Abstract
In this work, the rheological, mechanical and morphological properties of nylon 6/polypropylene compatibilized blends were investigated. Two types of polypropylene were used. One with MFI of 40 g/10 min (PP H103) and the other with MFI of 3.5 g/10 min (PP H503). The compatibilizers used were polypropylene grafted with 6% of acrylic acid (PPgAA) and polypropylene grafted with 1% of maleic anhydride (PPgMA). The blends composition was 80/20 (wt%) for the PA6/PP binary blends and 80/10/10(wt%) for the nylon 6/PPgAA/polypropylene and nylon 6/PPgMA/polypropylene ternary blends. Torque rheometry analysis showed that when PPgAA and PPgMA were added to nylon 6/polypropylene blends, there was an increase in the torque, indicating that reactive compatibilization has occurred. There is no influence of the polypropylene MFI on the mechanical properties of the uncompatibilized and compatibilized blends. The impact strength of the blends containing PPgMA were greater than those of the blends containing PPgAA. The blends containing PPgAA are unstable. SEM analysis showed that PPgMA improves considerably the adhesion between PA6/PP phases, leading to good mechanical properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Like most of the polymer blends, blends of nylon 6 (Ny6) and Polypropylene (PP) are immiscible and characterized by high interfacial tension and poor adhesion between the phases, leading to poor mechanical properties. To mitigate this problem, compatibilizers have been used. They may be a block or graft copolymers that will either interact chemically with both phases or will have chemical interaction with one phase and physical interaction with the other [1–11]. The obtained blends may present homogeneuos morphology. It is also possible to control the dimension of the particles, and hence improve the mechanical properties [5]. For Ny6/PP blends, many compatibilizers have been used in the literature. Dagli et al. [6] used polypropylene grafted with acrylic acid (PPgAA) as a compatibilizer. They concluded that acrylic acid can react with the end groups of Ny6 forming either amide groups or anhydride groups. Roeder et al. [7] studied the influence of PPgMA on interface domains of PP/NY 6 blends. They concluded that the addition of small quantities of PPgMA to the incompatible blends of PP/Ny6 increased the homogeneity of Ny6 phase dispersion in the PP matrix with a reduction in the size of domains and copolymer formation in the interface. It is known that maleic anhydride can react with the end groups of Ny6 forming either cyclic imide, amide acid or diamide structures [8]. Tedesco et al. [9] studied the effects of polypropylene functionalized with maleic anhydride (PPgMA) and polypropylene functionalized with glycidil methacrylate (PP-GMA) as compatibilizing agents on polypropylene/nylon 6 blends. They concluded that the effect of PPgMA for PP/nylon 6 blends was more effective than the PP-GMA. Tseng et al. [10] used a poly(oxypropylene)-amide grafted polypropylene (POPgPP) as a compatibilizer for PP/Ny6 blends, focusing on the effect of the hydrogen bonding to the miscibility, morphology, thermal behavior and mechanical properties. They concluded that POPgPP can facilitate the formation of hydrogen bonds with Ny6 and, therefore, the compatibilizing effect. Ohlsson et al. [11] studied the compatibility between Ny6 and PP by the use of maleic anhydride grafted SEBS (MAgSEBS). They concluded that even in high concentrations, unmodified SEBS was found to be a poor compatibilizer, affecting the properties of the matrix and the graft copolymer formed by the reaction between MAgSEBS and Ny6 influenced the morphology, forming an interphase separating Ny6 and PP phases. Besides PP other polymers have been used as a disperse phase. Araújo et al. [12–14] studied the effect of poly(methyl methacrylate-co-maleic anhydride) (MMA-MA) and poly(methyl methacrylate-co-glycidil methacrylate (MMA-GMA) as compatibilizers of the Ny6/ABS blends. The authors observed that the MMA-MA copolymer is an efficient alternative for the reactive compatibilization of the Ny6/ABS system because the mechanical and thermal properties were improved by a higher interaction between the two phases with incorporation of the MMA-MA compatibilizers. On the other hand, the results evidenced that the MMA-GMA copolymer is not very effective for Ny6/ABS system because this compatibilization agent did not promote significant toughening of Ny6/ABS blend. Valenza et al. [15] studied the blends of Nylon 6 and Linear Low Density Polyethylene (LDPE) functionalized with methacrylic acid derivatives. The results indicated that functional groups grafted in the polyethylene chains induce compatibilization. Different effects due to the chemical nature of the grafting groups of the blend structure were also observed. There is no work in the literature about the influence of the melt flow index (MFI) of the disperse phase on the final properties of the blends. The aim of this work is to study the effect of different polypropylenes (different MFI) and compatibilizers on the rheological, mechanical and morphological properties of Ny6/PP blends.
Experimental
Materials
Polypropylene PPH103(MFI = 40 g/10 min) and polypropylene PPH503 (MFI = 3.4 g/10 min) were supplied by Braskem. Polypropylene grafted with 6 wt.% of acrylic acid (MFI = 40 g/10 min) Polybond 1001(PPgAA) and polypropylene grafted with 1 wt.% of Maleic Anhydride (MFI = 110 g/10 min) Polybond 3200 (PPgMA) were supplied by Crompton. Nylon 6, Technyl C 216, natural was supplied by Rhodia.
Blends preparation
Prior to each processing step Ny6, PPgAA and PPgMA were dried under vacuum at 80 °C for 24 h. The mixtures were carried out in a counter-rotating intermeshing twin screw extruder at 240 °C in all zones and 50 rpm. The composition was 80/20 (wt.%) for Ny6/PP binary blends and 80/10/10 (wt.%) for Ny6/PPgAA/PP and Ny6/PPgMA/PP ternary blends.
Torque rheometry
Rheological measurements were performed in a Haake System 90 torque rheometer at 240 °C and 50 rpm during 20 min, under air atmosphere. The compositions of the blends were 80/20 (wt.%) for Ny6/PPgAA and Ny6/PPgMA binary blends and 80/05/15, 80/10/10, 80/15/05 (wt.%) for Ny6/PPgAA/PP and Ny6/PPgMA/PP ternary blends.
Samples preparation
Blends samples for tensile and impact strength tests were obtained by injection molding at 240 °C, using a FLUIDMEC, Model H3040 Apparatus.
Mechanical properties
Tensile tests were carried out in an Universal Testing Machine, LR 10KN, Lloyd Instruments, according to ASTM-D 638 at a cross head speed of 50 mm/min. Izod impact tests were performed according to ASTM-D 256 in notched samples at room temperature using a CEAST RESIL 5,5 machine and hammer of 2,75 J. For each test, 10 samples were used.
Scanning electron microscopy (SEM)
Fracture surfaces of the blends subjected to impact strength tests were coated with gold and analyzed by Scanning Electron Microscopy, using a SHIMADZU, SS X550 Super Scan Apparatus. The voltage used was 20.0 kV.
Results and discussion
Torque rheometry
Figure 1 shows the torque curves of the blends containing PPH103 and PPH503. According to Fig. 1a and b, when the compatibilizer PPgAA is present in Ny6/PPH103 and Ny6/PPH503 blends there is an increase in the torque. This is an evidence of graft reactions of Ny6–PPgAA copolymer, with an increase in the amide groups concentration, as reported by Dagli et al. [6]. After 10 min there is a slight decrease in the torque. According to Méier-Haak et al. [16], this happens because the acid groups of PPgAA lead to a degradation of the Ny6 chains reducing its molecular weight. In addition, at temperatures higher than 200 °C, the reaction between acids and polyamides is very fast. When PPgMA is present in the Ny6/PP blends, there is an increase in the torque, indicating that Ny6-PPgMA copolymer, containing imide groups, formation has occurred. After 10 min the torque remained constant for this system, i.e., it seems that the reaction is more stable. PPgMA copolymer seems to suitable for compatibilization since it can react only with one phase and be miscible with the other phase, being located in the interface between the two phases. This process reduces the interfacial tension between the blend components and delays the coalescence of the dispersed phase stabilizing the morphology. In both cases, the increase in the torque is an indicative of reactive compatibilization. PPgAA is more reactive than PPgMA. However, Ny6/PP blends with PPgAA are unstable.
Figure 2a and b show the torque rheometry curves of PA6/PPgAA/PPH103 and PA6/PPgAA/PPH503 respectively, with different PPgAA content (wt.%). The amounts of PPgAA used were 5, 10, 15 and 20 (wt.%). PPgAA was inserted after 5 min, when Nylon 6 and PP were already melt mixed, and it is indicated by an arrow (Fig. 2a, b). The amounts of Ny6 were kept constant, so that the number of amide end groups would be the same. It may be observed that increasing the PPgAA concentration, the torque of the blends also increases. This happens because increasing PPgAA concentration, there is more chances of reaction between the acid groups of PPgAA and the amino end groups of Ny6. It can also be observed that the higher the PPgAA concentration, the higher is the blend degradation. As discussed before, the acid groups of PPgAA lead to the degradation of Ny6 chains [16]. Regardless of Polypropylene melt flow index (MFI) the effect of PPgAA was the same. Figure 2c and d show the torque curves of PA6/PPgMA/PP H103 and PA6/PPgMA/PPH503 blends respectively, with different PPgMA content. The amounts of PPgMA used were 5, 10, 15 and 20 (wt.%). The amounts of Ny6 were kept constant. PPgMA was inserted after 5 min, when Ny6 and PP were melt-mixed and it is indicated by an arrow (Fig. 2c, d). It may be observed that the torque of the blends increased with the increase of PPgMA concentration. However this increase was less than that of the blends containing PPgAA, due to the fact that PPgAA has higher MFI, increasing the chances of reaction, and lower diffusion. There is almost no difference between the torque of the blend containing 15% of PPgMA and that of the blend containing 20% of PPgMA. As occurred with the blends containing PPgAA, the effect of PPgMA on the blends was the same, regardless of polypropylene MFI.
Mechanical properties and morphology
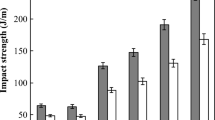
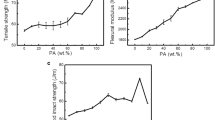
Table 1 shows the mechanical properties of Ny6, PP and their blends (uncompatibilized and compatibilized). It may be observed that the mechanical properties of the uncompatibilized blends were similar regardless of polypropylene MFI, indicating that there is no influence of the polypropylene melt flow index on the mechanical properties. Among the compatibilized blends, the mechanical properties of Ny6/PPgAA/PPH103 and Ny6/PPgAA/PPH503 blend were similar. Also, the mechanical properties of Ny6/PPgMA and Ny6/PPgMA/PPH503 blends were similar, indicating that there is no influence of polypropylene MFI. This is not the case for the blends molded by compression, as reported in previous works [17, 18]. The impact strength of the uncompatibilized and compatibilized blends were greater than those of the neat polymers. Among the compatibilized blends, the impact strength of the blends containing PPgMA was greater than those of the blends containing PPgAA. This is attributed to a stable reaction of PPgMA with nylon 6, and may be explained by analyzing the morphology. Figure 3a and b show the morphology of Ny6/PPH103 and Ny6/PPH503 blends, respectively. Ny6 clearly forms a continuous phase while PP forms large domains. It can be observed that the dispersed phase is debonded, leaving a dark region in the interface. Most of the dispersed phases were pulled out from the matrix during deformation. Hence, a failure has occurred at the Ny6/PP interface due to poor adhesion and high interfacial tension. For this reason, the mechanical properties were not sufficiently increased. When PPgAA was added to Ny6/PP blends (Fig. 3c and d), the particles average size decreased, but there was only a slight improvement in the adhesion between the phases. According to Kazmierczak et al. [19], the interfaces between blends components are weak element of the blends even in the presence of compatibilizing action of PPgAA. When PPgMA was added to Ny6/PP blends (Fig. 3e, f), there was a considerable decrease in the particles average size and improvement of the adhesion between Ny6/PP phases, leading to good mechanical properties. The presence of the PPgMA compatibilizer appears to restructure the PP domains. The Ny6/PPgMA/PPH103 blend presents a significant increase in the mechanical properties under impact.
Conclusions
The effect of different polypropylenes and compatibilizers on the rheological, mechanical and morphological properties of Nylon 6/PP blends was studied. Torque rheometry analysis showed that when PPgAA and PPgMA are added to Ny6/PP blends, there is an increase in the torque, which is an indicative of reactive compatibilization. The higher the PPgAA concentration, the higher is the degradation of the blends. There is no influence of the polypropylene MFI on the mechanical properties of uncompatibilized and compatibilized blends. The impact strength of uncompatibilized and compatibilized blends was greater than those of neat polymers. Among the compatibilized blends, the impact strength of the blends containing PPgMA was greater than those of the blends containing PPgAA, due to higher interaction with nylon 6 and polypropylene phases. Morphology analysis indicated that when PPgAA and PPgMA were added to Ny6/PP blends, there was a decrease in the particles average size and an improvement in the adhesion between Ny6/PP phases. The blends containing PPgAA are unstable, since the acid groups lead to a degradation of Ny6 chains. PPgMA was more effective than PPgAA indicating that the PPgMA copolymer is an efficient alternative for the reactive compatibilization of the Ny6/PP system.
References
Ebeling T, Norek S, Hasan A, Hiltner A, Baer E (1999) J Appl Polym Sci 71:1461
Zhahoui L, Zhang X, Tasaka S, Inagaki N (2001) Mater Lett 48:81
Piglowski J, Gancarz I, Wlazlak M, Kammer HW (2000) Polymer 41:6813
Campoy I, Arribas JM, Zaporta MAM, Marco C, Gómez MA, Fatou JG (1995) Eur Polym J 05:475
Sacchi A, Di Landro L, Pegoraro M, Severine F (2004) Eur Polym J 40:1705
Dagli SS, Xanthos M, Biesenberger JA (1994) Polym Eng Sci 34:1720
Roeder J, Oliveira RVB, Gonçalves MC, Soldi V, Pires ATN (2002) Polym Test 21:815
Xiaomin Z, Gang L, Dongmei W, Zhihui Y, Jinghua Y (1998) Polymer 39:15
Tedesco A, Barbosa RV, Nachtigall SMB, Mauler RS (2002) Polym Test 21:11
Tseng FP, Lin JJ, Tseng CR, Chang FC (2001) Polymer 42:713
Ohsson B, Hassander H, Törnell B (1998) Polymer 39:6705
Araújo EM, Hage E Jr, Carvalho AJF (2003) J Mater Sci 38:3515
Araújo EM, Hage E Jr, Carvalho AJF (2004) J Mater Sci 39:1173
Araújo EM, Hage E Jr, Carvalho AJF (2005) J Mater Sci 40:4239
Valenza A, Geuskens G, Spadaro G (1997) Eur Polym J 33:957
Meier-Haack J, Vako M, Lunkwitz K, Bleha M (2004) Desalination 163:215
Araújo WD, Araújo EM, Melo TJA (2004) In: Proceedings of Americas Regional Meeting. Florianópolis, November 2004, p 1
Agrawal P, Araújo WD, Araújo EM, Mélo TJA (2005) In: Proceedings of XX Brazilian Society of Microanalysis and Microscopy Congress. Águas de Lindóia, August 2005, p 1
Karmierczak T, Krasnikova NP, Galeski A, Pracella M (2000) Macro Symp 149:185
Acknowledgements
The authors would like to thank Crompton for the donation of the compatibilizers, to Rhodia for the donation of Nylon 6, CAPES, CNPq, PPG-EQ/UFCG and PPG-CEMat/UFCG for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agrawal, P., Oliveira, S.I., Araújo, E.M. et al. Effect of different polypropylenes and compatibilizers on the rheological, mechanical and morphological properties of nylon 6/PP blends. J Mater Sci 42, 5007–5012 (2007). https://doi.org/10.1007/s10853-006-0514-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0514-9