 to 500,000 can be used for propellants. These polymeric bonding agents are far more surface active than the small molecule bonding agents and can effectively prevent dewetting in the propellants. As with the basic thought of polymeric bonding agents, ETPEs can be given the ability of bonding functions and thus become a kind of polymeric bonding agents. These ETPEs with bonding functions not only can provide the binder matrix, but also play the role as the bonding agents. So they could decrease the dewetting and improve the mechanical property of propellants. The –CN group are usually incorporated into polymeric bonding agents because of the induced effect of –CN and –NO2 [8]. So we synthesized the GAP-based ETPE with bonding functions by introducing –CN group.
Experimental
Materials
Glycidyl azide polymer (GAP; OH equivalent: 26.71 mg KOH/g; Liming Research Institute of Chemical Industry, Henan China) was used after vacuum drying for 2 h at 90 °C. Hexamethylene diisocyanate (HMDI; Bayer.Co.) was used as received. Catalyst was obtained by dissolving dibutyltin dilaurate (DBTDL; Beijing Chemical Plant) into dibutyl phthalate. 1,4-butanediol (BDO; Beijing Chemical Plant) was used after vacuum drying for 4 h at 85 °C. N-(2-cyanoethyl) diethanolamine was made in our laboratory [9].
Synthesis of GAP-based ETPEs
A general pre-polymer method was employed to synthesize GAP-based ETPE using mixture of chain extenders including BDO and N-(2-cyanoethyl) diethanolamine.
The synthesized ETPEs are di-block copolymer (AB) n. A is the soft segment which gives the elastomeric behavior to the copolymer, and B is the hard segment which gives the thermoplastic behavior to the copolymer. In the GAP-based ETPEs, the hard segments B consist of hexamethylene diisocyanate (HMDI) chain extended with BDO and N-(2-cyanoethyl) diethanolamine, and the soft segment A is glycidyl azide polymer (GAP). The mole ratio of –NCO and –OH is 1:1. The hard segment content by weight (wt% B) was determined using the industry’s standard and the hard segment content of ETPEs in this paper was 30 %.
The mass fraction of N-(2-cyanoethyl) diethanolamine was 0, 25, 50, 75 and 100 % in the mixture chain extender, respectively. The samples with 0, 25, 50, 75 and 100 % N-(2-cyanoethyl) diethanolamine were named as ETPE-0, ETPE-25, ETPE-50, ETPE-75 and ETPE-100, respectively.
The synthesis process was illustrated in Fig. 1.
A stoichiometric amount of GAP was heated and stirred at 90 °C, and then a certain amount of catalyst and HMDI were added. The reaction mixture was stirred and mixed for 2 h at 90 °C. The different proportional chain extender (BDO and N-(2-cyanoethyl) diethanolamine) was added to the above NCO-terminated GAP pre-polymer at 60 °C, and the reaction was kept for 3–5 min. Then the product was cast in a mold to cure at 100 °C for around 10 h. Finally, the GAP-based ETPEs with different proportional chain extender were obtained.
Synthesis of RDX/ETPE propellants
To test the adhesion between binder (like ETPE) and solid ingredients (like RDX) in propellants, the RDX/ETPE propellants were made. The RDX/ETPE propellants were prepared as follows: The ETPE was dissolved in tetrahydrofuran, and the RDX was introduced in the ETPE solution at 80 % weight percentages. Then the solvent was evaporated in a vacuum oven at 60 °C. Finally, the mixture was mixed on open mill and tableted by flat-panel curing.
The propellants with ETPE-0, ETPE-25, ETPE-50, ETPE-75 and ETPE-100 were named as RDX/ETPE-0, RDX/ETPE-25, RDX/ETPE-50, RDX/ETPE-75 and RDX/ETPE-100, respectively.
Measurements
Infrared spectra were recorded on FTIR (Nicolet FTIR- 8700, Thermo) with a wavenumber resolution of 4 cm−1 and a single average of 32 scans at room temperature.
The molecular weights (\(\overline{{M_{\text{w}} }}\) and \(\overline{{M_{\text{n}} }}\)), and the polydispersity index (PDI = \(\overline{{M_{\text{w}} }} /\overline{{M_{\text{n}} }}\)) were obtained using GPC (LC-20A, Shimadzu). The operating temperature was 40 °C, the mobile phase was THF, the flow rate was 1.0 mL min−1 and the raw data were calibrated using a universal calibration with polystyrene standards.
Differential scanning calorimetric analysis (DSC) was made over the temperature range from −100 to 150 °C on Mettler DSC1 with a 10 K min−1 heating rate in a nitrogen atmosphere (40 mL min−1). 2–5 mg of each sample was placed in a small confined aluminum cell.
Thermo gravimetric analysis was carried out in TGA analyzer (TGA/DSC1SF/417-2, Mettler Toledo) at heating rates of 10 °C min−1 from room temperature to 600 °C in a nitrogen atmosphere (40 mL min−1).
The contact angle was measured using OCA contact angle analyzer (Datephysics Co.). The drop volume was 2 μL and the drop flow was medium speed. The surface free energy was calculated using SCA20 based on the Young’s equation [Eq. (1)].
The stress–strain test of elastomers was measured by using a tensile testing machine (Instron-6022, Shimadzu Co. Ltd) at a constant strain rate of 100 mm min−1 at room temperature. The dimensions of the samples were 20 mm (neck area length) × 4 mm (width) × 2 mm (thickness).
Results and discussion
Molecular weight analysis
The number-average molecular weight (\(\overline{{M_{\text{n}} }}\)) and polydispersity index (PDI; \(\overline{{M_{\text{w}} }} /\overline{{M_{\text{n}} }}\)) of synthesized GAP-based ETPEs are shown in Table 1. The \(\overline{{M_{\text{n}} }}\) of prepared ETPEs was between 35,000 and 41,000 g mol−1 and the PDI was between 1.97 and 2.06. So the synthesis products had high molecular weight and narrow molecular weight distribution.
FTIR analysis
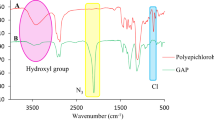
Figure 2 showed the infrared spectra of synthesized GAP-based ETPEs with different proportional chain extender. The infrared spectra of ETPEs did not show the absorption band at 3450 and 2265 cm−1 which corresponded to –OH and –NCO groups. The urethane characteristic bands at 3340 and 1710 cm−1 were also observed which corresponded to –NH stretching and C=O stretching of amide. The characteristic peaks of –N3 group from GAP at 2100 cm−1 were also shown [10, 11]. In addition, Fig. 2 showed the absorption at 2240 cm−1 which corresponded to the characteristic absorption of –CN.
Hydrogen bonding in ETPEs, which reflects the micro-phase separation, has an important effect on the properties of the elastomer. The donor of polyurethane is usually the –NH group of the urethane linkage. The hydrogen-bond acceptor may be in either the hard urethane segment (the carbonyl of the urethane group) or the soft segment (an ester carbonyl or ether oxygen) [12]. Traditionally, the hydrogen bond between –NH and carbonyl can reflect the aggregation ability of hard segments. One of the most useful methods of studying the hydrogen bonding is FTIR [13].
The stretching vibration mode of the –C=O in the ETPEs should be observed from 1800 to 1600 cm−1. In Fig. 3, the infrared spectra of the carbonyl stretching region of ETPEs were magnified. These curves can be fitted to three bands by Gaussian method, as shown in Fig. 3. It is well-known that hydrogen-bonded –C=O bands appear at lower wave numbers than the free bands [14]. It can be seen that three overlapping bands are apparently centered near 1715, 1690 and 1670 cm−1 corresponding to free, disordered hydrogen-bonded and ordered hydrogen-bonded C=O, respectively [15]. The degree of the carbonyl groups participating in hydrogen bonding can be described by the carbonyl hydrogen bonding index, R, as given in Eq. (2). The carbonyl hydrogen bonding index, R, can reflect the degree of hard segment linking hard segment (degree of phase separation) [16].
A is the intensity of the characteristic absorbance. The obtained values of R were given in Table 2.
The results indicated that the carbonyl hydrogen bonding index decreased with increasing percentage of N-(2-cyanoethyl) diethanolamine in Table 2. The reason could be the molecular chain of ETPEs cannot approach each other easily owing to steric hindrance of the bulk side chain. Figure 4 illustrates the chain of hydrogen-bonded urethane groups in ETPE-100. The schematic representation showed that after adding DBM, the space between the molecular chains of ETPEs increased, and then they cannot approach each other easily, which further caused the reductions in hydrogen bonding.
DSC analysis
The glass transition temperature of ETPEs was characterized using DSC. The DSC results of the ETPEs were given in Fig. 5 and Table 3. Briefly, two endotherms were found for ETPEs: T gs (the glass transition temperature of soft segment) was around −38.0 °C and T gh (the glass transition temperature of hard segment) was around 100.0 °C. As seen from Fig. 5 and Table 3, T gs moved to the higher temperature and T gh moved to the lower temperature with the percentage of N-(2-cyanoethyl) diethanolamine increasing, which indicated that the compatibility of hard and soft phases was improved. The reason was due to the decreased hydrogen bond and weaker ability of accumulation between hard segments.
TGA analysis
Thermal analysis is a frequent tool for propellants’ researches. Thermal decomposition could be correlated with important performance parameters such as heat of explosion, detonation velocity, and detonation energy [17]. So it is necessary to understand the thermal decomposition of the ETPEs. Figure 6 shows the thermal curves of ETPEs investigated using TGA measurement from 30 to 600 °C. The initial 5 % weight loss for total ETPEs occurred around 235 °C, suggesting that the ETPEs had good thermal stability properties and the introducing N-(2-cyanoethyl) diethanolamine into ETPEs did not affect their thermal stability properties of ETPEs.
Sensitivities of ETPEs
Sensitivity deserves significant attention by researchers because it is closely linked with the safety of handling and applying explosives. In this study, sensitivities towards impact and friction were tested using standard procedures [18–20], which are listed in Table 4.
The ETPEs were insensitive toward impact greater than 40 J and also insensitive towards friction greater than 360 N. The results showed that the ETPEs have safe and easy-to-handle processibility.
Interfacial characteristics
The interfacial property between oxidizer and binder can affect the mechanical properties of propellant. So it is important to study the interfacial property between oxidizer and ETPEs. In this paper, the surface free energy of ETPEs was obtained by measurement of contact angle for the ETPEs. The contact angle and the surface free energy which was calculated by using SCA20 were listed in Tables 5 and 6 separately.
The interfacial tension (γ sL) and work of adhesion (W a) between three different nitramine solid ingredients and ETPE were calculated by Eqs. (3) and (4) separately [22]. The γ sL and W a results of theoretic calculation were listed in Table 7.
As shown in Table 7, with the increasing percentage of N-(2-cyanoethyl) diethanolamine of the ETPEs, the γ sL between solid ingredients and ETPEs decreased and the W a improved generally. The W a value of ETPEs including –CN were greatly improved compared with ETPE-0 owing to the interactions between –NO2 and –CN.
Mechanical properties of ETPEs
The mechanical properties of ETPEs affect the mechanical properties of the final propellants. The sample of ETPE-100 was too soft to test the mechanical properties (tensile strength and elongation at break). The mechanical properties of four other ETPEs were shown in Fig. 7. With the increasing percentage of N-(2-cyanoethyl) diethanolamine, the maximum stress (σ m) obviously decreased and the elongation at break (ε b) improved. This results from the increasing of bulky side chain and the weaker hydrogen bonding. The analysis by FTIR also proved the point above.
Mechanical properties of RDX/ETPE
The adhesion between binder and fillers in propellant is affected by multiple factors, including mechanical properties of ETPEs and the W a between solid ingredients and ETPEs. The RDX/ETPE propellants were synthesized and the mechanical properties of them were tested to consider the results of two factors. The stress–strain curves are shown in Fig. 8. The sort of knee of stress–strain curves has been known to occur due to an extensive dewetting. Figure 8 showed the curves of propellants but RDX/ETPE-50 present knees, which meant that the ETPE-50 can prevent dewetting. Even better, the mechanical properties of RDX/ETPE-50 were higher than those of RDX/ETPE-0. The results showed that when the percentage of N-(2-cyanoethyl) diethanolamine was 50 %, the two kinds of influence worked together best. This indicated that the ETPE-50 with bonding functions can effectively prevent the dewetting and then improve the mechanical properties of propellants.
Conclusions
The energetic thermoplastic elastomers (ETPE) of glycidyl azide polymer with bonding functions were synthesized by using mixture of chain extenders including 1,4-butanediol and N-(2-cyanoethyl) diethanolamine. From FTIR results, with the percentage of N-(2-cyanoethyl) diethanolamine increasing, the ability of ETPEs to form hydrogen bond weakened, which further caused maximum stress of ETPEs to decrease and the elongation at break to improve. On the other hand, the interfacial interactions between solid ingredients and ETPEs were enhanced owing to the more –CN content. The two factors affect the adhesion between binder and fillers at the same time. The mechanical properties of RDX-ETPE propellants showed that the ETPE-50 with bonding functions can effectively prevent the dewetting and then improve the mechanical properties of propellants. That is, when the percentage of N-(2-cyanoethyl) diethanolamine was 50 %, the synthetic ETPEs had not only binding function but also bonding interaction.
References
Kanti Sikder A, Reddy S (2013) Review on energetic thermoplastic elastomers (ETPEs) for military science. Propell Explos Pyrot 38:14–28
Chen FT, Duo YQ, Luo SG et al (2003) Novel segmented thermoplastic polyurethanes elastomers based on tetrahydrofuran ethylene oxide copolyethers as high energetic propellant binders. Propell Explos Pyrot 28(1):7–11
Ampleman G, Brousseau P, Thiboutot S et al (2003) Obtained by dissolving an energetic copolyurethane thermoplastic elastomer of a polyglycidyl azide having methylenebis (p-phenylisocyanate) groups hydrogen bonded to crosslink in melted trinitrotoluene (TNT). US Patent 6,562,159
Diaz E, Brousseau P, Ampleman G et al (2003) Heats of combustion and formation of new energetic thermoplastic elastomers based on GAP, PolyNIMMO and PolyGLYN. Propell Explos Pyrot 28(3):101–106
Mulage KS, Patkar RN, Deuskar VD et al (2007) Studies on a novel thermoplastic polyurethane as a binder for extruded composite propellants. J Energ Mater 25(4):233–245
Allen HC (1982) Thermoplastic composite rocket propellant. US Patent 4,361,526
Fowkes FM, Mostafa MA (1978) Acid-base interactions in polymer adsorption. Ind Eng Chem Prod Res Dev 17(1):3–7
Kim CS (1990) Filler reinforcement of polyurethane binder using a neutral polymeric bonding agent. US Patent 4,915,755
Lang C, Jianru D, Wan X et al (2007) Synthesis of N-(2-Cyanoethyl) Diethanolamine. Petrochem Technol 36(10):1029
You JS, Kweon JO, Kang SC et al (2010) A kinetic study of thermal decomposition of glycidyl azide polymer (GAP)-based energetic thermoplastic polyurethanes. Macromo Res 18(12):1226–1232
Liu P, Ye L, Liu Y et al (2011) Preparation and properties of the main-chain-fluorinated thermoplastic polyurethane elastomer. Polym Bull 66(4):503–515
Kusy RP, Turner DT (1971) Radiation chemistry of polymers studied by depression of melting temperature. Macromolecules 4(3):337–341
Ning L, De-Ning W, Sheng-Kang Y (1996) Crystallinity and hydrogen bonding of hard segments in segmented poly (urethane urea) copolymers. Polymer 37(16):3577–3583
Zhang C, Ren Z, Yin Z et al (2008) Amide II and amide III bands in polyurethane model soft and hard segments. Polym Bull 60:97–101
Mattia J, Painter P (2007) A comparison of hydrogen bonding and order in a polyurethane and poly (urethane-urea) and their blends with poly (ethylene glycol). Macromolecules 40(5):1546–1554
Tien YI, Wei KH (2001) Hydrogen bonding and mechanical properties in segmented montmorillonite/polyurethane nanocomposites of different hard segment ratios. Polymer 42(7):3213–3221
Herder G, Weterings FP, de Klerk WPC (2003) Mechanical analysis on rocket propellants. J Therm Anal Calorim 72(3):921–929
Sabate CM, Delalu H, Jeanneau E (2012) Synthesis, characterization, and energetic properties of salts of the 1-cyanomethyl-1, 1-dimethylhydrazinium cation. Chem Asian J 7(5):1085–1095
Sabaté CM, Delalu H, Jeanneau E (2012) Energetic hydrazine-based salts with nitrogen-rich and oxidizing anions. Chem Asian J 7(9):2080–2089
Liu W, Lin QH, Yang YZ et al (2014) Energetic salts based on an oxygen-containing cation: 2, 4-diamino-1, 3, 5-triazine-6-one. Chem Asian J 9(2):479–486
Du MN, Luo YJ, Li GP (2007) Determination of surface free energy components of ε-CL-20 by thin-layer wicking technique. Chin J Energ Mater 15(3):269–272
Oprea S (2010) The effect of chain extenders structure on properties of new polyurethane elastomers. Polym Bull 65(8):753–766
Acknowledgments
We are grateful to the State Key Laboratory of Explosion Science and Technology (No. YBKT15-02).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Z., Wang, G., Wang, Z. et al. Synthesis and characterization of novel energetic thermoplastic elastomers based on glycidyl azide polymer (GAP) with bonding functions. Polym. Bull. 72, 1835–1847 (2015). https://doi.org/10.1007/s00289-015-1375-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1375-7