Abstract
In the previous study, we successfully prepared a chitin nanofiber film by regeneration from a chitin ion gel with an ionic liquid using methanol. In this study, we performed surface-initiated graft polymerization of γ-benzyl l-glutamate N-carboxyanhydride (BLG-NCA) from amino groups on a partially deacetylated chitin nanofiber (PDA-CNF) film. First, the chitin nanofiber film was immersed in 40 % NaOH aq. at 80 °C for 7 h for partial deacetylation. Then, the PDA-CNF film was immersed in a solution of BLG-NCA in ethyl acetate at 0 °C for 24 h for graft polymerization from amino groups on nanofibers to give a chitin nanofiber-graft-poly(γ-benzyl l-glutamate) (CNF-g-PBLG) film. The analytical results of the film indicated that graft polymerization of BLG-NCA occur on surface of nanofibers. Furthermore, the film was treated with 1.0 mol/L NaOH aq. to convert PBLG on nanofibers into poly(γ-l-glutamic acid sodium salt) (PLGA). Then, condensation of the resulting carboxylates with amino groups at the terminal ends of PLGAs or the remaining amino groups on nanofibers was performed using the condensing agent to produce a CNF-g-PLGA network film. The resulting film showed the good mechanical properties with high flexibility, which has potentials as promising materials for practical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chitin is an aminopolysaccharide composed of N-acetyl-d-glucosamine residues linked through β-(1→4)-glycosidic bonds, which occur mainly in exoskeletons of crustaceans, shellfish, and insects [1–4]. Although chitin is one of the most abundant biopolymers produced in nature, it still remains as an unutilized biomass resource primary because of its intractable bulk structure. Therefore, the researches concerning conversion of chitin into functional bio-based materials through its proper dissolution and processing have attracted much attention even in recent years [5]. However, chitin is insoluble in water and the most common organic solvents due to numerous intra- and intermolecular hydrogen bonds, causing difficulty in its feasibility and processability.
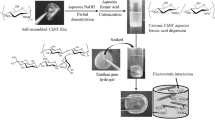
To efficiently provide new chitin-based materials through dissolution or gelation, we have focused on ionic liquids, which are low-melting point salts that form liquids at temperatures below the boiling point of water, because they have recently been found to be used as good solvents for polysaccharides such as cellulose [6–9]. Since it was reported that 1-butyl-3-methylimidazolium chloride of an ionic liquid dissolved cellulose in relatively high concentrations [10], various ionic liquids have been used for material processing of cellulose [6–9]. However, little has been reported regarding the dissolution of chitin with the ionic liquids [11–15]. In the previous papers, we reported that an ionic liquid, 1-allyl-3-methylimidazolium bromide (AMIMBr), dissolved chitin in the concentrations up to ca. 4.8 wt% and further formed ion gels with higher contents of chitin [16, 17]. Furthermore, we reported that chitin nanofiber films were facilely obtained by regeneration from the ion gels using methanol, followed by filtration (Fig. 1a) [18]. This approach accords to self-assembling generative (bottom-up) route, which is completely different from the common approaches for the production of chitin nanofibers according to top-down route that break down the starting native chitin [19–21]. Recently, we also performed the surface-initiated ring-opening graft copolymerization of l-lactide (LA)/ε-caprolactone (CL) from the chitin nanofiber film [22] because the ring-opening copolymerization has been known to produce a biodegradable and biocompatible poly(LA/CL) [23–25], which has a potential to act as a good component in bio-based materials. In this procedure, hydroxy groups in the chitin structure were acted as initiating groups and the graft copolymerization took place on surface of the nanofibers to produce chitin nanofiber-graft-poly(LA/CL) films. For grafting on the chitin nanofibers to yield further useful bio-based composite materials, in the present study, we have noted synthetic polypeptides as another biocompatible polymer. Because it has been well known that synthetic polypeptides with well-defined structures are synthesized by ring-opening polymerization of α-amino acid N-carboxyanhydrides (NCAs) accompanied with decarboxylation initiated from amino groups [26, 27], in this study, we investigated the surface-initiated graft polymerization of a NCA monomer from the chitin nanofiber film having amino initiating groups to give chitin nanofiber-graft-polypeptide materials. As the monomer, moreover, we selected γ-benzyl l-glutamate-NCA (BLG-NCA) because its ring-opening polymerization and subsequent hydrolysis of ester linkages gives poly(γ-glutamic acid) having carboxylic acid groups. In the previous papers related to the present study, graft polymerization of NCA monomers from amino groups on a water-soluble chitin (degree of deacetylation; 50 %) has been performed [28, 29].
In this paper, we report the surface-initiated ring-opening polymerization of BLG-NCA initiated from amino groups on surface of the partially deacetylated chitin nanofiber (PDA-CNF) film, which was prepared by alkaline treatment of the original chitin nanofiber film, to give chitin nanofiber-graft-poly(γ-benzyl l-glutamate) (CNF-g-PBLG) films (Fig. 1b). Furthermore, we found that a highly flexible chitin nanofiber-graft-poly(γ-l-glutamic acid sodium salt) (CNF-g-PLGA) network film was obtained by alkaline hydrolysis of ester linkages in the PBLG chains on the CNF-g-PBLG to convert into the PLGA chains, followed by condensation of the produced sodium carboxylate groups with amino groups present in the film (Fig. 1c).
Experimental
Materials
Chitin powder from crab shells was purchased from Wako Pure Chemical Industries, Ltd., Japan. The weight-average molecular weight value of the chitin sample was estimated by viscometric analysis to be 7 × 105 [30]. A monomer, BLG-NCA, was synthesized according to the literature procedure [31]. An ionic liquid, AMIMBr, was prepared by reaction of 1-methylimidazole with 3-bromo-1-propene according to the method modified from the literature procedure [32]. Chitin nanofiber films were prepared by the procedure as we performed in our previous publication [18]. All other reagents and solvents were used as received from commercial sources.
Partial deacetylation of chitin nanofiber (PDA-CNF) film [33]
The chitin nanofiber film (0.10 g, 0.49 mmol) was immersed in 40 % (w/v) NaOH aq. (20 mL) at 80 °C for 7 h. Then, the film was washed with water and methanol and dried under ambient atmosphere to give the PDA-CNF film.
Surface-initiated graft polymerization of BLG-NCA from PDA-CNF film
A typical experimental procedure for the surface-initiated graft polymerization was as follows. The chitin nanofiber film (0.010 g) was immersed in a solution of BLG-NCA (0.085 g, 0.32 mmol, 20 equiv. for an amino group) in ethyl acetate (2.0 mL) at 0 °C for 24 h. The resulting film was then washed with ethyl acetate and dried under ambient atmosphere to give CNF-g-PBLG film (0.0122 mg, grafting ratio; 18 wt%).
Alkaline treatment of CNF-g-PBLG film
The CNF-g-PBLG film (0.010 g, grafting ratio; 18 wt%) was immersed in 1.0 mol/L NaOH aq. (5.0 mL) at 60 °C for 5 h. The resulting film was then washed with water and methanol and dried under ambient atmosphere to give the CNF-g-PLGA film.
Condensation of sodium carboxylate groups with amino groups in CNF-g-PLGA film
The CNF-g-PLGA film (0.010 g) was immersed in a solution of N-hydroxysuccinimide (NHS, 0.016 g, 0.14 mmol) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC, 0.027 g, 0.14 mmol) in water (2.0 mL) at room temperature for 12 h. The resulting film was then washed with water and methanol and dried under ambient atmosphere to give CNF-g-PLGA network film.
Measurements
IR spectra were recorded on a SHIMADZU FTIR-8400 or Jasco IRT-3000 spectrometers. The SEM images were obtained using Hitachi S-4100H electron microscope. The powder X-ray diffraction (XRD) measurements were conducted using a PANalytical X’Pert Pro MPD with Ni-filtered CuKα radiation (λ = 0.15418 nm). The stress–strain curves were measured using a tensile tester (Little Senster LSC-1/30, Tokyo Testing Machine).
Results and discussion
Surface-initiated graft polymerization of BLG-NCA from chitin nanofiber film
As previously reported by us [18], the chitin nanofiber film was obtained through the gelation with AMIMBr, followed by regeneration using methanol and filtration according to Fig. 1a. Because the NCA monomer has been polymerized from an amino initiating group, partial deacetylation of acetamido groups in the chitin nanofiber film was conducted by the treatment with 40 % (w/v) NaOH aq. at 80 °C for 7 h to introduce amino groups in the film [33]. The degree of deacetylation of the produced PDA-CNF film was estimated by the ratio of the absorption due to amido II at 1,560 cm−1 to that due to the C–O stretching at 1,070 cm−1 in the IR spectrum to be 24 % [34]. The SEM image and the XRD pattern of the resulting PDA-CNF film (Figs. 2b, 3b) were comparable to those before the alkaline treatment (chitin nanofiber film, Figs. 2a, 3a), suggesting the preservation of the nanofiber morphology and the crystalline structure of antiparallel α-chitin arrangement, respectively.
When the attempts for the surface-initiated graft polymerization of BLG-NCA using the resulting PDA-CNF film were made under various conditions, we found that the following experimental manner was the most appropriate to perform the efficient polymerization. Thus, PDA-CNF film was immersed in a solution of BLG-NCA (5, 10, 20 equiv. for an amino group) in ethyl acetate at 0 °C for 24 h for the surface-initiated graft polymerization from amino groups [28]. Figure. 4b shows the IR spectrum of the produced CNF-g-PBLG film using 20 equiv. of BLG-NCA in comparison with that of PDA-CNF film (Fig. 4a). The C=O absorption due to ester linkage was detected at 1,735 cm−1 in the IR spectrum after the graft polymerization, strongly suggesting the presence of PBLG in the product, which was explanatorily bound to the nanofibers by covalent linkage. The grafting amounts (weight ratios to the original PDA-CNF film) of the PBLG chains, which were evaluated by the weight differences of the films before and after the graft polymerization, increased with increasing the feed ratios of BLG-NCA to an amino group (5 equiv.; 10 wt%, 10 equiv.; 14 wt%, 20 equiv.; 18 wt%). The SEM image of the CNF-g-PBLG film (Fig. 2c, grafting ratio; 18 wt%) indicated that morphology of nanofibers still remained, but some fibers were merged at the interfacial areas that was probably caused by the grafted PBLG chains present on the nanofibers. The XRD pattern of the CNF-g-PBLG film (Fig. 3c) was almost same as that of the PDA-CNF film, indicating that the crystalline structure of the chitin chains was not disrupted owing to the occurrence of the graft polymerization only on surface of the nanofibers.
Alkaline treatment and condensation of CNF-g-PBLG film
The alkaline treatment of the CNF-g-PBLG film and the subsequent condensation were conducted to produce the chitin nanofiber-polypeptide network film with superior mechanical property (Fig. 1c). First, the CNF-g-PBLG film (grafting ratio; 18 wt%) was immersed in 1.0 mol/L NaOH aq. at 60 °C for 5 h for the alkaline hydrolysis of benzyl esters to convert into the CNF-g-PLGA film having sodium carboxylate groups. The IR spectrum of the film after the alkaline treatment (Fig. 4c) did not exhibit the ester carbonyl absorption, supporting that benzyl esters were completely hydrolyzed to convert into sodium carboxylate groups. Then, the condensation of the introduced carboxylate groups with amino groups at the terminal end of the PLGA chains or the remaining amino groups on the nanofibers, which did not participate in the initiation of the graft polymerization was conducted using the NHS/EDC condensing agent (10 equiv. for a carboxylate group) in water at room temperature for 12 h to construct PLGA or PLGA/chitin networks in the film. The intensity ratio of the amido II absorption at 1,560 cm−1 to the C–O stretching absorption at 1,070 cm−1 in the IR spectrum of the product was 22 % larger than that of the film before the condensation [34]. This result indicated the progress of the desired condensation reaction to yield the CNF-g-PLGA network film. The SEM and XRD results of the produced network film (Figs. 2d, 3d) were quite similar to those of CNF-g-PBLG film (Figs. 2c, 3c).
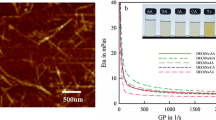
Finally, the mechanical properties of the chitin nanofiber, CNF-g-PBLG, and CNF-g-PLGA network films were evaluated by tensile testing (Fig. 5). The stress–strain curve of the CNF-g-PBLG film (Fig. 5b) showed the larger strain value at break than that of the chitin nanofiber film (Fig. 5a) with the comparable fracture stress values, indicating the more elastic nature of the former film. The stress–strain curve of the CNF-g-PLGA network film (Fig. 5c) exhibited the larger values both in fracture stress and strain than those of the other films (some horizontal bars of various lengths were detected in the curve probably because the measurement was conducted in the range almost nearby lower limit of the tensile machine). These data indicated the superior mechanical property of the CNF-g-PLGA network film. Indeed, the CNF-g-PLGA network film had the highly flexible nature, in which this was bended without breaking as shown in Fig. 6.
Conclusion
In this study, the surface-initiated graft polymerization of BLG-NCA initiated from amino groups on the PDA-CNF film, which was prepared by the alkaline treatment of the chitin nanofiber film, was conducted to give the CNF-g-PBLG film. The IR, SEM, and XRD results of the product indicated the presence of the graft PBLG chains on the nanofibers bound by covalent linkage. The PBLG chains on the film were converted into the PLGA chains by the alkaline hydrolysis of benzyl esters. Furthermore, the condensation of the carboxylate groups in the PLGA chains with amino groups at the terminal ends of the PLGA chains or the remaining amino groups on the nanofibers was conducted using the NHS/EDC condensing agent in water to construct PLGA or PLGA/chitin networks in the film. The resulting CNF-g-PLGA network film showed the superior mechanical property compared with the chitin nanofiber film as well as the CNF-g-PBLG film. Because the present composite films are fully composed of biocompatible polymeric chains, i.e., chitin and polypeptide, the materials have high potentials for the practical applications in the biomedical and environmental polymeric fields such as tissue engineering and bioplastics in the future.
References
Kurita K (2006) Chitin and chitosan: functional biopolymers from marine crustaceans. Mar Biotechnol 8:203–226
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Pillai CKS, Paul W, Sharma CP (2009) Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Polym Sci 34:641–678
Muzzarelli RAA (2011) Chitin nanostructures in living organisms. In: Gupta SN (ed) Chitin formation and diagenesis. Springer, New York, pp 1–34
Muzzarelli RAA (2011) Biomedical exploitation of chitin and chitosan via mechano-chemical disassembly, electro spinning, dissolution in imidazolium ionic liquids, and supercritical drying. Marine Drugs 9:1510–1533
Liebert T, Heinze T (2008) Interaction of ionic liquids with polysaccharides 5. Solvents and reaction media for the modification of cellulose. Bio Resources 3:576–601
Feng L, Chen ZIJ (2008) Research progress on dissolution and functional modification of cellulose in ionic liquids. Mol Liq 142:1–5
Pinkert A, Marsh KN, Pang S, Staiger MP (2009) Ionic liquids and their interaction with cellulose. Chem Rev 109:6712–6828
Zakrzewska ME, Lukasik EB, Lukasik RB (2010) Solubility of carbohydrates in ionic liquids. Energy Fuels 24:737–745
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellulose with ionic liquids. J Am Chem Soc 124:4974–4975
Wu Y, Sasaki T, Irie S, Sakurai K (2008) A novel biomass-ionic liquid platform for the utilization of native chitin. Polymer 49:2321–2327
Qin Y, Lu X, Sun N, Rogers RD (2010) Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers. Green Chem 12:968–971
Wang WT, Zhu J, Wang XL, Huang Y, Wang YZ (2010) Dissolution behavior of chitin in ionic liquids. J Macromol Sci Part B Phys 49:528–541
Jaworska MM, Kozlecki T, Gorak A (2012) Review of the application of ionic liquids as solvents for chitin. J Polym Eng 32:67–69
Bochek AM, Murav’ev AA, Novoselov NP, Zaborski M, Zabivalova NM, Petrova VA, Vlasova EN, Volchek BZ, Lavrent’ev VK (1012) Specific features of cellulose and chitin dissolution in ionic liquids of varied structure and the structural organization of regenerated polysaccharides. Russ J Appl Chem 85:1718–1725
Yamazaki S, Takegawa A, Kaneko Y, Kadokawa J, Yamagata M, Ishikawa M (2009) An acidic cellulose-chitin hybrid gel as novel electrolyte for an electric double Layer capacitor. Electrochem Commun 11:68–70
Prasad K, Murakami M, Kaneko Y, Takada A, Nakamura Y, Kadokawa J (2009) Weak gel of chitin with ionic liquid, 1-allyl-3-methylimidazolium bromide. Int J Biol Macromol 45:221–225
Kadokawa J, Takegawa A, Mine S, Prasad K (2011) Preparation of chitin nanowhiskers using an ionic liquid and their composite materials with poly(viny alcohol). Carbohydr Polym 84:1408–1412
Zeng JB, He YS, Li SL, Wang YZ (2012) Chitin whiskers: a overview. Biomacromolecules 13:1–11
Ifuku S, Saimoto H (2012) Chitin nanofibers: preparations, modifications, and applications. Nanoscale 4:3308–3318
Ifuku S (2012) Preparation of chitin nanofibers from crab shell and their applications. Kobunshi Ronbunshu 69:460–467
Setoguchi T, Yamamoto K, Kadokawa J (2012) Preparation of chitin nanofiber-graft-poly(l-lactide-co-ε-caprolactone) films by surface-initiated ring-opening graft copolymerization. Polymer 53:4977–4982
Albertsson AC, Varma IK (2002) Aliphatic polyesters: synthesis, properties and applications. Adv Polym Sci 157:1–40
Hakkarainen M (2002) Aliphatic polyesters: abiotic and biotic degradation and degradation products. Adv Polym Sci 157:113–138
Seyednejad H, Ghassemi AH, van Nostrum CF, Vermonden T, Hennink WE (2011) Functional aliphatic polyesters for biomedical and pharmaceutical applications. J Control Release 152:168–176
Kricheldorf HR (2006) Polypeptides and 100 years of chemistry of α-amino acid N-carboxyanhydrides. Angew Chem Int Ed 45:5752–5784
Deming TJ (2007) Synthetic polypeptides for biomedical applications. Prog Polym Sci 32:858–875
Kurita K, Kanari M, Koyama Y (1985) Studies on chitin. 11. Graft-copolymerization of γ-methyl l-glutamate NCA onto water-soluble chitin. Polym Bull 14:511–514
Kurita K, Iwawaki S, Ishii S, Nishimura S (1992) Introduction of poly(l-alanine) side chains into chitin as versatile spacer arms having a terminal free amino group and immobilization of NADH active sites. J Polym Sci, Part A Polym Chem 30:685–688
Poirier M, Charlet G (2002) Chitin fractionation and characterization in N, N-dimethylacetamide/lithium chloride solvent system. Carbohydr Polym 50:363–370
Fujita Y, Koga K, Kim HK, Wang XS, Sudo A, Nishida H, Endo T (2007) Phosgene-free synthesis of N-carboxyanhydrides of α-amino acids based on bisarylcarbonates as starting compounds. J Polym Sci Part A Polym Chem 45:5365–5369
Zhao D, Fei Z, Geldbach TJ, Scopelliti R, Laurenczy G, Dyson PJ (2005) Allyl-functionalised ionic liquids: synthesis, characterisation, and reactivity. Helv Chim Acta 88:665–675
Phongying S, Aiba S, Chirachanchai S (2007) Direct chitosan nanoscaffold formation via chitin whiskers. Polymer 48:393–400
Shigemasa Y, Matsuura H, Sashiwa H, Saimoto H (1996) Evaluation of different absorbance ratios from infrared spectroscopy for analyzing the degree of deacetylation in chitin. Int J Biol Macromol 18:237–242
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kadokawa, Ji., Setoguchi, T. & Yamamoto, K. Preparation of highly flexible chitin nanofiber-graft-poly(γ-l-glutamic acid) network film. Polym. Bull. 70, 3279–3289 (2013). https://doi.org/10.1007/s00289-013-1020-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-013-1020-2