Abstract
The synthesis of a novel complex system designed for colon-targeting drug delivery was reported. The complex was prepared by dialdehyde konjac glucomannan and adipic dihydrazides to form steady Schiff base, and crosslinking with 5-aminosalicylic acid (5-ASA) through glutaraldehyde as a cross-linking agent. The structure was characterized by Fourier transform infrared (FTIR) spectroscopy, 13C NMR, wide angle X-ray diffraction (WAXRD) and thermogravimetric analysis. In vitro release of 5-ASA from the complex showed that the total released 5-ASA after 24 h in buffer solution at pH 1.2, 6.8, and 7.4 were 4, 59, and 21%, respectively. The release rate of 5-ASA can be controlled by tuning the pH value more effectively. The results indicated that the novel pH-sensitive complex could be potentially useful for colon-targeting drug delivery system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oral colon-targeting drug delivery systems (OCTDDS) have been paid much attention during the past two decades. Drugs in OCTDDS can offer several advantages over the conventional drugs delivery systems (CDDS), including longer retention time in the body [1], lower noxious side effects, enhancing curative effect, and avoiding drugs degradation by the stomach and small intestine enzyme to improve the bioavailability of drugs such as protein-, peptide- based drugs, bacterin and chronic enteritis [2]. There are several types of controlled drug delivery systems, such as prodrugs [3], pH-sensitive [4], time-dependent [5], and microflora-activated [6] systems, based on cellulose, impulse stuff-bursa, methylcellulose acetate phthalate, azo-polymer, and polysaccharides. Although prodrugs can provide site-specific drug delivery, they are new chemical entities and their detailed toxicological studies need to be more investigated [7].
Polysaccharides playing an important role in OCTDDs due to their abundant, biocompatibility, biodegradation, nontoxicity and easily modified ability are suggested their applications in OCTDDs [8]. Chitosan [9], cyclodextrins [10], guar gum [11], alginate [12], and cellulose derivatives [13] have been studied for their potential as colon-specific drug carrier systems [8]. Recently, it was reported that KGM could hold potentialities in drug carrier delivery for its nonimmunogenicity and bioadhesive properties [14, 15]. However, all of above polysaccharides are only used as coating materials, by which drug is inevitable dissolved partially in stomach by the high pH value, when passing through stomach, and would reduce curative effect [16].
In this paper, a novel pH-sensitive complex was designed based on dialdehyde konjac glucomannan (DAKGM) prepared by konjac glucomannan using sodium periodate as oxidant. The structure and properties of the resulted products were characterized by FTIR, 13C NMR, thermogravimetric analysis (TGA), wide angle X-ray diffraction (WAXRD), and pH dependent evaluation. The release behavior of the system was investigated by using 5-aminosalicylic acid (5-ASA) as a model drug.
Experimental
Materials
Konjac glucomannan powder (KGM, Mw = 980,000, Mw/Mn = 1.7, a donation from Multi-Ring Health Products, Ltd, Beihai, China). Adipic dihydrazide (ADH) and 5-ASA were used without any further purification. All other chemicals used in this work were from commercial sources and of analytical grade.
Instruments
FTIR spectra were recorded using a Vector 33 spectrometer (Germany). KGM samples were mixed with KBr and pressed to a plate for measurement. 13C NMR measurement was conducted on Avance AV 400 spectrometer (Switzerland), at room temperature, using CF3COOD as solvent. TGA analysis was conducted with Netzsch 209F1 instrument (Germany) under a nitrogen atmosphere with the sample weight was about 8–10 mg. The scan of TG was carried out at a heating rate of 20 °C min−1 from 30 to 800 °C. X-ray diffraction of the films was analyzed using D-MAX 2200 VPC (Japan) diffractometer equipped with a scan rate of 4° min−1. The diffraction angle was ranging from 2θ = 3° to 2θ = 60°.
Preparation of DAKGM-ADH-ASA complex
Dialdehyde konjac glucomannan (DAKGM) was prepared as described in brief: 2 g (0.012 mol) of KGM was dissolved in 0.018 M aqueous sodium periodate (100 ml). The mixture was reacted for 9 h at room temperature in the dark. The reaction mixture was dialyzed in distilled water for 3 days till the dialysate was free from iodate (checked with silver nitrate). The dried samples were stored in a desiccator over silica gel for further use.
Preparation of DAKGM-ADH: DAKGM and Adipic dihydrazides (ADH) (mole rate 1:1 mol) were mixed with appropriate distilled water. The reactants were stirring for 2 h at 40 °C. The white precipitate was washed with distilled water, and then vacuum evaporation at 50 °C.
Preparation of DAKGM-ADH-ASA: DAKGM-ADH and 5-ASA were dispersed with ethanol in a three-necked round-bottomed flask and heated to 50 °C. Glutaraldehyde (glutaraldehyde:ethanol, 1:2 v/v) was added dropwise to the ethanol solution, while stirring with a magnetic stirrer. After 4 h, the yellow powder was gained and rinsed with distilled water and acetone alternatively. The resulting yellow powder was dispersed in ethanol and deoxidized by sodium borohydride. The crude sample was purified by repeated washing with distilled water until neutral, and then was extracted with acetone for 48 h to remove unreacted components and soluble fragments or byproducts. Finally, the ultimate product (K1) was dried at 60 °C for 24 h and stored in an airtight desiccator for further use.
Preparation of KGM–ASA blend
KGM (0.012 mol) was dissolved in distilled water under stirring for 8 h to form 1% (w/v) homogeneous solution at room temperature. Amount of ASA (0.006 mol) was added to the solution and continuous stirred for 8 h. The resulting mixture was high-speed centrifugal at 10,000 rpm for the removal of the residual water. The sedimentation (K2) was freeze-dried at −60 °C for 24 h.
In vitro drug release study
5-Aminosalicylic acid release from the complexes in a 100-mL flask at different pH (pH = 1.0, 6.8, 7.4, respectively), in vitro was performed in a thermostatic rotary shaker at shaking speed of 100 rpm and temperature at 37 °C. Samples at appropriate intervals, 4 mL were withdrawn and equal volume of same dissolution medium was added to maintain a constant volume. The amount of 5-ASA cumulative released from the matrix was determined with UV–visible spectrophotometer at 299 nm (Hitachi UV-3010, Japan) and calculated the drug release in complex. The percentage of cumulative drug release efficiency is determined from the following equation:
where L and L 0 are the content of 5-ASA released within a known content of complexes base on experiment data and calculation of concentration, respectively.
Results and discussion
Synthesis of the DAKGM-ADH-ASA complex
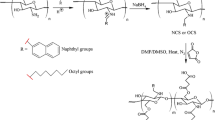
DAKGM-ADH-ASA complex was designed by KGM oxidated to DAKGM via sodium periodate as oxidant, then DAKGM with ADH formed to konjac glucomannan Schiff base, finally crosslinked with 5-ASA by glutaraldehyde crosslinker. The complex formation was illustrated in Scheme 1.
FTIR characterization of KGM samples
Figure 1 show FTIR spectra of KGM (a), DAKGM (b), DAKGM-ADH-ASA complex (c, K1), KGM–ASA blend (d, K2) and pure 5-ASA (e). In the IR spectrum of KGM (curve a), the broad peak at 3,398 cm−1 is attributed to the stretching of –OH groups. The peak at 2,889 cm−1 indicated –C–H stretching vibration. The absorption band of the carbonyl of acetyl groups is at 1,732 cm−1. The intense peak at 1,645 cm−1 is due to the in-plane deformation of the water molecule [17]. However, the oxidation leads to the appearance of two characteristic bands of DAKGM (curve b) around 1,733 and 888 cm−1 region. The former is also ascribed to the aldehyde symmetric vibrational band (carbonyl), which changed from a small shoulder in pure KGM to a distinct peak in DAKGM. The latter can be assigned to the hemiacetal structure between the aldehyde groups and neighboring hydroxyl groups [18]. The chemical interaction is reflected by changes in characteristic spectra peaks as follow in curve c (K1 complex). The peaks at 3,419 cm−1 is owed to symmetrical stretching of –N–H groups. This coupling reaction is followed by the disappearance of the aldehyde symmetric vibrational band (1,733 cm−1) and the appearance of a carbonyl band for the hydrazide at 1,665 cm−1, which was the absorption of carboxyl stretching of benzene [19]. The peaks near 1,571, 1,448 cm−1 were attributed to the symmetrical stretching of –C=C– and stretching vibrations absorption of –CH groups of benzene, respectively. Compared with the K1 complex, the results from the FTIR spectra of pure 5-ASA (curve e) is similar to that of the K2 blend (d), which was showed that it was physical blend, low compatibility between KGM and 5-ASA.
13C NMR spectrum of DAKGM-ADH-ASA complex
Figure 2 shows the 13C NMR spectrum of DAKGM-ADH-ASA complex in CF3COOD. The chemical shift of C atom in the copolymer was as follows: (a) 60–80 ppm (CH2 group of KGM), (b) 160 ppm (NH–CO), (c) 24–38 ppm ((CH2)4), (d) 168 ppm (CO–NH), (e) 24–38 ppm ((CH2)2), (f) 14 ppm (CH2), (e) 24–38 ppm ((CH2)2), and (g) 184 ppm (COOH). The FTIR and 13C NMR spectra confirmed that DAKGM was successfully crosslinked with ADH and 5-ASA.
Thermogravimetric analysis and X-ray diffraction analysis
Thermogravimetric analysis method is used to thermally characterize the complex in comparison with the pure polysaccharide. The thermograms recorded for KGM, DAKGM, DAKGM-ADH-ASA complex, KGM–ASA blend and 5-ASA are shown in Fig. 3. In the thermogram of KGM (curve a), two decomposition steps could be observed, the first occurs in the range of 50–120 °C, which is due to the loss of water. The second occurs in the range of 225–350 °C showing 65% weight loss could be attributed to the degradation of the saccharide structure of the molecule, including the dehydration of saccharide rings and decomposition of the acetylated units of KGM. In the dialdehyde konjac glucomannan (DAKGM) sample, the TGA curve of DAKGM (curve b) has three distinct stages, and shows three maximum peaks at 91, 204 and 290 °C. Similarly, the slight weight loss of DAKGM in the first stage is due to the loss of water. In the second stage, the weight loss starts at 162 °C and continues to 228 °C with a 21% weight loss due to the degradation of dialdehyde bond of DAKGM. The third stage of weight loss starts at 232 °C and it continues up to 369 °C indicating 52% weight loss because crosslinking of KGM exists in these remnants. In the thermogram of pure 5-ASA (curve e), it is showed that about 35% weight loss takes place at 170 °C for 5-ASA, and it can degrade completely at 235 °C. However, in the DAKGM-ADH-ASA complex sample, a residual weight of 40% was observed at 369 °C. The sample cannot degrade completely at 600 °C. Therefore, it was indicated that the chemical crosslinking of DAKGM and 5-ASA may provide feasible route for the improvement of the thermal stability. KGM–ASA blend shows a two step mechanism for thermal degradation. The first step weight loss is related to the decomposition of 5-ASA, occurs at about 170 °C. The second occurs within the range of 225–350 °C about 65% weight loss due to the degradation of the saccharide structure of the KGM molecule. The results in this work are somewhat similar to the previous reports of pure 5-ASA and pure KGM, respectively. It is because the two components (5-ASA, KGM) in blends have weak intermolecular interaction, namely, degrade independently, and then one has expected residual weight percent based on the additive rule.
To investigate the physical state of the drug within KGM-based complex, WAXRD patterns on the samples are carried out as shown in Fig. 4. Amorphous KGM does not show any diffraction, but exhibits a weak broad peak at 2θ of around 20°. Similarly, K1 complex exhibit a broad peak at 2θ of about 20°. These results showed that K1 complex is not exhibited any characteristic crystalline peak, indicating that they are amorphous. This could be a consequence of strong covalent bond interaction in the K1 complex. However, in the WAXRD of pure 5-ASA, some typical peaks at 2θ = 7.12, 12.92, 14.36, 16.90, 21.28, and 26.46° are observed. Upon mixing with KGM, these peaks intensity of K2 blend become weaker, so it is generalized that the interactions between KGM and 5-ASA make the blend partially miscible and decrease the crystallinity. These results of the WAXRD analysis are in agreement with results of IR and TG analysis.
In vitro release study
In vitro release studies were performed under experimental conditions that simulate the gastrointestinal fluids. Figures 5–7 show the drug-release behavior of 5-ASA in the HCl solution of pH 1.0 (simulated gastric fluid) and phosphate buffer solutions of pH 6.8 (simulated intestinal fluid) and 7.4 (simulated colon fluid), respectively. From Fig. 5, it can be seen that an initial rapid release of about 93% in the first 1 h of K2 blend. However, the release amount is quite low and remains essentially constant (3%) of the K1 complex. Under both conditions at pH 6.8 and pH 7.4, the amount of 5-ASA release is similar for the K2 blend, where sustained about 55–60% of the drug was released after 24 h (Figs. 6, 7). A large burst of drug release is mainly attributed to the presence of the drug at the surface of the blend.
Figure 8 shows the drug-release behavior of 5-ASA loaded K1 complex in different pH (1.0, 6.8 and 7.4). The maximum content of 5-ASA released from K1 complex reached 17% at 12 h in buffer solution of pH 7.4 and 56% in buffer solution of pH 6.8. On the contrary, at pH 1.0, the release amount is quite low and remains essentially constant of about 4%. It is very interesting to note that there is a steady release with stepwise increasing from 14 to 56 wt% during 12 h when at pH 6.8. This result indicates that drug delivery in our system at a mildly acidic pH value such as pH 6.8 may be long term and continuous, which is helpful for maintaining the concentration of drugs in the certain sites of the body within the optimum range.
Further, the release mechanism of 5-ASA from K1 and K2 blends was investigated by the Peppas equation [20, 21]:
where M t /M ∞ is the fraction of drug released, K is a constant depended on the system, t is the release time and n is the diffusion exponent, indicating the type of drug release mechanism. If n approaches to 0.45, the release mechanism could be Fickian diffusion. If n approaches to 0.89, the release mechanism could be zero order, and on the other hand if 0.45 < n < 0.89 non-Fickian transport (a synergistic effect of drug diffusion and matrices erosion) could be attained.
Table 1 shows the diffusion exponent n for K1 complex and K2 blend at different pH values. The results showed that the release of 5-ASA from K2 blend could be a Fickian (diffusion) mechanism being observed for the release at pH1.0, 6.8 and 7.4, respectively. It is correlative to several systems containing polysaccharides show release by diffusion mechanism [22, 23]. However, the drug release mechanism of 5-ASA from K1 complex could be independent on the pH value. Release of 5-ASA from the K1 complex shows release mechanisms via Fickian diffusion phenomena at pH1.0. While the release from K1 complex is mainly occurred by non-Fickian mechanism and the diffusion is relaxation controlled at 6.8 and 7.4, respectively.
The mentioned results indicate that covalent bond link is an important factor within molecular interaction and the level of 5-ASA release from this system is much stronger dependent on the pH value than of K2 blend.
Conclusion
A novel pH-sensitivity complex was synthesized by DAKGM crosslinking with 5-ASA by the crosslinker of glutaraldehyde, and the structure characteristics and thermal characteristics were investigated. 5-ASA in vitro release behavior was also studied. The results showed that the DAKGM-ADH-ASA complex was sensitive to pH. 5-ASA was released from the complex at pH 6.8 and 7.4 much higher than at pH 1.0. This investigation of konjac glucomannan-based, pH-responsive complex indicated that the rate of drug release can be modulated by the appropriate chemical modification of the crosslinking densities. The DAKGM-ADH-ASA complex could be a promising oral drug delivery to the intestinal environment.
References
Rösler A, Vandermeulen GWM, Klok HA (2001) Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv Drug Deliv Rev 53:95
Sinha VR, Kumria R (2003) Microbially triggered drug delivery to the colon. Eur J Pharm Sci 18:3
Dhaneshwar SS, Gairola N, Kandpal M, Vadnerkara G, Lokesh B (2007) Colon-specific, mutual azo prodrug of 5-aminosalicylic acid with L-tryptophan: synthesis, kinetic studies and evaluation of its mitigating effect in trinitrobenzenesulfonic acid-induced colitis in rats. Bioorg Med Chem 15:4903
Yin HQ, Lee ES, Kim D, Lee KH, Oh KT, Bae Y (2008) Physicochemical characteristics of pH-sensitive poly(L-Histidine)-b-poly(ethylene glycol)/poly(L-Lactide)-b-poly(ethylene glycol) mixed micelles. J Control Release 126:130
Prokop A, Kozlov E, Carlesso G, Davidson JM (2002) Hydrogel-based colloidal polymeric system for protein and drug delivery: physical and chemical characterization, permeability control and applications. Adv Polym Sci 160:119
Yang LB (2008) Biorelevant dissolution testing of colon-specific delivery systems activated by colonic microflora. J Control Release 125:77
Dhareshwar SS, Stella VJ (2008) Your prodrug releases formaldehyde: should you be concerned? No! J Pharm Sci 97:4184
Sinha VR, Kumria R (2001) Polysaccharides in colon-specific drug delivery. Int J Pharm 224:19
Nunthanid J, Huanbutta K, Luangtana-anan M, Sriamornsak P, Limmatvapirat S, Puttipipatkhachorn S (2008) Development of time-, pH-, and enzyme- controlled colonic drug delivery using spray- dried chitosan acetate and hydroxypropyl methylcellulose. Eur J Pharm Biopharm 68:253
Salmaso S, Semenzato A, Bersani S, Matricardi P, Rossi F, Caliceti P (2007) Dyclodextrin/PEG based hydrogels for multi-drug delivery. J Pharm 345:42
Huang YH, Yu HQ, Xiao CB (2007) pH-sensitive cationic guar gum/poly (acrylic acid) polyelectrolyte hydrogels: swelling and in vitro drug release. Carbohydr Polym 69:774
Ramesh Babu V, Krishna Rao KSV, Sairam M, Vijaya Kumar Naidu B, Hosamani MK, Aminabhavi MT (2006) pH sensitive interpenetrating network microgels of sodium alginate-acrylic acid for the controlled release of ibuprofen. J Appl Polym Sci 99:2671
Jantarat C, Tangthong N, Songkro S, Gary P, Martin GP, Suedee R (2008) S-propranolol imprinted polymer nanoparticle-on-microsphere composite porous cellulose membrane for the enantioselectively controlled delivery of racemic propranolol. Int J Pharm 349:212
Chen LG, Liu ZL, Zhuo RX (2005) Synthesis and properties of degradable hydrogels of konjac glucomannan grafted acrylic acid for colon-specific drug delivery. Polymer 46:6274
Du J, Dai J, Liu JL, Dankovich T (2006) Novel pH-sensitive polyelectrolyte carboxymethyl konjac glucomannan–chitosan beads as drug carriers. React Funct Polym 66:1055
Yang LB, Chu JS, Joseph AF (2002) Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int J Pharm 235:1
Zhang H, Yoshimura M, Nishinari K, Williams MAK, Foster TJ, Norton IT (2001) Gelation behaviour of konjac glucomannan with different molecular weights. Biopolymer 59:38
Kim UJ, Kuga S, Wada M, Okano T, Kondo T (2000) Periodate oxidation of crystalline cellulose. Biomacromolecules 1:1488
Lee KY, Bouhadir KH, Mooney DJ (2000) Degradation behavior of covalently cross-linked poly (aldehyde guluronate) hydrogels. Macromolecules 33:97
Sinclair GW, Peppas NA (1984) Analysis of non-Fickian transport in polymers using simplified exponential expression. J Membr Sci 17:329
Peppas NA, Ritger PL (1987) A simple equation for description of solute desorption. II. Fickian and anomalous desorption from swellable device. J Control Release 5:37
Saettone MF, Torraca MT, Pagano A, Giannaccini B, Rodriguez L, Cini M (1992) Controlled release of pilocarpine from coated polymeric ophthalmic inserts prepared by extrusion. Int J Pharm 86:159
Kumaresh SS, Tejraj MA (2002) Water transport and drug release study from cross-linked polyacrylamide grafted guar gum hydrogel microspheres for the controlled release application. Eur J Pharm Biopharm 53:87
Acknowledgments
The authors are grateful for the financial support of National Natural Science Foundation of China (Grant No. 50673104)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, DY., Li, GJ., Liao, ZF. et al. Preparation and in vitro controlled release behavior of a novel pH-sensitive drug carrier for colon delivery. Polym. Bull. 62, 183–193 (2009). https://doi.org/10.1007/s00289-008-0012-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-008-0012-0