Abstract
A method commonly used to induce compatibility between polyamides and polyolefins is by chemical modification of the polyolefins. This concept has been employed to produce compatibilized blend of nylon 6 (N6) and polypropylene copolymer (PPCP) by adding maleic anhydride grafted polypropylene (PP-g-MAH) as a third component. The blends of N6, PPCP and PP-g-MAH were prepared using a twin screw extruder. TEM of the blends revealed better dispersion and reduction in the size of the dispersed phase. In case of N6 rich blends, about 25% increase in tensile strength was observed with just three phb of PP-g-MAH. The maximum impact strength and flexural modulus were observed at around 30% PPCP. The incorporation of PP-g-MAH increased the flexural modulus of N6/PPCP blends significantly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polypropylene (PP) is a widely used commodity polymer because of its ease of processing, low cost, integral hinge property and high barrier property to moisture. However the high oxygen permeability limits its use. On the other hand nylon 6 (N6) in an engineering polymer with better mechanical properties and exhibits superior barrier for oxygen transport. N6 is processed at a higher temperature compared to PP. The major disadvantage of N6 is its hygroscopic nature hence a poor barrier for water and relatively higher cost. Blends of PP and N6 can combine the easy processibility of PP with good mechanical properties of N6. These blends can combine the integral hinge property, moisture resistance and easy processibility of PP with abrasion resistance and thermo-mechanical properties of N6 provided the blend is miscible. However PP and N6 form immiscible blends through the whole range of compositions leading to inferior properties necessitating the need for compatibilization.
Compatibilization of polymer blends to obtain miscible blends has been extensively studied over the last decade. In the absence of compatibilizers, PP/N6 blends lack interfacial adhesion leading to poor mechanical properties. The best known effect of compatibilization is reduction of the interfacial tension in the melt [1]. This causes an emulsifying effect and leads to an extremely fine dispersion of one phase in the other. A second effect is an increase in adhesion at phase boundaries giving improved stress transfer. To achieve this, the interaction between the compatibilizing copolymer chain, the polymer chains of the dispersed phase and the matrix phase is important. A third effect is the inhibition of coalescence of the dispersed phase by modifying the phase boundary interface.
A method commonly used to induce compatibility between polyamides and polyolefins is by chemical modification of the polyolefins. Pendent carboxyl groups are introduced into polyolefins by grafting with maleic anhydride (MAH), which forms chemical linkage with the polyamide via the terminal amino groups as shown in Fig. 1.
The existence of a reaction between the MAH and the N6—amino end groups was established by solvent extraction of the PP phase, estimation of the number of end groups and DSC analysis of the residue [2]. Other approaches to enhance the properties of polyolefin/polyamide blends include the addition of acrylic acid/butyl acrylate/styrene terpolymers and the addition of N6—polybutene multi-block copolymer [3–5]. Effect of processing conditions and properties of PP/nylon blends was studied by Pal and Kale [6]. In this work, PP and N6 were melt blended in a twin screw extruder at different screw speeds using PP-g-MAH as compatibilizer. Blending at higher shear rates was found to result in better properties than those prepared at lower shear rates.
The effect of different grades of polypropylenes (MFI 40 and 3.5 g/10 min) and compatibilizers (PP-g-acrylic acid with 6% of acrylic acid and PP-g-MAH with 1% of maleic anhydride) on the rheological, mechanical and morphological properties of N6/PP blends was studied by Agarwal et al. [7]. Kim and Kim [8] have reported the usage of PP-g-MAH as a compatibilizer to prepare blends of EPDM and N6. Compatibilization of PP/N6 (70/30) blends with commercial compatibilizers such as oxazoline grafted polymers, and hydroxyl functionalized copolymers prepared with metallocene catalyst has been reported by some workers [1].
The objectives of this work are (1) to prepare compatibilized blend of N6 and polypropylene copolymer (PPCP) using PP-g-MAH, (2) to study the effect of PPCP on mechanical and thermal properties of blend in N6 rich region and (3) to study the effect of PP-g-MAH on mechanical and thermal properties of N6 and PPCP blend.
Experimental work
Materials
Commercial grade Polypropylene copolymer (grade: PPCP C080, Reliance Industries Ltd., India) and nylon 6 (grade: Gujlon E35, GSFC, Gujarat, India) were used to prepare blends. Typical properties of these polymers are shown in Table 1. The compatibilizer maleic anhydride grafted polypropylene (PP-g-MAH) was a gift sample from Pluss Polymers, New Delhi (grade: OPTIM P423, 0.1–0.4% maleic anhydride, density 0.91 g/ml, MFI 170 g/10 min at 190 °C, 2.16 kg).
Blending procedure and specimen preparation
Blends of N6 and PPCP were prepared using a 30 mm diameter co-rotating intermeshing type twin screw extruder, having L/D of 40.
N6 was pre-dried at 105 °C for 3 h. The concentration of PP-g-MAH, ranging from 0 to 3 parts per hundred parts of blend (phb) were used. The temperature of the extruder was set at 220 and 240 °C in feeding zone and barrel zones, respectively. The test specimens were prepared using Windsor 75T injection molding machine. Various combinations of N6/PPCP blends prepared in this study are presented in Table 2.
Testing and characterization
The blend samples were tested for HDT (ASTM D 648), Vicat softening temperature (ASTM D 1525), tensile properties [ASTM D 638 with type-I tensile bar)], flexural properties (ASTM D 790) and impact strength (ASTM D 256). All the test samples were conditioned before testing according to standard ASTM D 618.
The transmission electron microscopy (TEM) studies of blends were performed on a Tecnai TEM. Digital image acquisition was performed using a Gatan Model 791 side mount camera. Images of representative areas were taken at appropriate magnifications. Samples for TEM observation were prepared by cutting, blocking and facing of samples on a Leica UCT ultra microtome at cryo temperature. Final microtomy of 100 nm sections was performed and the sections were stained with RuO4 for 2 min. Differential scanning calorimetric studies of the pure samples as well as blends were carried out using DSC Q 200 (TA Instruments, USA) at a scan rate of 20 °C/min. The thermal history of the samples were removed by heat-cool-heat procedure.
Results and discussion
Effect of PP-g-MAH on tensile properties of blend
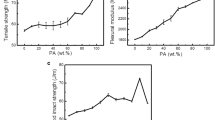
Figure 2a shows progressive decrease in tensile strength (TS) of blends with an increase in PPCP content. This trend is expected due to the fact that properties of PPCP which is inferior to that of N6 would begin to play its role [6]. The increase in TS at yield as a function of PP-g-MAH content is shown in Fig. 2b. With an increase in PP-g-MAH content up to two phb, TS at yield has increased in all the blend compositions and then shows a declining trend. The increase in TS is attributed to interaction of maleated part with amine end group of N6 enabling compatibilization of PPCP with N6.
The theoretical value of a property of a blend can be calculated from the rule of mixtures. If the blend components are completely miscible in each other, the theoretical value of the property will be in close agreement with that of experimental value. The results are presented in Table 3. The experimental value of the TS at yield for blends were less than theoretical value by 18–38% for most compositions (i.e. with and without compatibilizer). However blends with PP-g-MAH compatibilizer always exhibited an increase in TS compared to blends without compatibilizer in agreement with the earlier studies [6].
Elongation at break decreases with increase in PPCP content as shown in Fig. 3. Compatibilized blends with three phb PP-g-MAH exhibits higher elongation.
The increase in mechanical properties of the compatibilized blends is attributed to a stable reaction of PP-g-MAH with N6. This may be explained by analyzing the morphology of the blends. The TEM images of the selected blends are shown in Fig. 4.
In the TEM micrographs (Fig. 4), the darker continuous phase is N6 and the white dispersed domains are PPCP. Un-compatibilized blends 90/10/0 and 60/40/0 shows poor compatibility. With one phb of PP-g-MAH, compatibility appears to have improved slightly in 80/20/1. The smaller domains are seen but interface is still poor. In 70/30/2, the morphology is similar to 80/20/1 but larger white domains are seen due to relatively higher concentration of PPCP. The effect of compatibilizer can be seen in 60/40/0 and 60/40/3. In 60/40/3 blend system the compatibility of N6 with PP is better when compared to 60/40/0.
Effect of PP-g-MAH on the impact strength of blend
The variation of notched impact strength of the blend samples with PPCP composition in shown in Fig. 5. There is no clear pattern for impact strength values of N6/PPCP blend system. Since both N6 and PPCP are crystalline polymers, one would expect notch sensitivity and brittleness under impact condition despite compatibilization.
Accordingly, the N6/PPCP blends show lower notched izod impact strength than the individual components [9]. But at very high concentration of compatibilizer the impact strength value might increase due to the plasticization effect of low molecular weight PP-g-MAH [10]. The improvement also depends on the amount of compatibilizer added [7]. The maximum impact strength is found at 30% PPCP with three phb of compatibilizer.
Effect of PP-g-MAH on the flexural modulus of blend
We can see from Fig. 6 that with the increase in PPCP composition up to 30%, the flexural modulus of the blends increases and then it decreases. This can be attributed to the fact that properties of PPCP which is inferior to that of N6 would begin to play its role [6]. The incorporation of PP-g-MAH increases the flexural modulus of PA6/PP blends significantly. This is due the fact that the maleated part will interact with amine end group of N6, enabling compatibilization of PP and nylon 6. An amount of 30% PP with 3% by weight of PP-g-MAH is adequate [6].
Thermal properties of the blend
Heat deflection temperature (HDT) and Vicat softening temperature (VST) were investigated for the blends according to the ASTM Standard procedure.
It can be seen in Figs. 7 and 8 that the HDT and VST values of the polymer blends are intermediate to those of N6 (71 °C at 820 kPa) and PPCP. (105 °C at 455 kPa). There is no significant effect of compatibilizer on the thermal properties of the blend. At higher concentration of N6 the VST is high and it decreases with the increase in PPCP content. The HDT values show a similar trend.
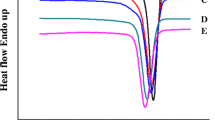
DSC thermograms of N6, PPCP and blends of these polymers are shown in Fig. 9. The results are summarized in Table 4. It is seen that crystalline melting temperature of PPCP decrease marginally with the addition of PP-g-MAH whereas Tm of N6 remains unchanged. The decreased Tm values of PPCP in blends are most likely due to increased interaction of N6 with PPCP in amorphous domain via the block copolymer formation.
Conclusions
PP-g-MAH has a compatibilizing action on N6/PPCP blend. The addition of PP-g-MAH has improved the interfacial adhesion between the two component phases due to low interfacial tension leading to better and finer dispersion and the reduction of the dispersed phase size. At higher concentration of N6, compatibilization with small amount of PPCP is required. Therefore the concentration PP-g-MAH at a level of 3 phb is adequate. The tensile strength value is improved by incorporation of compatibilizer. The impact strength shows intermediate value between that of N6 and PPCP. The optimum properties were observed at 30% PPCP. There is no significant effect of compatibilizer and PPCP on the thermal properties of the blend. At higher concentration of N6 the Vicat softening temperature is high and it decreases with an increase in PPCP concentration. The HDT value shows no regular trend. The morphology of the blend compatibility is markedly improved by the incorporation of PP-g-MAH. The particle size of the N6 and PPCP is decreased and the adhesion improved by addition of compatibilizer.
References
Hippi U (née Anttila) (1995) Novel functionalized polyolefins as compatibilizers in polyolefin/polyamide 6 blends and polyethylene/metal hydroxide composites. PhD thesis, Department of Polymer Technology, Helsinki University of Technology, Espoo
Ide FA, Hasegawa A (1974) Studies on polymer blend of nylon 6 and polypropylene or nylon 6 and polystyrene using the reaction of polymer. J Appl Polym Sci 18(4):963
Utracki LA (1998) Commercial polymer blends. Chapman & Hall, London, pp 230–282
Akkapeddi MK (2002) Commercial polymer blends. In: Utracki LA (ed) Polymer blends handbook, vol 2. Kluwer, Dordrecht, pp 1023–1115
Lindberg KAH, Johansson M, Bertilsson HE (1990) Effects from the addition of a multiblock copolymer to an incompatible blend. Plast Rub Proc Appl 14:195
Pal SK, Kale DD (2000) Effect of processing conditions and properties of PP/nylon 6 blends. J Poly Res 7(2):107–113
Agrawal P, Oliveira SI, Araújo EM, Melo TJA (2007) Effect of different polypropylenes and compatibilizers on the rheological, mechanical and morphological properties of nylon 6/PP blends. J Mater Sci 42(13):5007–5012
Kim MS, Kim BK (2004) Reactive melt blends of thermoplastic polyolefins, MAH-g-PP and nylon 6. Polym Adv Technol 15(7):419–424
Utracki LA (2003) Polymer blend handbook. Springer, New York, pp 1069–1070
Evstatiev O, Chow WS, Karger-Kocsis J, Friedrich K (2003) http://www.dechema.de/data/dechemaneu_/mbf/2262ab
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shashidhara, G.M., Biswas, D., Shubhalaksmi Pai, B. et al. Effect of PP-g-MAH compatibilizer content in polypropylene/nylon-6 blends. Polym. Bull. 63, 147–157 (2009). https://doi.org/10.1007/s00289-009-0074-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-009-0074-7