Abstract
Pathogen evolution towards the largest basic reproductive number,\(\mathcal R _0\), has been observed in many theoretical models, but this conclusion does not hold universally. Previous studies of host–pathogen systems have defined general conditions under which \(\mathcal R _0\) maximization occurs in terms of \(\mathcal R _0\) itself. However, it is unclear what constraints these conditions impose on the functional forms of pathogen related processes (e.g. transmission, recover, or mortality) and how those constraints relate to the characteristics of natural systems. Here we focus on well-mixed SIR-type host–pathogen systems and, via a synthesis of results from the literature, we present a set of sufficient mathematical conditions under which evolution maximizes \(\mathcal R _0\). Our conditions are in terms of the functional responses of the system and yield three general biological constraints on when \(\mathcal R _0\) maximization will occur. First, there are no genotype-by-environment interactions. Second, the pathogen utilizes a single transmission pathway (i.e. either horizontal, vertical, or vector transmission). Third, when mortality is density dependent: (i) there is a single infectious class that individuals cannot recover from, (ii) mortality in the infectious class is entirely density dependent, and (iii) the rates of recovery, infection progression, and mortality in the exposed classes are independent of the pathogen trait. We discuss how this approach identifies the biological mechanisms that increase the dimension of the environmental feedback and prevent \(\mathcal R _0\) maximization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The evolution of pathogen virulence has been an important area of study for the past two decades (Bull 1994; Ebert and Herre 1996; Frank 1996; Levin 1996; Brown et al. 2006; Alizon et al. 2009). One of the hypotheses put forth by theoretical studies about virulence evolution is the trade-off between virulence and other life history parameters of the pathogen (Bull 1994; Frank 1996; Levin 1996). Many theoretical studies have explored the evolutionary consequences of trade-offs in compartmental susceptible-infectious-recovered (SIR)-type host–pathogen models where the pathogen is horizontally transmitted, only a single strain of the pathogen can infect any given host, and selection is frequency independent (Anderson and May 1982; Bremermann and Thieme 1989; Thieme 2007; Metz et al. 2008; Gyllenberg et al. 2011). A classic result from the earliest of those studies is that under the above conditions evolution maximizes the basic reproductive number (Anderson and May 1982; Bremermann and Thieme 1989).
The basic reproductive number, \(\mathcal R _0\), is the number of secondary cases that arise from a single infected individual in a completely susceptible host population. When \(\mathcal R _0>1\), the pathogen is able to invade a susceptible population, resulting in an epidemic. When \(\mathcal R _0<1\), the pathogen is unable to invade and dies out. Larger values of \(\mathcal R _0\) imply that the pathogen is better able to invade a completely susceptible population. Thus, in systems where evolution maximizes \(\mathcal R _0\), the pathogen strain that is best able to invade a completely susceptible population is also the strain that is unable to be invaded by other strains at low density.
For well-mixed deterministic SIR-type systems where a host can only be infected by a single pathogen strain, \(\mathcal R _0\) maximization has been shown to occur in particular models (Dieckmann et al. 2002; Boots and Sasaki 2003; Thieme 2007; Medlock et al. 2009). However, \(\mathcal R _0\) maximization does not occur universally in that class of systems (Nowak 1991; Dieckmann et al. 2002; Thieme 2007; Metz et al. 2008; Gyllenberg et al. 2011). To help identify when pathogen evolution maximizes \(\mathcal R _0\), previous studies have derived sufficient conditions for the evolutionary maximization of \(\mathcal R _0\) in terms of \(\mathcal R _0\) itself (Mylius and Diekmann 1995; Metz et al. 2008; Gyllenberg et al. 2011). An important conclusion from these studies is that \(\mathcal R _0\) maximization, and optimization in general, requires the environmental feedbacks of the system to be effectively one dimensional (Mylius and Diekmann 1995; Metz et al. 2008).
Despite this existing body of general mathematical theory and the collection of specific studies demonstrating when \(\mathcal R _0\) maximization does and does not occur, it remains unclear under what general biological conditions evolution maximizes \(\mathcal R _0\) in SIR-type systems. Furthermore, it is unclear what constraints the \(\mathcal R _0\) maximization theory in Mylius and Diekmann (1995) and Metz et al. (2008) imposes on the functional forms used to model pathogen-related processes like transmission, progression of the infection, recovery, and mortality. Understanding these constraints on the functional forms is important for three reasons. First, such constraints allow one to identify when evolution maximizes \(\mathcal R _0\) simply from the structure of the equations of the dynamical system. Depending on how complex a model is, this can be simpler than determining if \(\mathcal R _0\) satisfies monotonicity conditions or computing invasability plots. Second, there is a clear mechanistic link between the functional forms used in theoretical models and particular characteristics of biological processes in natural systems. Thus, conditions on functional forms allows one to link particular biological phenomena to general theory. Finally, linking the functional form constraints to biological processes can identify what characteristics of natural systems inhibit and promote \(\mathcal R _0\) maximization. For example, constraints on functional forms can be used to identify what functional forms (and hence what biological processes) increase the dimension of environmental feedbacks. This in turn helps identify what kinds of systems are likely to exhibit \(\mathcal R _0\) maximization and evaluate how applicable results based on \(\mathcal R _0\) maximization theory are to natural systems.
This study presents a synthesis of results from the literature that results in a set of sufficient mathematical conditions under which evolution maximizes \(\mathcal R _0\) in SIR-type systems. We focus on well-mixed SIR-type systems where a host can only be infected by a single pathogen strain and consider both frequency independent and frequency dependent selection. The analysis is based on the next generation technique for computing \(\mathcal R _0\) in van den Driessche and Watmough (2002) and the sufficient condition for \(\mathcal R _0\) maximization in Mylius and Diekmann (1995). This approach yields conditions in terms of the functional forms used to model epidemiological processes and we directly relate these conditions to the characteristics of natural systems. We note that other optimization principles can arise in theoretical models (Metz et al. 2008; Gyllenberg et al. 2011), but in this study we will only focus on \(\mathcal R _0\) maximization.
The biological interpretation of our conditions yields three general biological conditions under which evolution maximizes \(\mathcal R _0\) when selection is frequency independent. First, there are no genotype-by-density or genotype-by-environment interactions. That is, the effects of the host class densities and the pathogen trait on system processes are independent. Second, the pathogen utilizes a single route of transmission (e.g. horizontal, vertical, or vector transmission). Third, if mortality is density dependent then: (i) there is a single infectious class that individuals cannot recover from, (ii) mortality in the infectious class is entirely density dependent, and (iii) the rates of recovery, infection progression, and mortality in the exposed classes are independent of the pathogen trait. The additional condition that arises when selection is frequency dependent is that there can be no genotype-by-genotype interactions between pathogen strains.
In the following we first review the theory underlying our results, focusing on the case where selection is frequency independent. We then apply the theory to epidemiological systems with a single infectious class and systems with one exposed and one infectious class. Next we present a summary of the results for more complicated systems involving multiple exposed and multiple infectious classes and vector transmission. Within each of the above cases we analyze specific examples. We then extend our conditions to include systems where selection is frequency dependent. We conclude with a discussion of how our conditions for \(\mathcal R _0\) maximization relate to the dimension of the environmental feedbacks in the systems.
2 Methods
2.1 Model
Here we introduce a general model for a direct transmission host–pathogen system with a single host species. A review of the following theory for more general systems (e.g., vector-borne pathogens) is included in Appendix A.
We divide the host population into susceptible \((S)\), infected (\(C_j\) for \(1 \le j \le n\)), and recovered \((R)\) classes. We assume that there is a single susceptible class and a single recovered class, but see Appendix B for two examples with multiple susceptible classes. Note that an infected class, \(C_j\), could be either an infectious class \((I)\) or an exposed class \((E)\), where exposed individuals are infected but not infectious. We assume all newly infected individuals enter class \(C_1\) and that infected individuals pass through each infected class sequentially as the infection progresses. The pathogen is assumed to be characterized by a one-dimensional trait parameter \(\theta \). We assume the dynamics of the system tend to either an endemic equilibrium or the disease free equilibrium for any fixed trait value. The disease free equilibrium is comprised of only susceptible individuals at density \(N\).
For a monomorphic pathogen population, the dynamics of the host–pathogen system are
where \(\mathcal C = (C_1,\ldots ,C_n)\). The functions \(\mathcal G _S\) and \(\mathcal U _S\) (\(\mathcal G _R\) and \(\mathcal U _R\)) are the rates at which individuals enter and leave the susceptible (recovered) class, respectively. The terms \(C_j\mathcal F ^{(j)}_{C_1}\) are the rates at which newly infected individuals enter class \(C_1\) due to transmission of the pathogen from individuals in class \(C_j\). The terms \(C_{j-1}\mathcal V ^{+}_{C_j}\) and \(C_{j}\mathcal V ^{-}_{C_j}\) are the rates at which already infected individuals enter and leave class \(C_j\), respectively. \(\mathcal V ^{-}_{C_j}\) is the sum of the per capita mortality rate, \(\mathcal D _{C_j}\), and the per capita rate at which individuals transfer out of class \(C_j\) due to recovery or progression of the infection, \(\mathcal T _{C_j}\). Throughout we assume \(\mathcal F ^{(j)}_{C_1}\), \(\mathcal V ^{+}_{C_j}\), and \(\mathcal V ^{-}_{C_j}\) are finite and positive when evaluated at points where \(C_j= 0\) for all \(j\).
2.2 The basic reproductive number
We now compute the basic reproductive number for the pathogen in system (1). The reproductive number of a pathogen with trait \(\theta ,\,\mathcal R (S,\mathcal C ,R,\theta )\), is the number of secondary infections that arise from a single infected individual in a population with host class densities \(S,\,\mathcal C \), and \(R\). The basic reproductive number of a pathogen with trait \(\theta ,\,\mathcal R _0(\theta )\), is the number of secondary infections that arise from a single infected individual in a completely susceptible population at the disease free equilibrium. The basic productive number can be computed as \(\mathcal R _0(\theta )=\mathcal R (N,0,0,\theta )\) where \(\mathcal C =0\) implies that \(C_j = 0\) for all \(j\).
We compute the reproductive number using the next generation technique in van den Driessche and Watmough (2002). Define \(\mathcal V _{C_j}(S,\mathcal C ,R,\theta ,\bar{C}_{j-1},\bar{C}_{j-1}) =-\bar{C}_{j-1}\mathcal V ^{+}_{C_j}(S,\mathcal C ,R,\theta ) + \bar{C}_j\mathcal V ^{-}_{C_j}(S,\mathcal C ,R,\theta )\). Let \(\mathbf{M}_F\) and \(\mathbf{M}_V\) be the matrices
evaluated at \(\bar{C}_j =0\) for all \(j\). The reproductive number is \(\mathcal R (S,\mathcal C ,R,\theta ) = \rho (\mathbf{M}_F \mathbf{M}_V^{-1})\), where \(\rho (\mathbf{M})\) is the spectral radius of a matrix M (Diekmann et al. 1990; van den Driessche and Watmough 2002). When \(\mathcal R (S,\mathcal C ,R,\theta )>1\), a pathogen with trait \(\theta \) can invade a population with class densities \(S,\,\mathcal C \), and \(R\).
2.3 Evolution and \(\mathcal R _0\) maximization
We assume evolution occurs at a much slower rate than the epidemiological dynamics in system (1). In order to apply our theory, we require that any successful invading pathogen replaces the resident pathogen in system (1). System (1) has this property when the trait values of the invading pathogen strains are sufficiently close to those of the resident pathogen strains (Geritz et al. 2002; Dercole and Rinaldi 2008). In particular, if the invading strain can invade the endemic equilibrium of the resident strain and the resident strain cannot invade the endemic equilibrium of the invader, then invasion implies replacement (Geritz 2005). The author is not aware of any conditions on system (1) that ensure invasion implies replacement for large differences between the trait values of the invading and resident strains. Finally, we assume only a single strain of the pathogen can infect any given host (i.e. no coinfection or superinfection) and recovered individuals are immune to all strains of the pathogen (i.e. total cross immunity).
We are interested in the case where nonresident strains of the pathogen can invade the endemic equilibrium of a resident strain. In the following we will focus on the evolutionary dynamics in system (1) when selection is frequency independent. When selection is frequency independent, the fitness of the invading pathogen strain is independent of the trait value of the resident and determined solely by the densities of the host classes (Hartl and Clark 2007). In this case the reproductive number of the invading strain only depends on the densities of the host classes and the invader trait value. Note that while the host class densities at the endemic equilibrium are determined by the trait value of the resident strain, the trait value of the resident strain only indirectly affects the fitness of the invading strain via the host class densities. We address the case where selection is frequency dependent in Appendix F. When selection is frequency dependent, the fitness of the invading strain depends explicitly on the trait value of the resident strain (Hartl and Clark 2007). In this case the reproductive number of the invading strain depends on the host class densities and both the resident and invader trait values.
Let \(S^{*},\,\mathcal C ^{*}\),and \(R^{*}\) be the susceptible, infected, and recovered host densities at the endemic equilibrium for a resident strain of the pathogen with trait \(\theta _r\). Let \(\bar{C}_j\) denote the densities of hosts infected with an invading pathogen strain with trait \(\theta _i\). When selection is frequency independent and the invader is rare, the epidemiological dynamics of the invader are
The invader can invade the endemic equilibrium of the resident strain if \(\mathcal R (S^{*},\mathcal C ^*,R^{*},\theta _i)>1\). Because we assume invasion implies replacement, in this case the invading strain becomes the new resident strain. Note that the reproductive number for a resident pathogen invading its own endemic equilibrium is \(\mathcal R (S^{*},\mathcal C ^*,R^{*},\theta _r)=1\). If there exists a resident strain such that \(\mathcal R (S^{*},\mathcal C ^*,R^{*},\theta _i)<1\) for all other strains, then that resident strain cannot be invaded by any other strain at low densities. Such strains are called evolutionary stable strategies (ESSs, Smith and Price 1973).
We are interested in sufficient conditions on the functional forms in system (1) such that an ESS also has the largest basic reproductive number. To determine these conditions, we use a sufficient condition for \(\mathcal R _0\) maximization derived in Mylius and Diekmann (1995). In particular, if the reproductive number for a pathogen with trait \(\theta \) can be written as
where \(g(S,R)\) is a positive function, then evolution always selects for the pathogen strain that maximizes the basic reproductive number; see Appendix A. Note that typically \(0\le g(S,R)\le 1\) for biological models. In the following we present the conditions on the functional forms of \(\mathcal F ^{(j)}_{C_1},\,\mathcal V ^{+}_{C_j}\), and \(\mathcal V ^{-}_{C_j}\) such that \(\mathcal R (S,\mathcal C ,R,\theta )\) factors as in Eq. (4). Note that because \(\mathbf{M}_F\) and \(\mathbf{M}_V\) depend only on the functions \(\mathcal F ^{(j)}_{C_1},\,\mathcal V ^{+}_{C_j}\), and \(\mathcal V ^{-}_{C_j}\), our conditions for \(\mathcal R _0\) maximization hold for any choice of dynamics for the susceptible and recovered classes.
3 Results
3.1 \(\mathcal R _0\) maximization in models with a single infectious class
We first consider systems with a single infectious class. The level of generality of our model includes systems where transmission is density dependent or frequency dependent and systems where immunity can be lost (SIS and SIRS systems) and recovery is not possible (SI systems). The model is
Here \(\mathcal F _I\) is the per capita recruitment rate of infectious individuals due to horizontal or vertical transmission and \(\mathcal V ^{-}_I\) is the per capita rate at which infected individuals exit the infectious class. The per capita exit rate \(\mathcal V ^{-}_I=\mathcal T _{I}+\mathcal D _{I}\) is the sum of the per capita rate at which infectious individuals transfer out of the infectious class into other classes \((\mathcal T _{I})\) and the per capita death rate \((\mathcal D _{I})\). Since there is only a single infected class in system (5), the transfer rate is equal to the recovery rate.
Using Eq. (2), the reproductive number for a pathogen with trait \(\theta \) in system (5) is
see Appendix B for details. The basic reproductive number for a pathogen strain is \(\mathcal R _{0}(\theta ) = \mathcal R (N,0,0,\theta )\). The reproductive number can be written in the form given by Eq. (4) when \(\mathcal F _{I}\) and \(\mathcal V ^{-}_{I}\) can be written as
-
(A1)
\(\mathcal F _{I}(S,I,R,\theta ) = f_{I}(S,I,R)\eta (\theta )\xi _f(S,I,R,\theta )\)
-
(A2)
\(\mathcal V ^{-}_{I}(S,I,R,\theta ) = v^{-}_{I}(S,I,R)\nu (\theta )\xi _v(S,I,R,\theta )\)
-
(A3)
\(\xi _f = \xi _v\)
Under these conditions the reproductive rate can be written as
In conditions (A1) and (A2), \(f_{I}\) and \(v^{-}_{I}\) represent the effects the densities of the host classes have on the recruitment and exit rates of infectious individuals. We will refer to \(f_{I}\) and \(v^{-}_{I}\) as the effects of the environment. The functions \(\eta \) and \(\nu \) represent the effects the pathogen trait has on the recruitment and exit rates of infected individuals. Finally, the terms \(\xi _f\) and \(\xi _v\) represent the effects of the interactions between the pathogen trait and the densities of the host classes. This term has multiple interpretations, e.g. genotype-by-density, genotype-by-environment, or phenotype-by-environment interactions, but throughout the text we will refer to these terms as genotype-by-environment interaction effects.
The biological interpretations and consequences of conditions (A1) through (A3) are the following. First consider condition (A3). Biologically, \(\mathcal F _{I}\) and \(\mathcal V ^{-}_{I}\) are only likely to have the same genotype-by-environment interaction terms when there are no genotype-by-environment interactions, i.e. \(\xi _f = \xi _v =1\). In this case, the effects of the environment and the trait on recruitment, recovery, and death are independent. Next consider condition (A1). When there are multiple routes of transmission (e.g., horizontal or vertical transmission), condition (A1) implies that either the environmental effects or the trait effects must be the same across all transmission routes; see Example 3 and Appendix B for details. In natural systems, we do not expect horizontal and vertical transmission pathways to have the same dependence on the densities of the host classes, nor do we expect the pathogen trait to affect all transmission routes the same. Thus, we expect evolution to maximize \(\mathcal R _0\) only in systems with a single transmission pathway.
To interpret condition (A2) we decompose \(\mathcal V _I\) into death \((\mathcal D _I)\) and transfer \((\mathcal T _I)\) rates. Condition (A2) is satisfied when one of the following holds
-
(A2.1)
\(\mathcal T _{I} = t_I(S,I,R)\tau (\theta )\xi _v(S,I,R,\theta )\) and \(\mathcal D _{I} =0\)
-
(A2.2)
\(\mathcal T _{I}=0\) and \(\mathcal D _{I} = d_I(S,I,R)\delta (\theta )\xi _v(S,I,R,\theta )\)
-
(A2.3)
\(\mathcal T _{I}=t_I(S,I,R)\tau (\theta )\xi _v(S,I,R,\theta ),\,\mathcal D _{I}=d_I(S,I,R)\delta (\theta )\xi _v(S,I,R,\theta )\), and \(\tau = \delta \)
-
(A2.4)
\(\mathcal T _{I}=t_{I}(S,I,R)\tau (\theta )\xi _v(S,I,R,\theta ),\,\mathcal D _{I}=d_{I}(S,I,R)\delta (\theta )\xi _v(S,I,R,\theta )\), and \(t_{I}=d_{I}\)
Condition (A2.1) implies that there is no mortality of infectious individuals. Condition (A2.2) implies that recovery is not possible and that infection ultimately leads to death. When death and recovery are both possible, condition (A2.3) requires the death and recovery rates to have the same trait dependence. Condition (A2.3) is unlikely to arise in nature as it implies that an increase in the death rate due to pathogen evolution is accompanied by an increase in the recovery rate.
Condition (A2.4) requires the death and recovery rates to have the same density dependence. To see when this arises, decompose the mortality rate into pathogen induced mortality that is independent of the host population density, \(\delta _1(\theta )\), and density dependent mortality, \(\delta _2(\theta )d(S,I,R)\). Thus the total death per capita rate is \(\mathcal D _I= \delta _1(\theta )+ \delta _2(\theta )d(S,I,R)\). An example from the literature is \(\mathcal D _I= \delta _1(\theta )+ \delta _2(S+I+R)\) (Thieme 2007; Gyllenberg et al. 2011). For \(\mathcal D _I\) to satisfy condition (A2.2), the density dependent mortality rate would either have to be independent of the host population density [\(d(S,I,R) =0\)] or the pathogen induced mortality would have to be negligible [\(\delta _1(\theta ) =0\)]. Note that we expect the per capita recovery rate to be independent of the host population density. Thus, for \(\mathcal D _I+\mathcal T _I\) to satisfy condition (A2.4), either mortality would have to be density independent or there could be no recovery [\(\mathcal T (S,I,R,\theta )=0\)]. In total, condition (A2.4) will be satisfied in natural systems either when mortality is density independent or when mortality is entirely density dependent and there is no recovery.
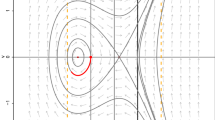
In the following we consider three examples. The first has a widely used and simple form that results in the maximization of \(\mathcal R _0\). In the second system the transmission function does not satisfy condition (A1) and evolution does not maximize \(\mathcal R _0\). Specific numerical examples of these two systems are included in Fig. 1. The third example illustrates why we do not expect \(\mathcal R _0\) to arise in systems where the pathogen is spread both vertically and horizontally.
Numerical examples where pathogen evolution maximizes \(\mathcal R _0(\theta )\) and where pathogen evolution does not maximize \(\mathcal R _0(\theta )\). a, b Basic reproductive number of a pathogen as a function of its trait, \(\theta \). c, d Pairwise invasability plots for the pathogen. Gray (white) regions denote strains of the pathogen that can (cannot) invade a given resident strain. The evolutionarily optimal trait value occurs at the intersection of the black curves. Arrows in (c) and (d) denote the trait values that maximize \(\mathcal R _0(\theta )\) in panels (a) and (b), respectively. Numerical examples are derived from Example 1 (a, c) and Example 2 (b, d) from the main text. Parameters are \(\beta (\theta ) = 4 + 2\theta ,\,\kappa (\theta ) = 1+\theta ,\,\omega =0.5,\,\mu (\theta ) = 2+\theta ^2\), and \(N=10\). The dynamical equation for the susceptible class is \(dS/dt = -\mathcal F (S,I,R)+\mu (\theta )I +\mu _R R+\rho R\) where \(\mu _R\) is the death rate of recovered individuals, \(\rho \) is the loss of immunity rate, and the total population size is assumed to be constant, \(S+I+R=N\)
Example 1:
Systems with functions of the form \(\mathcal F _{I} = f_1(S,I,R) \eta (\theta )\) and \(\mathcal V ^{-}_{I} = v_1(S,I,R) \nu (\theta )\) are widely used in the literature. In such systems, the reproductive number can easily be shown to factor as in Eq. (4),
Systems with these functional forms always satisfy conditions (A1) and (A2) and hence, result in the evolutionary maximization of \(\mathcal R _0\).
For example, the \(\mathcal R _0\) maximization hypothesis in Anderson and May (1982) and Bremermann and Thieme (1989) arose from models where the infectious class dynamics were given by
where \(\beta (\theta )\) is the mass action transmission coefficient, \(\mu (\theta )\) is the per capita mortality rate, and \(\omega (\theta )\) is the per capita recovery rate. Translating equation (7) into our notation yields \(f_I(S,I,R)=S,\,\eta (\theta )=\beta (\theta ),\,v^{-}_I(S,I,R)=1,\,\nu (\theta )=\mu (\theta )+\omega (\theta )\), and \(\xi _f=\xi _v=1\). Decomposing the exit rates into transfer and death rates yields \(t_I(S,I,R)=1,\,\tau (\theta )=\omega (\theta ),\,d_I(S,I,R)=1\), and \(\delta (\theta )=\mu (\theta )\). Note that the per capita mortality and recovery rates are independent of the host class sizes and thus satisfy condition (A2.4).
In this example,
which can be put in the form of Eq. (4) by setting
Hence, as was observed in Anderson and May (1982) and Bremermann and Thieme (1989), evolution maximizes \(\mathcal R _0\). In the numerical example in Fig. 1c, the evolutionary optimal strategy (i.e. the ESS denoted by the intersection of the two black curves) coincides with the trait value that maximizes the basic reproductive number (denoted by the black arrow).
Example 2:
Now consider a system where the infectious dynamics are given by
Here, the transmission function \(\beta S/[\kappa (\theta )+ S]\) represents how the transmission rate saturates as the density of susceptible individuals increases. The parameter \(\kappa (\theta )\) is the density of susceptible individuals at which the transmission rate is half of the maximum rate. In this system \(\mathcal F _I(S,I,R,\theta )=\beta (\theta )S/(\kappa (\theta )+S)\) and \(\mathcal V ^{-}_I(S,I,R,\theta )=\mu (\theta )+\omega (\theta )\). The reproductive number for the pathogen is
When \(\kappa \) does not depend on the trait \(\theta \), then system (7) satisfies conditions (A1) through (A3). In particular, \(f_I(S,I,R)=S/(\kappa +S),\,\eta (\theta )=\beta (\theta ),\,v^{-}_I(S,I,R)=1,\nu (\theta )=\mu (\theta )+\omega (\theta )\), and \(\xi _f=\xi _v=1\). In this case \(\mathcal R (S,I,R,\theta )\) can be written as in Eq. (4) with
Thus, evolution maximizes \(\mathcal R _0\).
When \(\kappa (\theta )\) does depend on the trait, \(\beta (\theta )S/(\kappa (\theta )+S)\) cannot be factored as in condition (A1). Thus, \(\mathcal R (S,I,R,\theta )\) cannot be written as in Eq. (4) because the function \(g(S,I,R)\) will depends on both \(S\) and \(\theta \). In this case, the value of the optimal strain will be determined by how the endemic equilibrium density of the susceptible population depends on the strain of the resident pathogen (Mylius and Diekmann 1995; Geritz et al. 1998). As seen in Fig. 1d, the evolutionary optimal strategy (denoted by the intersection of the black curves, \(\theta \approx 0.135\)) does not coincide with the trait value that maximizes the basic reproductive number (black arrow, \(\theta \approx 0.283\)).
Example 3
Assume the pathogen can be transmitted vertically and horizontally. To simplify the dynamics, we assume that the host population dynamics follow logistic growth with carrying capacity \(K\). The equation for the infectious class is
where \(r(\theta )\) is the per capita birth rate of infectious hosts and all other parameters are defined as in Example 1. In Eq. (13), \(\mathcal F _I(S,I,R,\theta )=r(\theta ) - (S+I+R)/K+\beta (\theta )S\) and \(\mathcal V ^{-}_I(S,I,R,\theta )=\mu (\theta )+\omega (\theta )\).
The reproductive number for system (20) is
If either \(r(\theta )\) or \(\beta (\theta )\) depend on the trait, then \(\mathcal F _I(S,I,R,\theta )\) does not factor as in condition (A1). In this case it is not possible to write \(\mathcal R (S,I,R,\theta )\) as in Eq. (4) because \(g(S,I,R)\) will depend on \(S,\,I,\,R\) and \(\theta \). If \(r\) and \(\beta \) do not depend on the pathogen trait, then \(\mathcal F _I(S,I,R,\theta )\) can be factored as \(f_I(S,I,R)=r-(S+I+R)/K+\beta S\) and \(\eta _I(\theta )=1\). However, this case only arises in the biologically unlikely scenario where the pathogen trait has no affect on pathogen transmission or host recruitment. This example illustrates why we do not expect evolution to maximize \(\mathcal R _0\) when vertical and horizontal transmission pathways are both present.
3.2 \(\mathcal R _0\) maximization in models with an exposed class
To demonstrate how the conditions for \(\mathcal R _0\) maximization in model (5) generalize to more complex models, we consider a system with an exposed class, \(E\), and an infectious class, \(I\). The generality of the model includes systems where immunity can be lost and recovery is not possible. For notational ease in this section we set \(X = (S,E,I,R)\). The model is
Note that infectious individuals cannot return to the exposed class (i.e. there is no \(I\mathcal V ^{+}_E\) term) and that all newly infected individuals enter the exposed class (i.e. there is no \(I\mathcal F _I\) term).
The reproductive number for a pathogen with strain \(\theta \) in system (15) is
The basic reproductive number is \(\mathcal R _0(\theta ) = \mathcal R (N,0,0,0,\theta )\). \(\mathcal R (X,\theta )\) factors as in Eq. (4) when
-
(B1)
\(\mathcal F _{E} = f_{E}(X)\eta _{E}(\theta )\xi ^{+}_{E}(X,\theta )\)
-
(B2)
\(\mathcal V ^{-}_{E}=v^{-}_{E}(X)\nu ^{-}_{E}(\theta )\xi ^{-}_{E}(X,\theta )\)
-
(B3)
\(\mathcal V ^{+}_{I}=v^{+}_{I}(X)\nu ^{+}_{I}(\theta )\xi ^{+}_{I}(X,\theta )\)
-
(B4)
\(\mathcal V ^{-}_{I}=v^{-}_{I}(X)\nu ^{-}_{I}(\theta )\xi ^{-}_{I}(X,\theta )\)
-
(B5)
\(\xi ^{+}_{E} = \xi ^{-}_{E}\) and \(\xi ^{+}_{I} = \xi ^{-}_{I}\)
Under these conditions, \(\mathcal R (X,\theta )\) can be written as in Eq. (4),
Conditions (B1) through (B5) have a particular structure. First, each functional response must factor into terms representing environmental effects (\(f_{E},\,v^{-}_{E},\,v^{+}_{I}\), and \(v^{-}_{I}\)), pathogen trait effects (\(\eta _{E},\,\nu ^{-}_{E},\,\nu ^{+}_{I}\), and \(\nu ^{-}_{I}\)), and genotype-by-environment interaction effects (\(\xi ^{\pm }_E\) and \(\xi ^{\pm }_I\)). In addition, for each infected class the genotype-by-environment interaction terms of all the functional forms must be the same (\(\xi ^{+}_E=\xi ^{-}_E\) and \(\xi ^{+}_I=\xi ^{-}_I\)).
The biological interpretation of conditions (B1) through (B5) is the following. First consider condition (B5). Since the rate of disease progression out of the exposed class is equal to the entry rate into the infectious class, the equivalence of the genotype-by-environment interactions within classes implies that the genotype-by-environment interactions are the same across all classes. We do not expect the genotype-by-environment interaction effects to be the same for the transmission, entry, and exit rates. Thus, condition (B5) implies that there are no genotype-by-environment interactions \((\xi ^{\pm }_E = \xi ^{\pm }_I =1)\). In this case the effects of the environment and the trait are independent.
Condition (B1) implies that the pathogen utilizes a single route of transmission. Thus, transmission cannot occur both vertically and horizontally. Conditions (B2) through (B4) can be interpreted by decomposing \(\mathcal V ^{-}_{E}\) and \(\mathcal V ^{-}_{I}\) into transfer and death rates. This decomposition yields conditions analogous to conditions (A2.1) through (A2.4) for each infected class. We expect conditions (B2) through (B4) to be satisfied in two different cases. In the first case, the per capita mortality, disease progression, and recovery rates of all class are density independent. In the second case, mortality is density dependent, but additional constraints on the infected classes must hold. First, the per capita mortality, transfer, and recovery rates of the exposed class must be independent of the pathogen trait. Second, recovery is not possible from the infectious class. Third, the mortality rate of the infectious class is entirely density dependent and factors as in condition (A2.2).
In the following we consider two examples that illustrate the two cases above. In the first, mortality is density independent and evolution maximizes \(\mathcal R _0\). The second illustrates the conditions under which \(\mathcal R _0\) maximization occurs when mortality is density dependent.
Example 4:
When mortality is density independent, the dynamics of the infected classes are
where \(\beta \) is the transmission coefficient, \(1/\nu _E\) is the average time between infection and the onset of infectiousness, \(\rho _E\) and \(\rho _I\) are the per capita recovery rates, and \(\mu _E\) and \(\mu _I\) are per capita death rates. All parameters are potential functions of the pathogen trait. Here, \(f_E(X)=S,\,\eta _E(\theta )=\beta (\theta ),\,v^{-}_E(X)=v^{+}_I(X)=v^{-}_I(X)=1,\,\nu ^{-}_E(\theta )=\nu _E(\theta )+\rho _E(\theta )+\mu _E(\theta ),\,\nu ^{+}_I(\theta )=\nu _E(\theta ),\,\nu ^{-}_I(\theta )=\nu _I(\theta )+\rho _I(\theta )+\mu _I(\theta )\), and \(\xi ^{\pm }_E=\xi ^{\pm }_I=1\). Note that decomposing the exit rates into transfer and mortality rates yields \(\tau _E(\theta )=\nu _E(\theta )+\rho _E(\theta ),\,\delta _E(\theta )=\mu _I(\theta ),\,\tau _I(\theta )=\rho _E(\theta )\), and \(\delta _I(\theta )=\mu _I(\theta )\). Since \(d_E(X) = d_I(X) = t_E(X) = t_I(I)=1\), system (18) satisfies the condition for SEIR systems analogous to condition (A2.4).
Since the functional forms in system (18) satisfy conditions (B1) through (B5), the reproductive number factors as
where \(g(S,I,R) = S/N\) as in Eq. (4). Thus, evolution always maximizes \(\mathcal R _0\).
Example 5:
Now consider a system where mortality is density dependent,
where \(M=S(t)+E(t)+I(t)+R(t)\) is the host population size at time \(t\). Here, \(m_E(M)\) and \(m_I(M)\) are the per capita density dependent mortality rates. All other parameters are interpreted as in system (18). The reproductive number for system (20) is
The functions \(\mathcal F _E=\beta (\theta )S\) and \(\mathcal V ^{+}_I=\nu _E(\theta )\) factor as \(f_E(X)=S,\,\eta _E(\theta )=\beta (\theta ),\,v^{+}_I(X)=1,\,\nu ^{+}_I(\theta )=\nu _E(\theta )\), and \(\xi ^{+}_E=\xi ^{+}_I=1\). However, in general \(\mathcal V ^{-}_E=\nu _E + \rho _E + \mu _E +m_E(M,\theta )\) and \(\mathcal V ^{-}_E=\rho _I + \mu _I + m_I(M,\theta )\) do not satisfy conditions (B2), (B4), and (B5). Hence, in general Eq. (21) cannot be written as in Eq. (4).
System (20) satisfies conditions (B1) through (B5) when one of the following is holds
-
(C1)
\(m_E=0\) and \(m_I=0\)
-
(C2)
\(m_E=0,\,\rho _I= \mu _I=0\), and \(m_I(M,\theta )=m_1(M)m_2(\theta )\)
-
(C3)
\(\nu _E,\,\rho _E,\,\mu _E\), and \(m_E\) do not depend on \(\theta ,\,\rho _I= \mu _I=0\), and \(m_I(M,\theta )=m_1(M)m_2(\theta )\)
Other possibilities exist if \(\rho _I(\theta )= \mu _I(\theta )=\delta _I(\theta )\) or if \(\rho _E(\theta )= \mu _E(\theta )=\delta _E(\theta )\). However, we do not expect these cases to arise in natural systems because they imply that the pathogen trait has the same effect on recovery and mortality rates. Note that under conditions (C2) and (C3) mortality in the infectious class is entirely due to density dependent processes.
When condition (C1) is satisfied, systems (18) and (20) are equivalent. Hence evolution maximizes \(\mathcal R _0\). When condition (C2) is satisfied, the per capita mortality rate of the exposed class is independent of the host population size, there is no recovery from the infectious class, and mortality in the infectious class is entirely due to density dependent processes. In this case \(v^{-}_E(X)=v^{+}_I(X)=1,\,\nu ^{-}_E(\theta )=\nu _E(\theta )+\rho _E(\theta )+\mu _E(\theta ),\,\nu ^{+}_I(\theta )=\nu _E(\theta ),\,v^{-}_I(X)=m_1(M),\,\nu ^{-}_I(\theta )=m_2(\theta )\), and \(\xi ^{\pm }_E=\xi ^{\pm }_I=1\). Following Eq. (17), the reproductive number can then be written as
where \(g(S,E,I,R)= S m_1(M)/[N m_1(N)]\) as in Eq. (4).
When condition (C3) is satisfied, all recovery and mortality rates of the exposed class are independent of the pathogen trait, there is no recovery from the infectious class, and mortality in the infectious class is entirely due to density dependent processes. In this case, \(\mathcal V ^{-}_E\) and \(\mathcal V ^{-}_E\) satisfy conditions (B2) through (B5) where \(v^{-}_E(X)=\nu _E+\rho _E+\mu _E+m_E(M),\,\nu ^{-}_E(\theta )=1,\,v^{-}_I(X)=m_1(M),\,\nu ^{-}_I(\theta )=m_2(\theta )\), and \(\xi ^{-}_E=\xi ^{-}_I=1\). The reproductive number for system (20) can then be written as
3.3 Maximization in models with vectors and multiple infected classes
We now summarize the main conclusions for systems with multiple exposed and multiple infectious classes and systems with vector-borne pathogens. All analytical results are contained in Appendices C, D, and E.
There are three general mathematical conditions under which evolution maximizes \(\mathcal R _0\). First, each functional response must factor into three components representing environmental, trait, and genotype-by-environment interaction effects, e.g. \(\mathcal F (X,\theta ) = f(X)\eta (\theta )\xi _f(X,\theta )\). Second, for each species the pathogen infects, the genotype-by-environment interaction terms must be the same across all classes of that species. For example, in vector-borne systems the genotype-by-environment terms for all host classes must be the same and the genotype-by-environment terms for all vector classes must be the same. Third, for systems with multiple infectious classes, the per capita rates at which infectious individuals enter \((\mathcal V ^{+}_{C_j})\) and exit \((\mathcal V ^{-}_{C_j})\) a particular infectious class must have either the same dependence on the pathogen trait or the same dependence on the densities of the host classes. This last condition is similar to, but more restrictive than, conditions (A2.1) through (A2.4) for system (5).
The biological consequences of the conditions are similar to those for the previous models. The first condition implies that the pathogen can only utilize one transmission pathway. Thus the pathogen can only be spread via horizontal, vertical, or vector-borne transmission; see Example 6. The constraints imposed by the second condition suggest that \(\mathcal R _0\) maximization will arise only in systems where there are no genotype-by-environment interactions. In this case, the trait and the host class densities affect pathogen related processes independently.
The biological consequences of the third condition depend on the structure of the host population. When each species has a single infectious class, the consequences are the same as those in system (15). That is, either mortality is density independent or mortality is density dependent and the conditions illustrated in Example 5 must hold. When multiple infectious classes are present in a given species, the entry and exit rates for all infectious classes of that species must have either the same dependence on the trait or the same dependence on the host classes. We do not expect the pathogen trait to have the same effect on the entry and exit rates for all infected classes. In principle the entry and exit rates of all infectious classes can have the same density dependence, but biologically we expect this only in the case where the per capita recovery, mortality, and infection progression rates are density independent. Thus, for any species with multiple infectious classes, \(\mathcal R _0\) maximization is only expected if mortality is density independent.
The following two examples consider vector-borne pathogens. Let \(\hat{S},\,\hat{E},\,\hat{I}\), and \(\hat{R}\) denote the densities of the susceptible, exposed, infectious and recovered vector classes, respectively. Let \(\hat{N}\) be the density of susceptible vectors at the disease free equilibrium. For notational ease, let \(X=(S,E,I,R,\hat{S},\hat{E},\hat{I},\hat{R})\). The first example is from a study by Medlock et al. (2009) where evolution maximizes \(\mathcal R _0\). The second example includes both direct and vector transmission and does not maximize \(\mathcal R _0\).
Example 6:
The equations for the infected classes in Medlock et al. (2009) are
where \(\beta \) and \(\hat{\beta }\) are the transmission coefficients, \(1/\nu _E\) and \(1/\nu _{\hat{E}}\) are the average times between exposure to the pathogen and the onset of infectiousness, \(\rho _I\) and \(\rho _{\hat{I}}\) are the recovery rates, and \(\mu _E,\,\mu _I,\,\mu _{\hat{E}}\) and \(\mu _{\hat{I}}\) are the mortality rates. All parameters are potentially functions of \(\theta \). Note that mortality is density independent and that the pathogen only utilizes one route of transmission.
In this system, the functional forms in the host class equations factor into components that depend on the host class densities [\(f_E(X) = S\) and \(v^{-}_E(X) = v^\pm _I(X) = 1\)] and components that depend on the pathogen trait [\(\eta _E(\theta ) = \beta (\theta )S,\,\nu ^{-}_E(\theta ) =\nu _E+\mu _E,\,\nu ^{+}_I(\theta ) =\nu _E,\,\nu ^{-}_I(\theta ) = \rho _I+\mu _I\)]. Furthermore, there are no genotype-by-environment interaction terms, i.e. \(\xi _E^\pm = \xi _I^\pm =1\). The functional forms in the vector class equations factor in an analogous way. Consequently, the reproductive number of the pathogen can be written as in Eq. (4),
where \(g(X) = S\hat{S}/(N\hat{N})\). As was found in Medlock et al. (2009), evolution maximizes \(\mathcal R _0\).
Example 7
Now assume the pathogen utilizes both horizontal and vector-borne transmission routes. The dynamics of the exposed class are
where \(\beta _1\) is the direct transmission coefficient and \(\beta _2\) is the vector transmission coefficient. The equations for the \(I,\,\hat{E}\), and \(\hat{I}\) classes are as in system (24). The reproductive number of the pathogen is
where \(\mathcal R _{1}\) is defined as in Eq. (25) with \(\beta =\beta _2\).
As shown in Example 6, all of the transfer functions factor into components that depend only on the host and vector classes and components that only depend on the pathogen trait. The contributions to newly infected individuals from direct transmission \((\beta _1 SI)\) and vector transmission \((\beta _2 S\hat{I})\) also factor. However, because the pathogen utilizes two modes of transmission, the reproductive number \(\mathcal R _{2}(X,\theta )\) factors as in Eq. (4) only if the parameters depend on \(\theta \) in such a way that \(\hat{\beta }\beta _2\hat{\nu }_E =\beta _1^2\nu _E\) and \((\nu _E+\mu _E)(\nu _I+\mu _I) = (\hat{\nu }_E+\hat{\mu }_E)(\hat{\nu }_I+\hat{\mu }_I)\). We do not expect these conditions to be satisfied in natural systems. Thus, we do not expect evolution to maximize \(\mathcal R _{0}\) when there are multiple routes of transmission.
3.4 Frequency dependent selection
The above conditions can be extended to include the case where selection is frequency dependent. Here we present the results for a system with a single infectious class and discuss how the results can be extended for more complex systems. Additional details and the analysis of a particular example are included in Appendix F.
Consider a system with a single infectious class. Let \(\theta \) denote the resident’s trait and let \((S^*,I^*,R^*)\) denote the endemic equilibrium of the resident. When selection is frequency dependent, the dynamics of an invading pathogen with strain \(\theta _i\) at the endemic equilibrium of the resident are
Here, \(I_i\) is the density of individuals infected with the invading strain, \(F\) is the per capita transmission rate of the invading strain, and \(V^{-}\) is the per capita exit rate. The reproductive number of the invading strain is
The basic reproductive number of the invading strain is \(\mathcal R (\theta _i)= \mathcal R (N,0,0,\theta _i,\theta _i)\). The reproductive number factors as in Eq. (4) under the following conditions
-
(D1)
\(F_{I}(S,I,R,\theta ,\theta _i) = f_{I}(S,I,R,\theta )\eta (\theta _i)\xi _f(S,I,R,\theta ,\theta _i)\)
-
(D2)
\(V^{-}_{I}(S,I,R,\theta ,\theta _i) = v^{-}_{I}(S,I,R,\theta )\nu (\theta _i)\xi _v(S,I,R,\theta ,\theta _i)\)
-
(D3)
\(\xi _f = \xi _v\)
-
(D4)
\(f_{I}(S,0,0,\theta _1) = f_{I}(S,0,0,\theta _2)\) and \(v^{-}_{I}(S,0,0,\theta _1) = v^{-}_{I}(S,0,0,\theta _2)\) for all \(\theta _1\) and \(\theta _2\)
These conditions are similar to conditions (A1) and (A3) but two key differences arise. First, the terms that define the environmental effects (\(f_I\) and \(v^{-}_I\)) now depend on the trait value of the resident. Note that from the perspective of the invader, the resident is part of the environment. Second, condition (D4) imposes an additional constraint on the effects of the host density and the invading pathogen strain in completely susceptible populations.
The biological interpretations of the conditions are the following. Condition (D3) implies that the genotype-by-environment interactions are the same for transmission, mortality, and recovery. Condition (D3) also implies that the genotype-by-genotype interactions are the same. Here, genotype-by-genotype interactions refer to interactions between the traits of the resident and invading strain. We do not expect genotype-by-environment and genotype-by-genotype interaction to be the same for all processes in natural systems. Thus, condition (D3) is only likely to be satisfied in systems where the effects of the invader’s trait are independent of the host class densities and the resident’s trait, i.e. \(\xi _f = \xi _v=1\). Condition (D1) implies that the pathogen can only utilize one transmission pathway. Condition (D3) implies that either mortality is density independent or mortality is density dependent and recovery is not possible. Condition (D4) implies the effects of host density and the pathogen trait are independent in completely susceptible populations.
The conditions for \(\mathcal R _0\) maximization in any system with frequency independent selection can be extended to the frequency dependent selection case in an analogous way. First, all terms representing environmental effects need to depend on both the host class densities and the trait value of the resident. Second, terms representing trait effects can only depend on the invader’s trait. Third, a condition like condition (D4) must hold for all functional responses. The biological interpretation of these conditions remains essentially unchanged.
4 Discussion
In this study we presented a set of sufficient conditions for \(\mathcal R _0\) maximization in terms of the functional forms used to model epidemiological processes. We also discussed how those mathematical conditions relate to the biological characteristics of natural systems. Our analysis yields three mathematical conditions under which evolution maximizes \(\mathcal R _0\) in SIR-type systems. First, each functional response must factor into terms representing environmental, trait, and genotype-by-environment interaction effects [conditions (A1) through (A2) for system (5)]. Second, for each species the pathogen infects, the effects of genotype-by-environment (or genotype-by-density) interactions must the same for all epidemiological processes [condition (A3)]. Third, the per capita mortality, recovery, and infection progression rates must have the same density dependence or the same trait dependence [conditions (A2.1) through (A2.4)].
These mathematical conditions yield three general biological constraints on when evolution is expected to maximize \(\mathcal R _0\) in natural systems. First, the pathogen can only utilize one transmission pathway, e.g. horizontal, vertical, or vector transmission. Second, there are no genotype-by-environment interaction effects. That is, the host class densities and the pathogen trait have independent effects on all epidemiological processes. Third, either the per capita mortality, recovery and disease progression rates are density independent or mortality is density dependent and (i) there is a single infectious class that individuals cannot recover from, (ii) mortality in the infectious class is entirely density dependent, and (iii) the rates of recovery, infection progression, and mortality in the exposed classes are independent of the pathogen trait.
It is important to note that the conditions presented in this study are only sufficient conditions for \(\mathcal R _0\) maximization. Other studies have considered particular systems that do not satisfy our conditions but result in \(\mathcal R _0\) maximization (Thieme 2007; Metz et al. 2008; Gyllenberg et al. 2011). However, while our conditions do not capture all of the cases in which \(\mathcal R _0\) maximization occurs, our conditions do identify what characteristics of natural systems inhibit or promote \(\mathcal R _0\) maximization. We now interpret our results in the context of one-dimensional environmental feedbacks. In particular, Metz et al. (2008) found that \(\mathcal R _0\) maximization required a one-dimensional environmental feedback. We focus on the biological mechanisms that yield higher dimensional environmental feedbacks and inhibit \(\mathcal R _0\) maximization.
When multiple transmission pathways are present, each pathway yields an additional environmental feedback. Previous studies have shown that evolution does not maximize \(\mathcal R _0\) when a pathogen can be transmitted both horizontally and vertically (Nowak 1991; Lipsitch et al. 1996). For this case, the susceptible class is the feedback variable for horizontal transmission and the infectious class, via the density regulated birth rate, is the feedback variable for vertical transmission. Our results show that this conclusion extends to indirectly transmitted pathogens as well. In particular, when vector transmission is also possible, the susceptible vector population becomes a feedback variable. This suggests we should not expect evolution to maximize \(\mathcal R _0\) when a pathogen utilizes multiple pathways.
Previous work has also shown that host heterogeneity can inhibit \(\mathcal R _0\) maximization (Dwyer et al. 1997; Gandon et al. 2001). In our models, host heterogeneity is represented via multiple susceptible classes; see Appendix B. When the effects of the pathogen trait on transmission differ across susceptible classes, then each susceptible class acts as an independent feedback variable and evolution will not maximize \(\mathcal R _0\). However, if the pathogen trait affects transmission uniformly (e.g. increasing transmission by a constant factor across all susceptible classes) then host heterogeneity will not prevent \(\mathcal R _0\) maximization. Thus, the underlying structure dictating the heterogeneity and its interaction with the pathogen trait will determine if \(\mathcal R _0\) maximization is possible.
Density dependent mortality has been shown to add additional feedback variables (Thieme 2007; Metz et al. 2008; Gyllenberg et al. 2011). However, this is true only when the pathogen trait affects pathogen-induced mortality, recovery, density dependent mortality, or all of the above in a given class. For example consider system (5) where there is a single infectious class. The feedback variable for density independent pathogen-induced mortality and recovery is the susceptible class. The feedback variable for density dependent mortality is the total population size. If density dependent mortality is present with density independent mortality or recovery, then the environmental feedback will have dimension two. But, if recovery is not possible and density independent mortality is negligible, then the environmental feedback will be one-dimensional. Furthermore, as shown in system (15) and Example 5, density dependent and density independent sources of mortality can arise in exposed classes so long as those processes are not affected by the pathogen trait. Thus, our results show that the dimension of the environmental feedback depends on the characteristics of the system when mortality is density dependent.
Our approach also shows that the structure of the host population can influence the dimension of the environmental feedback. Differences in the progression of infection can inhibit \(\mathcal R _0\) maximization. When newly infected hosts can enter different infected classes, multiple life cycle pathways are accessible to the pathogen. Each new pathway can potentially result in the pathogen trait affecting the environmental feedback. When this occurs, the environmental feedback has dimension two. Differences in the infectiousness of infectious individuals can also inhibit \(\mathcal R _0\) maximization. In particular, if transmission processes differ mechanistically across the infectious classes of a given species, then each transmission mechanism will create a new feedback; see Appendix C. In this case the environmental feedback will depend on the pathogen trait and have a dimension greater than one. This is particularly important for pathogens of organisms in stage or age structured populations. If the pathogen can be spread during different life stages, then the transmission mechanisms could differ, preventing \(\mathcal R _0\) maximization.
The conditions for \(\mathcal R _0\) maximization for every model we considered included a requirement for the genotype-by-environment interaction effects to be the same for all functional responses. Genotype-by-environment interactions arise when the effect of the environment is conditional on the pathogen trait. In most cases, this implies that the feedback must be at least two dimensional. Many previous studies have implicitly assumed there are no genotype-by-environment interactions via density dependent, frequency dependent, or mass action transmission functions and density independent mortality and recovery rates (Anderson and May 1982; Bremermann and Thieme 1989; Lenski and May 1994; Dieckmann et al. 2002; Boots and Sasaki 2003; Basu and Galvani 2009; Medlock et al. 2009). Each of these cases found that evolution maximized \(\mathcal R _0\). However, those transmission functions do not accurately capture the dynamics of all host–pathogen systems (McCallum et al. 2001; Smith et al. 2009). Other functional forms like the negative binomial (Knell et al. 1996; Barlow 2000; Briggs and Godfray 1995) and asymptotic transmission functions (Barlow 2000; Diekmann and Kretzschmar 1991; Roberts 1996; Heesterbeek and Metz 1993) have been used to model host–pathogen systems. Depending on how the pathogen trait is incorporated, these transmission functions may contain terms that represent genotype-by-environment interactions. In such cases, we do not expect evolution to maximize \(\mathcal R _0\).
Finally, we return to our initial assumptions about complete cross immunity and single strain infections. Previous studies have shown that superinfection and coinfection of multiple strains (May and Nowak 1995; Nowak and May 1994; Mosquera and Adler 1998) and assumptions about cross immunity (Gog and Grenfell 2002) can also affect evolutionary outcomes. In these cases the infectious classes of other strains can become environmental feedback variables. Furthermore, interactions between pathogen strains can result in evolution selecting for strains that do not maximize \(\mathcal R _0\). These genotype-by-genotype interactions are related to additional conditions for \(\mathcal R _0\) maximization that arise when selection is frequency dependent. In particular, as with genotype-by-environment interactions, genotype-by-genotype interactions tend to create higher dimensional environmental feedbacks. Understanding how prevalent genotype-by-genotype interactions are in host–pathogen systems is an important area of future research that can determine how applicable \(\mathcal R _0\) maximization theory is to natural systems.
The conditions in this study hold for systems where any pathogen strain can invade the resident as long as invasion implies replacement. Other studies have focused on gradient dynamic evolutionary models where only pathogen strains close to the resident can attempt to invade the resident (Abrams et al. 1993; Dieckmann and Law 1996). In these models, the pathogen evolves in the direction of increasing fitness by climbing the fitness gradient. As shown in Appendix G, the conditions under which evolution maximizes \(\mathcal R _0\) in a system with a single infectious class are very similar to those of system (5). In particular, each functional response must factor into terms representing environmental and trait effects and there can be no genotype-by-environment interaction terms. This suggests that conclusions about \(\mathcal R _0\) maximization in those host–pathogen evolutionary models may also be useful in identifying other mechanisms through which higher dimensional environmental feedbacks arise.
In total, the conditions for \(\mathcal R _0\) maximization presented in this study have shown that there are many biological mechanisms that inhibit \(\mathcal R _0\) maximization in natural systems. This suggests that additional theoretical studies involving more realistic functional forms and fewer simplifying biological assumptions are necessary to understand if \(\mathcal R _0\) maximization in natural systems. The approach taken in this study may also be useful in identifying biological conditions under which optimization principles beyond \(\mathcal R _0\) maximization arise. An important area of research is understanding if optimization principles of any kind are expected to arise in natural systems.
References
Abrams PA (2001) Modelling the adaptive dynamics of traits involved in inter- and intraspecific interactions: an assessment of three models. Ecol Lett 4:166–175
Abrams PA (2005) ‘Adaptive dynamics’ vs. ‘adaptive dynamics’. J Evol Biol 18:1162–1165
Abrams PA, Matsuda H, Harada Y (1993) Evolutionarily unstable fitness maxima and stable fitness minima of continuous traits. Evol Ecol 7:465–487
Alizon S, Hurford A, Mideo N, van Baalen M (2009) Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J Evol Biol 22:245–259
Anderson RM, May RM (1982) Coevolution of hosts and parasites. Parasitology 85:411–426
Barlow ND (2000) Non-linear transmission and simple models for bovine tuberculosis. J Anim Ecol 69: 703–713
Basu S, Galvani AP (2009) The evolution of tuberculosis virulence. Bull Math Biol 71:1073–1088
Boots M, Sasaki A (2003) Parasite evolution and extinctions. Ecol Lett 6:176–182
Bremermann HJ, Thieme HR (1989) A competitive exclusion principle for pathogen virulence. J Math Biol 27:179–190
Briggs CJ, Godfray HCJ (1995) The dynamics of insect-pathogen interactions in stage-structured populations. Am Nat 145:855–887
Brown NF, Wickham ME, Coombes BK, Finlay BB (2006) Crossing the line: selection and evolution of virulence traits. PLoS Pathog 2:e42
Bull JJ (1994) Virulence. Evolution 48:1423–1437
Capasso V, Serio V (1978) Generalization of the kermack-mckendrick deterministic epidemic model. Math Biosci 42:43–61
Dercole F, Rinaldi S (2008) Analysis of evolutionary processes: the adaptive dynamics approach and its applications. Princeton University Press, Princeton
Dieckmann U, Law R (1996) The dynamical theory of coevolution: a derivation from stochastic ecological processes. J Math Biol 34:579–612
Dieckmann U, Metz JAJ, Sabelis MW, Sigmund K (2002) Adaptive dynamics of infectious diseases: adaptive dynamics of pathogen-host interactions. In: Pursuit of virulence management. Cambridge University Press, Cambridge
Diekmann O, Kretzschmar M (1991) Patterns in the effects of infectious diseases on population growth. J Math Biol 29:539–570
Diekmann O, Heesterbeek JAP, Metz JAJ (1990) On the definition and the computation of the basic reproduction ratio \(R_0\) in models for infectious diseases in heterogeneous populations. J Math Biol 28:365–382
Dwyer G, Elkinton JS, Buonaccorsi JP (1997) Host heterogeneity in susceptibility and disease dynamics: tests of a mathematical model. Am Nat 150:685–707
Ebert D, Herre EA (1996) The evolution of parasitic diseases. Parasitol Today 12:96–101
Frank SA (1996) Models of parasite virulence. Q Rev Biol 71:37–78
Gandon S, Mackinnon MJ, Nee S, Read AF (2001) Imperfect vaccines and the evolution of pathogen virulence. Nature 414:751–756
Geritz SAH (2005) Resident-invader dynamics and the coexistence of similar strategies. J Math Biol 50: 67–82
Geritz SAH, Kisdi É, Meszéna G, Metz JAJ (1998) Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evol Ecol 12:35–57
Geritz SAH, Gyllenberg M, Jacobs FJA, Parvinen K (2002) Invasion dynamics and attractor inheritance. J Math Biol 44:548–560
Gog JR, Grenfell BT (2002) Dynamics and selection of many-strain pathogens. Proc Natl Acad Sci USA 99:17,209–17,214
Gyllenberg M, Metz J, Service R (2011) When do optimization arguments make evolutionary sense? In: Chalub FACC, Rodrigues JF (eds) The mathematics of Darwin’s legacy. Springer, Basel, pp 44–118
Hartl DL, Clark AG (2007) Principles of population genetics. Sinauer Associates, Sunderland
Heesterbeek JAP, Metz JAJ (1993) The saturating contact rate in marriage and epidemic models. J Math Biol 29:539–570
Knell RJ, Begon M, Thompson DJ (1996) Transmission dynamics of Bacillus thuringiensis infecting Plodia interpunctella: a test of the mass action assumption with an insect pathogen. P Roy Soc Lond B Biol 263:75–81
Lande R (1982) A quantitative genetic theory of life history evolution. Ecology 63(3):607–615
Lenski RE, May RM (1994) The evolution of virulence in parasites and pathogens: reconciliation between two competing hypotheses. J Theor Biol 169:253–265
Levin BR (1996) The evolution and maintenance of virulence in microparasites. Emerg Infect 2:93–102
Lipsitch M, Siller S, Nowak MA (1996) The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution 50:1729–1741
May RM, Nowak MA (1995) Coinfection and the evolution of parasite virulence. Proc Roy Soc Lond B: Biol 261:209–215
McCallum H, Barlow N, Hone J (2001) How should pathogen transmission be modelled? Trends Ecol Evol 16:295–300
Medlock J, Luz PM, Struchiner CJ, Galvani AP (2009) The impact of transgenic mosquitoes on Dengue virulence to humans and mosquitoes. Am Nat 174:565–577
Metz JAJ, Mylius SD, Diekmann O (2008) When does evolution optimize? Evol Ecol Res 10:629–654
Mosquera J, Adler FR (1998) Evolution of virulence: a unified framework fro coinfection and superinfection. J Theor Biol 195:293–313
Mylius SD, Diekmann O (1995) On evolutionary stable life histories, optimization and the need to be specific about density dependence. Oikos 74:218–224
Nowak MA (1991) The evolution of viruses. Competition between horizontal and vertical transmission of mobile genes. J Theor Biol 150:339–347
Nowak MA, May RM (1994) Superinfection and the evolution of virulence. Proc Roy Soc B: Biol Sci 255:81–89
Roberts MG (1996) The dynamics of bovine tuberculosis in possum populations and its eradication or control by culling or vaccination. J Anim Ecol 65:451–464
Smith JM, Price GR (1973) The logic of animal conflict. Nature 246:15–18
Smith MJ, Telfer S, Kallio ER, Burthe S, Cook AR, Lambin X, Begon M (2009) Hostpathogen time series data in wildlife support a transmission function between density and frequency dependence. Proc Natl Acad Sci USA 106:7905–7909
Thieme HR (2007) Pathogen competition and coexistence and the evolution of virulence. In: Takeuchi Y, Iwasa Y, Sato K (eds) Mathematics for life science and medicine, biological and medical physics, biomedical engineering. Springer, Berlin, pp 123–153
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180:29–48
Acknowledgments
I thank Lauren Childs and Paul Hurtado for suggestions on a previous version of the manuscript. I thank Mark Lewis and two anonymous reviewers for suggestions and comments on the manuscript. This work was partially supported by the National Science Foundation under Award No. DMS-1204401 awarded to MHC. This work was also supported by a Career Award at the Scientific Interface from the Burroughs Wellcome Foundation and a grant from the James S. McDonnell Foundation awarded to Joshua S. Weitz.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A: Review of theory
Here we review the next generation technique in van den Driessche and Watmough (2002) for computing a pathogen’s reproductive number and the optimization theory in Mylius and Diekmann (1995). Note that in order to account for vector transmission systems, the notation in this appendix is slightly different from that in the main text.
1.1 A.1 Computing the reproductive number
Let \(S\) and \(R\) denote the susceptible and recovered classes. Note that \(S\) and \(R\) can be multidimensional variables, e.g. as is the case in vector-borne systems where there are susceptible hosts and susceptible vectors. Denote all infected classes by \(C_j\) for \(1 \le j \le n\). Let \(\theta \) be the one dimensional trait that characterizes the pathogen. The equations for the general host–pathogen system are
where \(\mathcal C =(C_1,\ldots ,C_n)\). The functions \(\mathcal G _S\) and \(\mathcal U _S\) denote the rates at which hosts enter and leave the susceptible classes, respectively. The corresponding rates for the recovered class are \(\mathcal G _R\) and \(\mathcal U _R\). The functions \(C_k\mathcal F ^{(k)}_{C_j}\) denote the rates at which newly infected individuals enter class \(C_j\) due to transmission caused by individuals in class \(C_k\). \(\mathcal F ^{(k)}_{C_j}=0\) if class \(C_k\) is an exposed class or does not contribute to the influx of newly infected individuals in class \(C_j\). Note that at this point we do not make any assumptions about which classes newly infected individuals enter. \(C_{j-1}\mathcal V ^{+}_{C_j}\) and \(C_j\mathcal V ^{-}_{C_j}\) are the rates at which already infected individuals enter and leave class \(C_j\).
We assume all functions are positive and finite when evaluated at point where \(C_j=0\) for all \(j\). We assume that in the absence of the pathogen, system (30) converges to a disease free equilibrium where \(C_j = R=0\) and \(S=N\). We assume that for any fixed value of the pathogen trait, system (30) tends to an endemic equilibrium or to the disease free equilibrium.
For notational convenience define
where \(\bar{\mathcal{C }} = (\bar{C}_1,\ldots ,\bar{C}_n)\). Let \(\mathbf{M}_F\) and \(\mathbf{M}_V\) be the matrices
Then the reproductive number for a pathogen with trait \(\theta \) in a population with host class densities \(S,\,\mathcal C \), and \(R\) is
where \(\rho (\mathbf{M})\) is the spectral radius of a matrix M (Diekmann et al. 1990; van den Driessche and Watmough 2002). The basic reproductive number for a pathogen invading the disease free equilibrium is \(\mathcal{R }_0(\theta )=\mathcal{R }(N,0,0,\theta )\) where \(\mathcal C =0\) implies that \(C_j=0\) for all \(j\).
1.2 A.2 Evolution
To study the evolutionary dynamics of the pathogen in system (30), we assume that evolution occurs on a slower time scale than the epidemiological dynamics of the system and assume that selection is frequency independent. We then ask when a nonresident strain of the pathogen can invade the endemic equilibrium of the resident strain. We assume that all pathogen strains can potentially invade the resident. We further assume that an individual host cannot be infected by more than one strain of the pathogen and that recovered individuals are immune to all pathogen strains. Finally, we assume that any invading pathogen strain that successfully invades the resident strain replaces the current resident strain and becomes the new resident strain.
Let \(S^{*},\,\mathcal C ^{*}\), and \(R^{*}\) be the densities of the susceptible, infected, and recovered classes, respectively, at the endemic equilibrium of the resident pathogen with trait value \(\theta _r\). When selection is frequency independent, an invading strain of the pathogen with trait \(\theta _{i}\) can only invade the endemic equilibrium of the resident if \(\mathcal R (S^{*},\mathcal C ^*,R^{*},\theta _i)>1\). Note that the reproductive number for a pathogen invading its own endemic equilibrium is \(\mathcal R (S^{*},\mathcal C ^*,R^{*},\theta _r)=1\). Our goal is to find trait values that are Evolutionary Stable Strategies (ESSs, Smith and Price 1973) and that maximize \(\mathcal R _0\).
Under the above assumptions and assuming frequency independent selection, evolution maximizes \(\mathcal R _0\) when \(\mathcal R (S,I,R,\theta )\) can be written as
where \(g(S,R)\) is a positive function (Mylius and Diekmann 1995). The following proof is taken from Mylius and Diekmann (1995). We know that
Substitution into Eq. (35) yields
Thus, a pathogen can invade the endemic equilibrium of the resident, i.e. \(\mathcal R (S^{*},\mathcal C ^*,R^{*},\theta _i)>1\), if the basic reproductive number of the invader is greater than the resident. This result is a special case of one theorem in Metz et al. (2008). In particular, if the basic reproductive number can be written in the form \(\mathcal R (X,\theta ) = \exp [\alpha (\psi (\theta ),X))]\) where \(\alpha \) is increasing in \(\psi \), then evolution maximizes \(\mathcal R _0\) (Metz et al. 2008).
Appendix B: Systems with a single infectious class
In the following we derive results for host–pathogen systems where infected individuals pass through a single infectious class. We first consider system (5) where there is one susceptible, one infectious class, and one recovered class. Then we address two different types of systems where there are multiple susceptible classes (i.e. host heterogeneity). In the first case, we consider systems where all infectious hosts are the same. Biologically, this would arise when host are heterogeneous in their susceptibility to the pathogen and homogeneous in how they transmit the pathogen once infected. In the second case, we consider systems where newly infected individuals from different susceptible classes enter different infectious classes. This would arise in natural systems where hosts have different genotypes that affect both their susceptibility to the pathogen and their transmissibility of pathogen to other hosts (e.g. clonal species).
1.1 B.1 One susceptible and one infectious class
The model with a single susceptible class and a single infectious class is
Since there is only one infected class, \(\mathcal V ^{+}_I =0\). We assume \(\mathcal F _{I}\) and \(\mathcal V ^{-}_{I}\) are finite and positive when evaluated at points where \(I= 0\). We define \(\mathcal V _I(S,I,R,\bar{I}) = \bar{I}\mathcal V ^{-}_I\).
We compute the reproductive number, \(\mathcal R (S,I,R,\theta )\), for a pathogen with trait \(\theta \) using the next generations technique from van den Driessche and Watmough (2002). For system (38), the matrices \(\mathbf{M}_F\) and \(\mathbf{M}_V\) are the scalar functions \(\mathcal F _{I}(S,I,R,\theta )\) and \(\mathcal V ^{-}_{I}(S,I,R,\theta )\), respectively. Thus, \(\mathcal R (S,I,R,\theta )\) is
The basic reproductive number for a pathogen invading the disease free equilibrium is \(\mathcal R _0(\theta )=\mathcal R (N,0,0,\theta )\).
The reproductive number factors as \(\mathcal R (S,I,R,\theta ) = g(S,I,R) \mathcal R _0(\theta )\) under the following conditions
-
(a1)
\(\mathcal F _{I}(S,R,\theta ) \,= f_{I}(S,I,R)\eta _{I}(\theta )\xi (S,I,R,\theta )\)
-
(a2)
\(\mathcal V ^{-}_{I}(S,R,\theta ) = v^{-}_{I}(S,I,R)\nu ^{-}_{I}(\theta )\xi (S,I,R,\theta )\).
The interpretation of the conditions is included in the main text. Note that condition (A3) from the main text is present in conditions (a1) and (a2) via the shared genotype-by-environment interaction term \(\xi (S,I,R,\theta )\).
The function \(\mathcal V ^{-}_I\) can be decomposed into mortality, \(\mathcal D _I\), and transfer, \(\mathcal T _I\), rates as \(\mathcal V ^{-}_I =\mathcal D _I + \mathcal T _I\). Transfer rates include recovery and progression of the disease (when there are multiple infected classes) as well as stage structure in the host population. Under this decomposition, the reproductive number is
and condition (a2) becomes
-
(a2.1)
\(\mathcal T _{I} = t_{I}(S,I,R)\tau (\theta )\xi (S,I,R,\theta )\) and \(\mathcal D _{I}=0\)
-
(a2.2)
\(\mathcal T _{I}=0\) and \(\mathcal D _{I} = d_{I}(S,I,R)\delta (\theta )\xi (S,I,R,\theta )\)
-
(a2.3)
\(\mathcal T _{I}=t_{I}(S,I,R)\tau (\theta )\xi (S,I,R,\theta ),\,\mathcal D _{I}=d_{I}(S,I,R)\delta (\theta )\xi (S,I,R,\theta )\), and \(\tau = \delta \)
-
(a2.4)
\(\mathcal T _{I}=t_{I}(S,I,R)\tau (\theta )\xi (S,I,R,\theta ),\,\mathcal D _{I}=d_{I}(S,I,R)\delta (\theta )\xi (S,I,R,\theta )\), and \(t_{I}=d_{I}\)
The biological interpretation of these conditions is also included in the main text.
1.2 B.2 Multiple susceptible classes
We now consider systems with multiple susceptible classes. We assume there are \(n_S\) susceptible classes denoted by \(S_j\). At the disease free equilibrium, the density of the class \(S_j\) is \(N_j\).
1.2.1 Single Infectious Class
First we consider the case where all newly infected individuals from any susceptible class enter the same infectious class \(I\). The dynamics of the infectious class are given by
where \(X = (S_1,\ldots ,S_{n_S},I,R)\). The functions \(I\mathcal F _j\) are the recruitment rates of susceptible individuals in class \(S_j\) into the infectious class. We assume \(\mathcal F _{j}\) and \(\mathcal V ^{-}_{I}\) are positive and finite when evaluated at any point where \(I=0\).
The reproductive number for the pathogen is
where \(X = (S_1,\ldots ,S_{n_S},I,R)\). The basic reproductive number for the pathogen invading the disease free equilibrium is \(\mathcal R _0(\theta ) = \mathcal R (N_1,\ldots ,N_{n_S},0,0,\theta )\). For \(\mathcal R (X,\theta )\) to factor as in Eq. (35) and for evolution to maximize \(\mathcal R _0\), condition (a1) becomes
-
(a1.1)
\(\mathcal F _{j} = f_{j}(X)\eta _j(\theta )\xi (X,\theta )\)
-
(a1.2)
Either \(f_j=f_k\) for all \(j,k\) or \(\eta _j(\theta )=\eta _k(\theta )\) for all \(j,k\)
As with condition (a1), the factorization in condition (a1.1) implies that there are no genotype-by-environment interaction effects and that the pathogen only utilizes a single transmission pathway for each susceptible class. The equalities in condition (a1.2) also have biological interpretations. If the equality \(f_j=f_k\) in condition (a1.2) is satisfied, then the recruitment rates from each susceptible class must have the same density dependence. Biologically, this is unlikely to occur in any system. If the equality \(\eta _j(\theta )=\eta _k(\theta )\) in condition (a1.2) is satisfied, then the pathogen trait must affect the recruitment from each susceptible class the same. Biologically, this would arise in systems where pathogen evolution that increased the transmission rate by fifty percent for one class also increase the transmission rate by fifty percent for all other classes as well. Note that this condition implies that \(\mathcal R _0\) maximization will not occur in systems where there is a trade-off between the recruitment rates from two different susceptible classes.
1.2.2 Multiple Infectious Classes
Now consider the case where individuals from different susceptible classes enter different infectious classes. We will focus on the case where there are only two susceptible classes because the analysis of the reproductive number becomes analytically intractable for more than two susceptible classes. In the case where there are two susceptible classes, newly infected individuals that were in class \(S_1\) enter into class \(I_1\) and newly infected individuals that were in class \(S_2\) enter into class \(I_2\). Individuals in classes \(S_1\) and \(I_1\) cannot transfer to classes \(S_2\) or \(I_2\) and vice versa. We refer to hosts in classes \(S_1\) and \(I_1\) as clonal type 1. Similarly, we refer to hosts in classes \(S_2\) and \(I_2\) as clonal type 2.
Let \(X = (S_1, S_2, I_1, I_2, R)\). The dynamics of the infectious classes are given by
where the functions \(I_j \mathcal F _{I_j}\) denotes recruitment due to transmission between individuals of the same clonal type and \(I_k\mathcal H _{I_j}\) denotes recruitment due to transmission between individuals of different clonal types. For example, \(I_2\mathcal H _{I_1}\) denotes the recruitment of newly infected individuals of clonal type 1 due to the transmission of the pathogen from individuals of clonal type 2. Note that we will ignore the special case where \(\mathcal H _{I_j}=0\). In that case, transmission cannot occur between clonal types and we would expect the pathogen to evolve independently in each clonal population. We assume \(\mathcal F _{I_j},\,\mathcal H _{I_j}\), and \(\mathcal V ^{-}_{I_j}\) are positive and finite when evaluated at any point where \(I_1 = I_2 =0\).
The reproductive number for the pathogen is
The basic reproductive number for the pathogen invading the disease free equilibrium is \(\mathcal R _0(\theta ) = \mathcal R (N_1,N_2,0,0,0,\theta )\). Evolution maximizes \(\mathcal R _0\) when all of the following conditions hold
-
(b1)
\(\mathcal F _{I_j}(X,\theta ) = f_{I_j}(x)\eta _{I_j}(\theta )\xi _j(X,\theta )\)
-
(b2)
\(\mathcal V ^{-}_{I_j}(X,\theta ) = v^{-}_{I_j}(x)\nu _{I_j}(\theta )\xi _j(X,\theta )\)
-
(b3)
\(\mathcal H _{I_j}(X,\theta ) = h_{I_j}(x)\eta _{I_j}(\theta )\xi _j(X,\theta )\)
and when one of the following is satisfied
-
(b4.1)
\(f_{I_j} = h_{I_k}\) and \(v^{-}_{I_j} = v^{-}_{I_k}\) for all \(j\) and \(k\)
-
(b4.2)
\(\eta _{I_j} = \eta _{I_k}\) and \(\nu ^{-}_{I_j}=\nu ^{-}_{I_k}\) for all \(j\) and \(k\)
As with conditions (a1) and (a2), conditions (b1) through (b3) require the derivatives of the functions to factor into components that represent the effects of the densities of the uninfected classes, the trait, and genotype-by-environment interactions. The biological interpretation of conditions (b1) through (b3) is the same as that for conditions (a1) and (a2).
Conditions (b4.1) and (b4.2) require all recruitment rates to have either the same dependence on the uninfected classes or the same dependence on the pathogen trait. Those conditions also require the exit rates for the two infectious classes to have the same dependence either on the densities of the uninfected classes or the pathogen trait. Biologically, we do not expect condition (b4.1) to be satisfied as it implies that all recruitment rates into \(I_1\) and \(I_2\) must depend on both susceptible classes. Condition (b4.2) is satisfied in systems where the trait affects the recruitment and exit rates for both infectious classes the same. For example, evolution resulting in a fifty percent increase in transmissibility to one susceptible class would automatically result in a fifty percent increase in transmissibility to all susceptible classes. Note that this implies that there are no nonlinear interactions between the pathogen trait and the host clonal type. It is unclear how often these conditions will be satisfied by natural systems.
Appendix C: Systems with multiple infectious classes
In this appendix we consider host–pathogen systems where there are \(n_I\) infected class, all of which are infectious. We first consider systems where all newly infected individuals enter through the first infectious class. Then we consider systems where newly infected individuals enter the class of the infectious individual that infected them. We do not include an analysis for systems where newly infected individuals enter any infectious class because the calculations become analytically intractable for more than two infectious classes. Finally, we examine a system with two infectious classes where newly infected individuals enter the first infectious class and can revisit that class.
1.1 C.1 Single entry class
Consider a system with a single susceptible class, a single recovered class, and \(n_I\) infectious classes. We assume all newly infected individuals enter through class \(I_1\). We also assume that infectious individuals move through the classes sequentially. Note that individuals can recover from any infectious class.
Let \(X=(S,I_1,\ldots ,I_{n_I},R)\). The equations for the infectious classes are
where \(I_j\mathcal F _{I_1}^{(j)}\) is the rate newly infected individuals enter class \(I_1\) due to transmission caused from individuals from class \(I_j\). We assume the following functions are finite when evaluated at \(I_j = 0\) for all \(j\): \( \mathcal F _{I_1}^{(j)}\), \(\mathcal V ^{+}_{I_{j}}\), and \(\mathcal V ^{-}_{I_j}\). An example of system (45) is
where the \(\gamma _j\) are the transmission coefficients, \(1/\nu _j\) is the mean time spent in infectious class \(I_j,\,\mu _j\) is the stage specific per capita death rate, \(\omega _j\) is the recovery rate for individuals in class \(I_j\), and all parameters are potential functions of \(\theta \).
The reproductive number for a pathogen with trait \(\theta \) can be computed using the methods in van den Driessche and Watmough (2002); see Appendix A. The matrix \(\mathbf{M}_{F}\) is composed of zeros except along the first row where the terms are \(\mathcal F _{I_1}^{(j)}(X,\theta )\). In the matrix \(\mathbf{M}_{V}\), the main diagonal has entries \(\mathcal V ^{-}_{I_j}(X,\theta )\) and the subdiagonal has entries \(\mathcal V ^{+}_{I_{j}}(X,\theta )\). All other entries of \(\mathbf{M}_{V}\) are zero. The only nonzero eigenvalue of the product \(\mathbf{M}_F \mathbf{M}_V^{-1}\) is the reproductive number,
where all functions are evaluated at \((X,\theta )\). The basic reproductive number for a pathogen invading the disease free equilibrium is \(\mathcal R _0(\theta ) =\mathcal R (N,0,\ldots ,0,\theta )\). \(\mathcal R (X,\theta )\) factors when the following conditions hold
-
(c1)
\(\mathcal F _{I_1}^{(j)} = f_{I_1}^{(j)}(X)\eta _{I_1}^{(j)}(\theta )\xi _1(X,\theta )\)
-
(c2)
\(\mathcal V ^{-}_{I_j}\,\,=v^{-}_{I_j}(X)\nu ^{-}_{I_j}(\theta )\xi _j(X,\theta )\)
-
(c3)
\(\mathcal V ^{+}_{I_j}\,\,=v^{+}_{I_j}(X)\nu ^{+}_{I_j}(\theta )\xi _j(X,\theta )\)
-
(c4)
\(f^{(j)}_{I_1} = f^{(k)}_{I_1}\) for all \(j,k\) or \(\eta ^{(j)}_{I_1} = \eta ^{(k)}_{I_1}\) for all \(j,k\).
-
(c5)
\(v^{-}_{I_j} \,\,= v^{+}_{I_j}\) for all \(j\ge 2\) or \(\nu ^{-}_{I_j} = \nu ^{+}_{I_j}\) for all \(j\ge 2\).
System (46) is an example of a system that satisfies all conditions.
Conditions (c1) through (c5) have a particular structure. First, each function factors into terms representing the effects of the environment (\(f_{I_1}^{(j)},\,v^{-}_{I_j}\), and \(v^{+}_{I_j}\)), the trait (\(\eta _{I_1}^{(j)},\,\nu ^{-}_{I_j}\) and \(\nu ^{+}_{I_j}\)), and genotype-by-environment interactions \((\xi _j)\). Second, for each infectious class, the genotype-by-environment interaction effects are the same for all rates. Third, all transmission rates have the same dependence either on the trait or the host classes. The same holds for the entry \((\mathcal V ^{+}_{I_j})\) and exit \((\mathcal V ^{-}_{I_j})\) rates for each infectious class. Note that this structure will arise in all later cases where there are multiple infectious classes.
We now give the biological interpretation of conditions (c1) through (c5). Note that the entry rate into class \(I_j\) is equal to the transfer rate out of class \(I_{j-1}\). Because the genotype-by-environment interaction terms are the same within each class, this implies that the genotype-by-environment interaction terms must be the same across all classes. We do not expect this to be the case in natural systems, thus \(\mathcal R _0\) maximization will only occur in systems where there are no genotype-by-environment interactions. The equality of the transfer rates in combination with condition (c5) implies that the entry and exit rates for all classes must have either the same dependence on the trait or the same dependence on the densities of the host classes. We do not expect all of the entry and exit rates to have the same dependence on the trait nor do we do not expect the per capita transfer rates to depend on the host population size. Thus, evolution will maximize \(\mathcal R _0\) under two cases: either (i) the per capita transfer rates and death rates for all classes are constant with respect to the host classes or (ii) the pathogen trait only affects the transfer and mortality rates of the first infectious class. System (46) satisfies the first condition. The second condition could arise in stage-structured or age-structured host populations. In particular, this can arise when the pathogen is only contracted during a single stage, the host pays a trait dependent fitness cost only during that stage, and the pathogen can be transmitted for part or the rest of the host’s life. Note that while the contraction of the pathogen earlier in life can result in a loss of fitness in later stages, that fitness loss cannot depend on the pathogen trait.
1.2 C.2 Multiple entry classes
We now consider the case where newly infected individuals enter into the class of the individual that infected them. For example, a susceptible individual that was infected by an individual in class \(I_j\) enters through class \(I_j\). Then, each individual passes through classes \(I_k\) for \(k>j\) sequentially. The equations for the infected classes are
where \(X=(S,I_1,\ldots ,I_{n_I},R)\). \(\mathcal F ^{(j)}_{I_j}\) is the rate at which newly infected individuals enter class \(I_j\) due to transmission of the pathogen from individuals in class \(I_j\). We assume the following functions are finite and nonzero when evaluated at \(I_j = 0\) for all \(j\): \( \mathcal F ^{(j)}_{I_j},\,\mathcal V ^{+}_{I_j}\), and \(\mathcal V ^{-}_{I_j}\). A example of such a system is
where \(\gamma _j\) is the transmission coefficient for class \(I_j,\,1/\nu _j\) is the mean times spent in class \(I_j,\,\mu _j\) is the stage specific per capita death rate, and \(\omega _j\) is the recovery rate for individuals in class \(I_j\). All of the parameters are potentially functions of \(\theta \).
Using the methods in van den Driessche and Watmough (2002), we have that the matrix \(\mathbf{M}_{F}\) is a diagonal matrix with entries \(F^{(j)}_{I_j}\). The matrix \(\mathbf{M}_{V}\) has entries \(\mathcal V ^{-}_{I_j}\) along the diagonal, \(-\mathcal V ^{+}_{I_{j}}\) along the subdiagonal, and zeros elsewhere. The eigenvalues of the product \(\mathbf{M}_F \mathbf{M}_V^{-1}\) are the collection
The reproductive number for the pathogen is the maximum of these values. The basic reproductive number is the maximum of the values evaluated at \((X,\theta )=(N,0,\ldots ,0,\theta )\).
Evolution maximizes \(\mathcal R _0\) under two cases. For the first case, assume one element of the set (50) is greater than all others for all values of \(S,\,I_j,\,R\), and \(\theta \). That is, for some \(k\), assume \(\mathcal F ^{(k)}_{I_k}/\mathcal V ^{-}_{I_k} > F^{(j)}_{I_j}/\mathcal V ^{-}_{I_j}\) for all \(j\). Biologically, this means that stage \(I_k\) is the most important stage for lifetime transmission of the pathogen. This condition also means that stage \(I_k\) is most important stage regardless of the pathogen’s trait value. In this case, \(\mathcal R (X,\theta )\) factors under conditions (a1) and (a2) in Appendix B. For the second case, assume for all \(j\) that \(F^{(j)}_{I_j}(X,\theta ) = f(X)\eta (\theta )\xi _j(X,\theta )\) and \(\mathcal V ^{-}_{I_j} = v(X) \nu (\theta ) \xi _j(X,\theta )\). In this case, all of the transmission rates have the same dependence on the host classes and the trait. Similarly, all of the exit rates have the same dependence on the host classes and the trait. This case is biologically degenerate in the sense that the trait value that maximizes fitness for a particular infectious class maximizes fitness for all classes. We do not expect the second to case to arise in natural systems.
1.3 C.3 Revisiting infectious classes
We now consider a system where there are only two infectious classes. We assume newly infected individuals enter into the first infectious class and that infectious individuals can return to the first infectious class after entering the second infectious class. This particular system shows the additional constraints that arise when individuals can enter previous infectious classes. This case can arise in systems where the disease or pathogen goes into remission. The equations for the infectious classes are
where \(X = (S,I_1,I_2,R)\). We assume the following functions are finite and nonzero when evaluated at \(I_j = 0\) for all \(j=1,2\): \( \mathcal F ^{(j)}_{I_j},\,\mathcal V ^{+}_{I_j}\), and \(\mathcal V ^{-}_{I_j}\).
Following the methods in Appendix A, the top row of the matrix \(\mathbf{M}_{F}\) has entries \(F^{(j)}_{I_j}\) and the bottom row is zeros. The matrix \(\mathbf{M}_{V}\) has entries \(\mathcal V ^{-}_{I_j}\) along the diagonal, \(-\mathcal V ^{+}_{I_{2}}\) in the bottom left entry and \(-\mathcal V ^{+}_{I_{1}}\) in the top right entry. The eigenvalue of the product \(\mathbf{M}_F \mathbf{M}_V^{-1}\) is the reproductive number
The basic reproductive number for a pathogen invading the disease free equilibrium is \(\mathcal R _0(\theta ) =\mathcal R (N,0,0,0,\theta )\). \(\mathcal R (X,\theta )\) factors when the following conditions hold
-
(d1)
\(\mathcal F _{I_1}^{(j)} = f_{I_1}^{(j)}(X)\eta _{I_1}^{(j)}(\theta )\xi (X,\theta )\)
-
(d2)
\(\mathcal V ^{-}_{I_j}=v^{-}_{I_j}(X)\nu ^{-}_{I_j}(\theta )\xi (X,\theta )\)
-
(d3)
\(\mathcal V ^{+}_{I_j}=v^{+}_{I_j}(X)\nu ^{+}_{I_j}(\theta )\xi (X,\theta )\)
and one of the following conditions holds
-
(d4.1)
\(f^{(1)}_{I_1} = f^{(2)}_{I_1}\) and \(v^{-}_{I_j} = v^{+}_{I_j}\)
-
(d4.2)
\(\eta ^{(1)}_{I_1} = \eta ^{(2)}_{I_1}\) and \(\nu ^{-}_{I_j} = \nu ^{+}_{I_j}\)
The biological consequences and interpretation of conditions (d1) through (d3) are the same as in the previous subsection of this appendix. Condition (d4.2) is unlikely to hold because we do not expect the pathogen trait to have the same effect on the per capita transfer, recovery and mortality rates. The first equality in condition (d4.1) implies that pathogen transmission does not differ mechanistically for different infectious classes. The second equality in condition (d4.1) implies the per capita entry and exit rates of the classes must have the same dependence on the host classes. This is only likely to occur when when mortality is not density dependent and the per capita recovery and transfer rates are independent of the host class sizes. Thus, when infected individuals can revisit previous infectious classes, evolution maximizes \(\mathcal R _0\) only when all pathogen related processes except for transmission are density independent.
Appendix D: Systems with multiple exposed and infectious classes
In this appendix we consider host–pathogen systems with multiple exposed classes. We first consider the case where there are \(n_E\) exposed classes and one infectious class. Then we consider the case where there are multiple exposed and multiple infectious classes. In all cases we assume that all of the exposed classes come before the infectious classes. We also assume each newly infected individual enters through the first exposed class and then passes through the other infected class sequentially. Finally, we consider the case where individuals can revisit a previously visited exposed class in a model with one exposed and one infectious classes.
1.1 D.1 One infectious class
Let \(n_E\) be the number of exposed classes. Assume there is one infectious class. The equations for the infected classes are
where \(X=(S,E_1,\ldots ,E_{n_E},I,R)\). \(I_1 \mathcal F _{E_1}\) is the rate at which newly infected individuals enter class \(E_1\) due to transmission via individuals in the infected class \(I_1\). Note that \(\mathcal V ^{+}_{E_1}=0\) because only newly infected individuals enter the first exposed class. We assume that the following are finite when evaluated at at \(E_j= I_1 = 0\) for all \(j\): \(\mathcal F _{E_1},\,\mathcal V ^{+}_{E_{j}},\,\mathcal V ^{-}_{E_j},\,\mathcal V ^{+}_{I_1}\), and \(\mathcal V ^{-}_{I_1}\). A simple example system (53) is
where \(\gamma _1\) is the transmission coefficient, \(1/\kappa _j\) is the mean time spent in exposed class \(E_j,\,\lambda _j\) is per capita death rate of exposed class \(E_j\), and \(\epsilon _j\) is the recovery rate for individuals in class \(E_j\). The interpretation of the remaining parameters is as in system (49). All of the parameters are potentially functions of \(\theta \).
We compute the reproductive number using the methods in van den Driessche and Watmough (2002). In the matrix \(\mathbf{M}_{F}\), the top right entry is \(\mathcal F _{E_1}(X,\theta )\) and all other entries are zero. In the matrix \(\mathbf{M}_{V}\), the main diagonal has entries \(\mathcal V ^{-}_{E_j}(X,\theta )\) and \(\mathcal V ^{-}_{I_1}(X,\theta )\) and the subdiagonal of the matrix has entries \(-\mathcal V ^{+}_{E_{j}}(X,\theta )\) and \(-\mathcal V ^{+}_{I_1}(X,\theta )\). All other entries of \(\mathbf{M}_{V}\) are zero. The only nonzero eigenvalue of the product \(\mathbf{M}_F \mathbf{M}_V^{-1}\) is the reproductive number,
The basic reproductive number of the pathogen is \(\mathcal R _0(\theta )=\mathcal R (N,0,\ldots ,0,\theta )\).
The reproductive number \(\mathcal R (X,\theta )\) factors and evolution to maximizes \(\mathcal R _0\) under the following conditions
-
(f1)
\(\mathcal F _{E_1} = f_{E_1}(X)\eta _{I_1}(\theta )\xi _1(X,\theta )\)
-
(f2)
\(\,\mathcal V ^{-}_{E_1}=v^{-}_{E_1}(X)\nu ^{-}_{E_1}(\theta )\xi _1(X,\theta )\)
-
(f3)
\(\,\mathcal V ^{+}_{E_j}=v^{+}_{E_j}(X)\nu ^{+}_{E_j}(\theta )\xi _j(X,\theta ) \text{ for} j\ge 2\)
-
(f4)
\(\,\mathcal V ^{-}_{E_j}=v^{-}_{E_j}(X)\nu ^{-}_{E_j}(\theta )\xi _j(X,\theta ) \text{ for} j \ge 2.\)
-
(f5)
\(\,\,\,\mathcal V ^{+}_{I_1}=v^{+}_{I_1}(X)\nu ^{+}_{I_1}(\theta )\xi _I(X,\theta )\)
-
(f6)
\(\,\,\,\mathcal V ^{-}_{I_1}=v^{-}_{I_1}(X)\nu ^{-}_{I_1}(\theta )\xi _I(X,\theta )\)
The structure for conditions (f1) through (f6) is the following. Each function factors into terms representing the environmental, trait, and genotype-by-environment interaction effects. For any given infected class, the genotype-by-environment interaction effects are the same across all rates. Note that the transfer rate into any infected class is equal to the infection progression rate out of the previous infected class. Thus, as a consequence of decomposing the exit rates into recovery, mortality, and infection progression rates, all classes must have the same dependence either on the pathogen trait or the densities of the uninfected host classes. The biological consequences for the these conditions are similar to those of the previous cases.
1.2 D.2 Multiple infectious classes
Let us now consider a system with \(n_E\) exposed classes and \(n_I\) infectious classes. The equations for the infected classes are
where \(X=(S,E_1,\ldots ,E_{n_E},I_1,\ldots ,I_{n_I},R)\). The functions \(I_j\mathcal F _{E_1}^{(j)}\) are the rates at which newly infected individuals enter class \(E_1\) due to transmission of the pathogen via individuals in \(I_j\). All other functions are interpreted as in systems (45) and (53). Note that \(\mathcal V ^{+}_{E_1}=0\) because only newly infected individuals enter the first exposed class.
We assume the following functions are finite when evaluated at \(E_j = I_j = 0\) for all \(j\): \(\mathcal F ^{(j)}_{E_1},\,\mathcal V ^{+}_{E_{j}},\,\mathcal V ^{-}_{E_j},\,\mathcal V ^{+}_{I_{j}}\), and \(\mathcal V ^{-}_{I_j}\). A example of a system satisfying these conditions is
where all terms are interpreted as in systems (46) and (54). All of the parameters are potentially functions of \(\theta \).
We compute the basic reproductive number using the methods in van den Driessche and Watmough (2002). In the matrix \(\mathbf{M}_{F}\), the top row is zeros for the first \(n_E\) entries and the remaining \(n_I\) entries of the top row are \(\mathcal F _{E_1}^{(j)}(X,\theta )\). All other entries are zero. In the matrix \(\mathbf{M}_{V}\), the main diagonal has entries \(\mathcal V ^{-}_{E_j}(X,\theta )\) and \(\mathcal V ^{-}_{I_j}(X,\theta )\) and the subdiagonal of the matrix has entries \(-\mathcal V ^{+}_{E_{j}}(X,\theta )\) and \(-\mathcal V ^{+}_{I_j}(X,\theta )\). All other entries of \(\mathbf{M}_{V}\) are zero.
The only nonzero eigenvalue of the product \(\mathbf{M}_F \mathbf{M}_V^{-1}\) is the basic reproductive number,
The basic reproductive number for a pathogen is \(\mathcal R _0(\theta )\). \(\mathcal R (X,\theta )\) factors and evolution maximizes \(\mathcal R _0(\theta )\) when the following conditions are met:
-
(g1)
\(\,\,\mathcal V ^{+}_{E_j} = v^{+}_{E_j}(X)\nu ^{+}_{E_j}(\theta )\xi _{E_j}(X,\theta )\) for \(2\le j \le n_E\)
-
(g2)
\(\,\,\mathcal V ^{-}_{E_j} = v^{-}_{E_j}(X)\nu ^{-}_{E_j}(\theta )\xi _{E_j}(X,\theta )\) for \(2\le j \le n_E\)
-
(g3)
\(\mathcal F _{E_1}^{(j)} = f^{(j)}_{E_1}(X)\eta ^{(j)}_{E_1}(\theta )\xi _{E_1}(X,\theta )\) for \(1\le j\le n_I\)
-
(g4)
\(\,\,\mathcal V ^{-}_{E_1} = v^{-}_{E_1}(X)\nu ^{-}_{E_1}(\theta ) \xi _{E_1}(X,\theta )\)
-
(g5)
\(\,\,\,\mathcal V ^{+}_{I_i} = v^{+}_{I_i}(X)\nu ^{+}_{I_i}(\theta )\xi _{I_i}(X,\theta )\) for \(1\le i \le n_I\)
-
(g6)
\(\,\,\,\mathcal V ^{-}_{I_i} = v^{-}_{I_i}(X)\nu ^{-}_{I_i}(\theta )\xi _{I_i}(X,\theta )\) for \(1\le i \le n_I\)
-
(g7)
\(\,f^{(j)}_{E_1} = f^{(k)}_{E_1}\) for all \(j,k\) or \(\eta ^{(j)}_{E_1} = \eta ^{(k)}_{E_1}\) for all \(j,k\).
-
(g8)
\(\,\,\,\,v^{-}_{I_j} = v^{+}_{I_j}\) for all \(j\) or \(\nu ^{-}_{I_j} = \nu ^{+}_{I_j}\) for all \(j,k\).
The structure for conditions (g1) through (g8) is a combination of the structure for conditions (c1) through (c5) and conditions (f1) through (f6). As was the case for all models, each function must factor into terms representing genotype, environment, and genotype-by-environment interaction affects. In addition, for each infected class the genotype-by-environment interaction terms must be the same for all processes. This further implies that genotype-by-environment interaction terms must be the same for all classes. Condition (g7) implies that all transmission functions must either have the same dependence on the trait or have the same dependence on the host classes. Conditions (g8) implies that the entry and exit rates for each infectious classes have the same dependence either on the host classes or the trait. Because entry rates into one class are equal to disease progression rates out of the previous class, condition (g8) implies that the entry and exit rates of all infectious classes have either the same dependence on the host classes or the same dependence on the pathogen trait. The biological constraints that arise from conditions (g1) through (g8) are the same as those that arise from conditions (c1) through (c5) and conditions (f1) through (f6). Note that system (57) satisfies conditions (g1) through (g8) and thus, evolution maximizes \(\mathcal R _0\) in that system.
1.3 D.3 Revisiting exposed classes
We now consider a system where there is one exposed class and one infectious class. We assume newly infected individuals enter into the exposed class and that infectious individuals can return to the exposed class. This particular system shows the additional constraints that arise when individuals can enter previous exposed classes. This case can arise in systems where the disease or pathogen goes into remission. The equations for the infectious classes are
where \(X = (S,E_1,E_1,R)\). We assume the following functions are finite and nonzero when evaluated at \(I_1 = E_1 = 0\): \(\mathcal F _{E_1},\,\mathcal V ^{+}_{E_1},\,\mathcal V ^{+}_{I_1},\,\mathcal V ^{-}_{E_1}\), and \(\mathcal V ^{-}_{I_1}\).
Following the methods in Appendix A, the top right entry of matrix \(\mathbf{M}_{F}\) is \(F_{E_1}\) and the remaining entries are zeros. The matrix \(\mathbf{M}_{V}\) has entries \(\mathcal V ^{-}_{E_1}\) and \(\mathcal V ^{-}_{I_1}\) along the diagonal, \(-\mathcal V ^{+}_{I_{1}}\) in the bottom left entry and \(-\mathcal V ^{+}_{E_{1}}\) in the top right entry. The eigenvalue of the product \(\mathbf{M}_F \mathbf{M}_V^{-1}\) is the reproductive number
The basic reproductive number for a pathogen invading the disease free equilibrium is \(\mathcal R _0(\theta ) =\mathcal R (N,0,0,0,\theta )\). \(\mathcal R (X,\theta )\) factors when the following conditions hold
-
(h1)
\(\mathcal F _{E_1} = f_{E_1}(X)\eta _{E_1}(\theta )\xi (X,\theta )\)
-
(h2)
\(\mathcal V ^{-}_{E_1}=v^{-}_{E_1}(X)\nu ^{-}_{E_1}(\theta )\xi (X,\theta )\)
-
(h3)
\(\mathcal V ^{+}_{E_1}=v^{+}_{E_1}(X)\nu ^{+}_{E_1}(\theta )\xi (X,\theta )\)
-
(h4)
\(\mathcal V ^{-}_{I_1}=v^{-}_{I_1}(X)\nu ^{-}_{I_1}(\theta )\xi (X,\theta )\)
-
(h5)
\(\mathcal V ^{+}_{I_1}=v^{+}_{I_1}(X)\nu ^{+}_{I_1}(\theta )\xi (X,\theta )\)
and one of the following conditions holds
-
(h6.1)
\(v^{-}_{I_j} = v^{+}_{I_j}\)
-
(h6.2)
\(\nu ^{-}_{I_j} = \nu ^{+}_{I_j}\)
Note that with a change in notation, these conditions are a subset of conditions (d1) through (d4) for system (46) in Appendix C. The biological consequences and interpretation of conditions (h1) through (h6) are the same after removing the conditions regarding multiple infectious classes. Of particular importance is that condition (h6.2) is unlikely to be satisfied in natural systems and condition (h6.1) is only likely to arise when the per capita mortality, recovery, and transfer rates of the system are do not depend on the density of the host classes, i.e. they are density independent.
Appendix E: Vector transmission systems
We now consider systems where the pathogen is spread via a transmission vector. We will assume that transmission does not occur between conspecifics (i.e. no direct transmission). Thus, a susceptible host can only become infected through contact with an infectious vector and a susceptible vector can only become infected through contact with an infectious host. The reason behind this assumption is illustrated at the end of the first subsection. In that subsection we consider a system where direct and vector transmission occur and find that the biological assumptions necessary to have evolution maximize \(\mathcal R _0\) are unlikely even in a simple case. Since incorporating both direct and vector-borne modes of transmission into any of the models results in biologically unlikely conditions, in the following we assume that direct transmission does not occur.
We use the following notation in this appendix. Vector classes are denoted with carets \((\,\hat{\,}\,)\). Let \(n_{\hat{E}}\) be the number of exposed vector classes and \(n_{\hat{I}}\) be the number of infectious vector classes. The disease free equilibrium is \((N,0,\ldots ,0,\hat{N},0\ldots ,0)\) where \(\hat{N}\) is the disease free equilibrium density of the vector.
We first consider a system where the host and vector have a single infectious class. We then consider systems where there are multiple exposed and infectious host classes and a single infectious vector class. Finally, we consider systems where there are multiple exposed and infectious classes in the host and multiple exposed classes in the vector populations. Note that due to symmetry, these cases also cover the case where there are multiple exposed and infectious vector classes and only one infectious host class. We do not consider the case where there are multiple infectious classes in both the vector and the host because the computations of \(\mathcal R \) become analytically intractable.
1.1 E.1 SIR host and SIR vector systems
1.1.1 Vector Transmission
We assume that there is one infected class for the host and vector populations. The system is
where \(X = (S,I,R,\hat{S}, \hat{I}, \hat{R})\). Note that since there is a single infectious class for both host populations, \(\mathcal V ^{+}_{I} = \mathcal V ^{+}_{\hat{I}}=0\). We assume the following functions are finite when evaluated at \(I=\hat{I}=0\): \( \mathcal F _{I},\,\mathcal F _{\hat{I}},\,\mathcal V ^{-}_{I}\), and \(\mathcal V ^{-}_{\hat{I}}\).
Using the methods from van den Driessche and Watmough (2002), the only nonzero eigenvalue of the product \(\mathbf{M}_F \mathbf{M}_V^{-1}\) is the reproductive number
The basic reproductive number for a pathogen invading the disease free equilibrium is \(\mathcal R _0(\theta ) = \mathcal R (N,0,\hat{N},0,\theta )\). When the following conditions are satisfied, \(\mathcal R \) factors as \(\mathcal R (X,\theta ) = g(X) \mathcal R _0(\theta )\),
-
(i1)
\(\mathcal F _{I}(X,\theta ) = f_{I}(X)\eta _{I}(\theta )\xi (X,\theta )\)
-
(i2)
\(\mathcal V _{I}(X,\theta ) = v_{I}(X)\nu _{I}(\theta )\xi (X,\theta )\).
-
(i3)
\(\mathcal F _{\hat{I}}(X,\theta ) = f_{\hat{I}}(X)\eta _{\hat{I}}(\theta )\hat{\xi }(X,\theta )\)
-
(i4)
\(\mathcal V _{\hat{I}}(X,\theta ) = v_{\hat{I}}(X)\nu _{\hat{I}}(\theta )\hat{\xi }(X,\theta )\).
Conditions (i1) through (i4) are analogous to conditions (a1) and (a2) in Appendix B. In particular, each functional form must factor into terms representing environmental, trait, and genotype-by-environment interaction effects. The genotype-by-environment effects must be the same across all rates for each species. The biological interpretation of these conditions is the same as conditions (a1) and (a2) with the added consequence that transmission cannot be both direct and vector-borne (see below).
1.1.2 Direct and Vector Transmission
We now consider the case where direct transmission between conspecifics can occur. The equations for the infected classes are
where \(X=(S,I,R,\hat{S},\hat{I},\hat{R})\). The functions \(I\mathcal H _I\) and \(\hat{I}\mathcal H _{\hat{I}}\) denote transmission due to contact with conspecifics. In addition to the assumptions made about system (61), we \(\mathcal H _{I}\) and \(\mathcal H _{\hat{I}}\) are finite and nonzero when evaluated at \(I=\hat{I}=0\).
The reproductive number, \(\mathcal R (X,\theta )\), for the pathogen in system (63) is
The basic reproductive number for a pathogen invading the disease free equilibrium is \(\mathcal R _0(\theta ) =\mathcal R (N,0,\hat{N},0,\theta )\).
The reproductive number factors as \(\mathcal R = g(X) \mathcal R _0(\theta )\) under the following conditions
-
(j1)
\(\mathcal F _{I}(X,\theta ) = f_{I}(X)\eta _{I}(\theta )\xi (X,\theta )\)
-
(j2)
\(\mathcal V _{I}(X,\theta ) = v_{I}(X)\nu _{I}(\theta )\xi (X,\theta )\)
-
(j3)
\(\mathcal F _{\hat{I}}(X,\theta ) = f_{\hat{I}}(X)\eta _{\hat{I}}(\theta )\hat{\xi }(X,\theta )\)
-
(j4)
\(\mathcal V _{\hat{I}}(X,\theta ) = v_{\hat{I}}(X)\nu _{\hat{I}}(\theta )\hat{\xi }(X,\theta )\)
-
(j5)
\(\mathcal H _{I}(X,\theta ) = h_{I}(X)\zeta _{I}(\theta )\xi (X,\theta )\)
-
(j6)
\(\mathcal H _{\hat{I}}(X,\theta ) = h_{\hat{I}}(X)\zeta _{\hat{I}}(\theta )\hat{\xi }(X,\theta )\)
One of the following must also hold
-
(j7.1)
\(v_{I}=v_{\hat{I}}\) and \(h_{I}^2 = h_{\hat{I}}^2 = f_{I}f_{\hat{I}}\)
-
(j7.2)
\(\nu _{I}=\nu _{\hat{I}}\) and \(\zeta _{I}^2 = \zeta _{\hat{I}}^2 = \eta _{I}\eta _{\hat{I}}\)
Condition (j7.1) is unlikely to be met in natural systems since in most cases \(h_I\) and \(h_{\hat{I}}\) will not depend on the host and vector classes in the same way. Condition (j7.2) is unlikely to be met in natural systems because it requires the trait to affect horizontal and vector-based transmission similarly. In total, this suggest that evolution does not maximize \(\mathcal R _0\) when a pathogen can be transmitted directly between conspecifics and indirectly via vectors.
1.2 E.2 Multiple exposed and infectious host classes, one infectious vector class
We now assume there are \(n_E\) exposed host classes and \(n_I\) infectious host classes. We assume that there is only one infectious vector class and that susceptible vectors become infectious when they come in contact with infectious hosts, i.e. exposed hosts cannot transmit the pathogen to vectors. The equations for the infected classes are
where \(X=(S,E_1,\ldots ,E_{n_E},I_1,\ldots ,I_{n_{I}},R,\hat{S},\hat{I},\hat{R})\) and \(\mathcal F _{\hat{I}_1}^{(j)}\) is the rate at which vectors become infected due to contact with hosts in class \(I_{j}\). We assume all functions in system (65) are positive and finite when evaluated at points where the densities of all infected class are zero.
An example of system (65) is
Here, \(\gamma \) is the transmission coefficient for vector to host transmission and \(\hat{\gamma }_j\) is the transmission coefficient for \(I_j\) to vector transmission. In system (66), \(1/\kappa _j,\,1/\nu _k\), and \(1/\hat{\nu }\) are the average times spent in each class; \(\lambda _j,\,\mu _k\), and \(\hat{\mu }\) are the death rates of each class; and \(\epsilon _j,\,\omega _k\), and \(\hat{\omega }\) are the recovery rates of each class.
We compute the reproductive number for a pathogen using the methods in van den Driessche and Watmough (2002). In the matrix \(\mathbf{M}_{F}\), the top right entry is \(\mathcal F _{E_1}\), the bottom row has entries \(F^{(j)}_{\hat{I}_1}\), and all other entries are zero. In the matrix \(\mathbf{M}_{V}\), the main diagonal has entries \(\mathcal V ^{-}_{J_j}\) and the subdiagonal of the matrix has entries \(-\mathcal V ^{+}_{J_{j}}\) where \(J \in \{I,E,\hat{I}\}\). All other entries of \(\mathbf{M}_{V}\) are zero. The only positive eigenvalue of the product \(\mathbf{M}_F \mathbf{M}_V^{-1}\) is the reproductive number, \(\mathcal R (X,\theta )\). Below we give the square of \(\mathcal R (X,\theta )\),
The basic reproductive number for a pathogen invading the disease free equilibrium is \(\mathcal R _0(\theta ) = \mathcal R (N,0,\ldots ,0,\hat{N},0,\theta )\). The conditions for \(\mathcal R (X,\theta )\) to factor and evolution to maximize \(\mathcal R _0\) are
-
(k1)
\(\mathcal F _{E_1}(X,\theta ) = f_{E_1}(X)\eta _{E_1}(\theta )\xi _{E_1}(X,\theta )\)
-
(k2)
\(\mathcal V ^{-}_{E_1}(X,\theta ) = v^{-}_{E_1}(X)\nu ^{-}_{E_1}(\theta )\xi _{E_1}(X,\theta )\)
-
(k3)
\(\mathcal V ^{+}_{E_j}(X,\theta ) = v^{+}_{E_j}(X)\nu ^{+}_{E_j}(\theta )\xi _{E_j}(X,\theta )\) for \(2\le j \le n_E\)
-
(k4)
\(\mathcal V ^{-}_{E_j}(X,\theta ) = v^{-}_{E_j}(X)\nu ^{-}_{E_j}(\theta )\xi _{E_j}(X,\theta )\) for \(2\le j \le n_E\)
-
(k5)
\(\mathcal F _{\hat{I}_1}^{(k)}(X,\theta ) = f_{\hat{I}_1}^{(k)}(X)\eta _{\hat{I}_1}^{(k)}(\theta )\xi _{\hat{I}_1}(X,\theta )\) for \(1\le k\le n_I\)
-
(k6)
\(\mathcal V ^{-}_{\hat{I}_1}(X,\theta )=v^{-}_{\hat{I}_1}(X)\nu _{\hat{I}_1}(\theta ) \xi _{\hat{I}_1}(X,\theta )\)
-
(k7)
\(\mathcal V ^{+}_{I_j}(X,\theta ) = v^{+}_{I_j}(X)\nu ^{+}_{I_j}(\theta )\xi _{I_j}(X,\theta )\) for \(1\le j \le n_I\)
-
(k8)
\(\mathcal V ^{-}_{I_j}(X,\theta )=v^{-}_{I_j}(X)\nu ^{-}_{I_j}(\theta )\xi _{I_j}(X,\theta )\) for \(1\le j \le n_I\)
-
(k9)
\(f_{\hat{I}_1}^{(j)} = f_{\hat{I}_1}^{(k)}\) for all \(j,k\) or \(\eta _{\hat{I}_1}^{(j)} = \eta _{\hat{I}_1}^{(k)}\) for all \(j,k\).
-
(k10)
\(v^{-}_{I_j} = v^{+}_{I_j}\) for all \(j\ge 2\) or \(\nu ^{-}_{I_j} = \nu ^{+}_{I_j}\) for all \(j\ge 1\).
where \(x=(S,R,\hat{S},\hat{R})\). The structure and interpretation of conditions (k1) through (k10) is essentially identical to that of conditions (g1) through (g8).
1.3 E.3 Multiple exposed and infectious host classes, multiple exposed vector classes
We now compute the reproductive number for the case where there are multiple exposed and infectious host classes, multiple exposed vector classes, and a single infectious vector class. The notation is the same as the previous section except that we denote the number of exposed vector classes by \(n_{\hat{E}}\). The equations for the infected classes are
where \(X\) is shorthand all host and vector classes. We assume all of the functions in system (68) are positive and finite when evaluated at any point where the densities of all infected classes are zero.
We now compute the reproductive number. In the matrix \(\mathbf{M}_{F}\), the top right entry is \(\mathcal F _{E_1}\), the \(\hat{E}_1\) row has entries \(\mathcal F ^{(j)}_{\hat{I}_1}\), and all other entries are zero. In the matrix \(\mathbf{M}_{V}\), the main diagonal has entries \(\mathcal V ^{-}_{J_j}\) and the subdiagonal of the matrix has entries \(-\mathcal V ^{+}_{J_{j}}\) where \(J \in \{I,E,\hat{E},\hat{I}\}\). All other entries of \(\mathbf{M}_{V}\) are zero. The only positive eigenvalue of the product \(\mathbf{M}_F \mathbf{M}_V^{-1}\) is the reproductive number, \(\mathcal R (X,\theta )\). The square of the reproductive number is
The basic reproductive number for a pathogen with trait \(\theta \) is \(\mathcal R _0(\theta ) \!=\! \mathcal R (N,0\ldots ,0,\hat{N},0,\ldots ,0,\theta )\).
The conditions under which \(\mathcal R (X,\theta )\) factors are below. With a slight change in notation, the first ten conditions are the same as conditions (k1) through (k10). Two additional conditions also arise due to the addition of the exposed vector classes.
-
(m1)
\(\mathcal F _{E_1}(X,\theta ) = f_{E_1}(X)\eta _{E_1}(\theta )\xi _{E_1}(X,\theta )\)
-
(m2)
\(\mathcal V ^{-}_{E_1}(X,\theta ) = v^{-}_{E_1}(X)\nu ^{-}_{E_1}(\theta )\xi _{E_1}(X,\theta )\)
-
(m3)
\(\mathcal V ^{+}_{E_j}(X,\theta ) = v^{+}_{E_j}(X)\nu ^{+}_{E_j}(\theta )\xi _{E_j}(X,\theta )\) for \(2\le j \le n_E\)
-
(m4)
\(\mathcal V ^{-}_{E_j}(X,\theta ) = v^{-}_{E_j}(X)\nu ^{-}_{E_j}(\theta )\xi _{E_j}(X,\theta )\) for \(2\le j \le n_E\)
-
(m5)
\(\mathcal F _{\hat{E}_1}^{(k)}(X,\theta ) = f_{\hat{E}_1}^{(k)}(X)\eta _{\hat{E}_1}^{(k)}(\theta )\xi (X,\theta )\) for \(1\le k\le n_I\)
-
(m6)
\(\mathcal V ^{-}_{\hat{E}_1}(X,\theta )=v_{\hat{E}_1}(X)\nu _{\hat{I}_1}(\theta ) \xi (X,\theta )\)
-
(m7)
\(\mathcal V ^{+}_{I_i}(X,\theta ) = v^{+}_{I_i}(X)\nu ^{+}_{I_i}(\theta )\xi _{I_i}(X,\theta )\) for \(1\le i \le n_I\)
-
(m8)
\(\mathcal V ^{-}_{I_i}(X,\theta )=v^{-}_{I_i}(X)\nu ^{-}_{I_i}(\theta )\xi _{I_i}(X,\theta )\) for \(1\le i \le n_I\)
-
(m9)
\(f_{\hat{E}_1}^{(j)} = f_{\hat{E}_1}^{(k)}\) for all \(j,k\) or \(\eta _{\hat{E}_1}^{(j)} = \eta _{\hat{E}_1}^{(k)}\) for all \(j,k\).
-
(m10)
\(v^{-}_{I_j} = v^{+}_{I_j}\) for all \(j\ge 2\) or \(\nu ^{-}_{I_j} = \nu ^{+}_{I_j}\) for all \(j\ge 2\)
-
(m11)
\(\mathcal V ^{+}_{\hat{E}_j}(X,\theta )= v^{+}_{\hat{E}_j}(X)\nu ^{+}_{\hat{E}_j}(\theta )\xi _{\hat{E}_j}(X,\theta )\) for \(2\le j \le n_{\hat{E}}\)
-
(m12)
\(\mathcal V ^{-}_{\hat{E}_j}(X,\theta )= v^{-}_{\hat{E}_j}(X)\nu ^{-}_{\hat{E}_j}(\theta )\xi _{\hat{E}_j}(X,\theta )\) for \(2\le j \le n_{\hat{E}}\)
The structure of these conditions is the same as the previous cases. The biological consequences are the same as conditions (k1) through (k10).
Appendix F: Systems with frequency dependent selection
Here we show how our approach and results can be extended to systems where selection is frequency dependent. In the following we first present our frequency dependent selection model. We show how to calculate the reproductive number and restate the theorem from Mylius and Diekmann (1995) in our notation. Next we analyze a system with a single infectious class. This illustrates how the conditions for the frequency dependent selection case and the frequency independent selection case are related. We then discuss how the conditions for \(\mathcal R _0\) maximization in the frequency independent cases can be extended to incorporate frequency dependent selection. We also give a biological interpretation to our conditions. Finally, we present an example where frequency independent selection does not result in \(\mathcal R _0\) maximization whereas frequency dependent selection does result in \(\mathcal R _0\) maximization.
We begin with the epidemiological dynamics for the resident strain,
We assume for every fixed value of \(\theta \) that the dynamics of system (70) converge to either an endemic equilibrium or the disease free equilibrium. Thus, in the limit where evolution is much slower than the epidemiological dynamics of the system, the dynamics of the invading strain are given by
where \(\theta \) is the resident trait, \(\theta _i\) is the invader trait, and \((S^*,\mathcal C ^*,R^*)\) is the endemic equilibrium of the resident strain. The state variables \(\bar{C}_j\) are the densities of hosts infected with the invading strain of the pathogen. The functions \(F^{(k)}_{C_j},\,V_{C_j}^+\), and \(V_{C_j}^-\) describe the epidemiological processes of the invading strain when at low densities. They are written such that they explicitly state how the resident trait value affects the epidemiological dynamics of the invader. They are related to the functions \(\mathcal F ,\,\mathcal V _{C_j}^+\), and \(\mathcal V _{C_j}^-\) in the follow way
We assume that all functions are finite and positive when evaluated at points where \(C_j = 0\) for all \(j\).
The reproductive number for a pathogen can be computed using the method in van den Driessche and Watmough (2002). Define \(V_{C_j}(S,\mathcal C ,R,\bar{C}_{j-1},\bar{C}_{j}) = -\bar{C}_{j-1}V^{+}_{C_j}+\bar{C}_{j}V^{-}_{C_j}\). Let \(\mathbf{M}_F\) and \(\mathbf{M}_V\) be the matrices
Then the reproductive number for a pathogen with trait \(\theta \) in a population with host class densities \(S,\,\mathcal C \), and \(R\) is
where \(\rho (\mathbf{M})\) is the spectral radius of a matrix M. The basic reproductive number for a pathogen invading the disease free equilibrium is \(\mathcal R _0(\theta )=\mathcal R (N,0,0,\theta ,\theta )\) where \(\mathcal C =0\) implies that \(C_j=0\) for all \(j\). When selection is frequency dependent, the \(\mathcal R _0\) maximization theorem from Mylius and Diekmann (1995) can be restated as: If the reproductive number of a pathogen can be written in the form \(\mathcal R (X,\theta ,\theta _i) = g(X,\theta )\mathcal R _0(\theta _i)\) then evolution maximizes \(\mathcal R _0(\theta )\).
To illustrate the class of functions such that \(\mathcal R _0\) maximize occurs, we consider a system with a single infectious class. The dynamics of the invading strain are
The reproductive number for a pathogen with trait \(\theta _i\) in a population with resident trait \(\theta \) is
\(\mathcal R (S,I,R,\theta ,\theta _i)\) factors when the following conditions are met
-
(n1)
\( F_{I}(S,I,R,\theta ,\theta _i) = f_I(S,I,R,\theta )\eta _I(\theta _i)\xi _f(S,I,R,\theta ,\theta _i)\)
-
(n2)
\( V_{I}^{-}(S,I,R,\theta ,\theta _i) = v_I^{-}(S,I,R,\theta )\nu _I^{-}(\theta _i)\xi _v(S,I,R,\theta ,\theta _i)\)
-
(n3)
\(\xi _f = \xi _v\)
-
(n4)
\(f_I(S,0,0,\theta _1) = f_I(S,0,0,\theta _2)\) and \(v_I^{-}(S,0,0,\theta _1) = v_I^{-}(S,0,0,\theta _2)\) for all \(\theta _1\) and \(\theta _2\)
Under these conditions the reproductive number of the invader at the endemic equilibrium of the resident can be written as
where in the last line we used the equalities in conditions (n3) and (n4).
The structure of conditions (n1) through (n4) is the following. Each function must factors into terms that represent the effects of the environment (host classes and resident trait), the invader trait (i.e. the genotype), and the genotype-by-environment interactions. The genotype-by-environment interaction effects must be the same for both processes. Finally, condition (n4) states that the effects of the environment on the epidemiological processes of the system are independent of the pathogen trait in a completely susceptible population.
There are two key structural differences between conditions (n1) through (n4) and conditions (A1) through (A3) in the main text. First, when selection is frequency dependent the functions factor into a term that is a function of the host classes and the resident trait (the environment) and a term that is only a function of the invader’s trait. The second structural difference is condition (n4). When analyzing more complex epidemiological models, these structural differences are the only differences that arise between the \(\mathcal R _0\) maximization conditions for frequency independent and frequency dependent selection models. Thus, for all of the conditions presented in the appendices for models where selection is frequency independent, the conditions can be modified to address frequency dependent selection by making two changes. First, the notation in all of the conditions needs to be changed such that the term representing the “environmental effects” includes the resident trait value. For example, let \(F(S,I,R,\theta ,\theta _i)\) be a function representing some epidemiological process. Instead of writing that \(F\) factors as \(F(S,I,R,\theta _i) = f(S,I,R)\eta (\theta _i)\xi (S,I,R,\theta _i)\) as in the frequency independent selection case, we now write \(F(S,I,R,\theta ,\theta _i) = f(S,I,R,\theta )\eta (\theta _i)\xi (S,I,R,\theta ,\theta _i)\). Second, a condition analogous to condition (iv) needs to be appended. Note that this condition would require all terms representing environmental effects to satisfy \(f(S,0,0,\theta _1) = f(S,0,0,\theta _2)\) for all \(\theta _1\) and \(\theta _2\).
The biological interpretation of conditions (n1) through (n4) are similar to those of the conditions (A1) through (A3) in the main text. Condition (n3) implies that the genotype-by-environment interactions are the same for pathogen transmission, host recovery, and host mortality. Since we do not expect this to be the case in most natural systems, this implies that \(\mathcal R _0\) maximization only occurs when there are no genotype-by-environment effects. Condition (n3) also implies that the genotype-by-genotype interactions between the resident and invader traits are the same for pathogen transmission, host recovery, and host mortality. Since we do not expect this to be the case in most natural systems, this implies that \(\mathcal R _0\) maximization only occurs when there are no genotype-by-genotype effects. In total, condition (n3) implies that the effects of the invader trait and the effects of the environment (i.e. both the host class densities and the resident trait) are independent. After decomposing the exist rate, \(\mathcal V _{I}^{-}\), into transfer and death rates, the biological consequences of condition (n2) are the same as those for conditions (A2.1) through (A2.4) in the main text. Condition (n4) implies that the effects of the invader’s trait and the environmental effects due to the host density are independent in a completely susceptible population.
Example:
We consider a system with a single infectious class where the transmission rate of the infectious class decreases as the density of the infectious class increases. A similar functional form has been used to account for how the crowding of infectious individuals or host behavioral changes (e.g. fear) decrease transmission rates (Capasso and Serio 1978). The dynamics of the infectious class infected by the resident are
where \(k(\theta )\) defines the level of inhibition infectious individuals exhibit on the transmission rate. For large values of \(k(\theta )\) the level of inhibition is large and for small values of \(k(\theta )\) the level of inhibition is small. For notational convenience, let \(X = (S,I,R)\). The reproductive number of the pathogen is
The basic reproductive number is
We first consider the case where selection is frequency independent. In this case, the dynamics of the invading strain, \(\bar{I}\), at the endemic equilibrium of the resident, \((S^*,I^*,R^*)\), are given by
This implies that \(\mathcal F _I(X,\theta )= \beta (\theta )S/[1+k(\theta )I]\) and \(\mathcal V ^{-}_I(X,\theta ) = \mu (\theta )+\rho (\theta )\). When \(k(\theta )\) does not depend on the trait \(\theta \), i.e. it is constant, then (7) satisfies conditions (A1) through (A3) from the main text. In this case \(\mathcal R (X,\theta )\) can be written as in Eq. (4),
When \(k(\theta )\) does depend on the trait, \(\beta (\theta )S/(1+k(\theta )I)\) cannot be factored as in condition (A1) from the main text. Because the function \(g(S,I,R)\) will depends on \(S,\,I\), and \(\theta \), it follows that \(\mathcal R (S,I,R,\theta )\) cannot be written as in Eq. (4). Thus, when \(k(\theta )\) depends on the trait and the selection is frequency independent, \(\mathcal R _0\) maximization is not guaranteed.
Now consider the case where selection is frequency dependent. We will focus on the case where \(k(\theta )\) depends on the pathogen trait. The dynamics of the invading strain at the endemic equilibrium of the resident are given by
where \(\theta \) is the trait value of the resident strain and \(\theta _i\) is the strait value of the invading strain. Note that the trait value in the denominator of the transmission function is determined by the resident strain. This is biologically reasonable because the (fear-driven or crowding-based) behavioral dynamics of the host population should be dictated by the resident strain, not the invader which is initially at low density.
Using the notation in system (74), we have that \(F_I(S^*,I^*,R^*,\theta ,\theta _i) \!=\! \beta (\theta _i)S^*/[1+k(\theta )I^*]\) and \(V_I^{-}(S^*,I^*,R^*,\theta ,\theta _i)= \mu (\theta _i)+\rho (\theta _i)\). These two functions factor as in conditions (n1) through (n3) with \(f_I(X,\theta ) = S/[1+k(\theta )I^*],\,\eta _I(\theta _i) =\beta (\theta _i),\,v_I^{-}(X,\theta ) = 1,\,\nu _{I}^{-}=\mu (\theta _i)+\rho (\theta _i)\), and \(\xi _f=\xi _v=1\). Furthermore, condition (n4) is satisfied because
and \(v_I^{-}(S,0,0,\theta _1) = 1 = v_I^{-}(S,0,0,\theta _2)\). This implies that reproductive number can be written as
Thus, when selection is frequency dependent evolution always maximizes \(\mathcal R _0\) in system (76).
Appendix G: Gradient evolutionary models
As opposed to the previous models where evolution allowed for any pathogen strain to arise, here we consider gradient dynamic models of evolution. In these models, only strains of the pathogen with a trait value close to the trait value of the resident can arise through mutation and potentially invade the system. The models follow from the quantitative genetic approach derived in Lande (1982) and Abrams et al. (1993). The theory assumes that evolution drives the trait in the direction of increasing fitness, determined by the fitness gradient, at a rate that is proportional to both the additive genetic variance of the trait and the fitness gradient. These models are a first approximation to many types of models (e.g. adaptive dynamic and clonal models) and capture a range of behavior observed at the phenotypic level without having to specify gene level processes Abrams (2001, 2005). We consider them here because of their wide use and because the conditions necessary for evolution to maximize the basic reproductive number are similar to those of the previous models.
In the following we will only consider epidemiological systems with a single infectious class. We first consider the case where selection is frequency independent. We then consider the case where selection is frequency dependent. In both cases, we assume that ecological processes are occurring at a faster timescale than the evolutionary processes.
1.1 G.1 Frequency independent selection
The gradient dynamic model for a system with a single infectious class and frequency independent selection is
where \(\sigma ^2\) is the additive genetic variance of the pathogen population. The first three equations of system (83) describe the epidemiological dynamics of the system. The last equation describes the evolutionary dynamics of the system. In the trait equation, \(\frac{1}{I}\frac{dI}{dt}\) is the per capita fitness of a pathogen with trait \(\theta \). The derivative of the per capita fitness defines the fitness gradient and the trait evolves in the direction of increasing fitness. Note that because selection is frequency independent, the fitness gradient is just the derivative of fitness with respect to the trait. We assume that for any fixed value of the trait, the epidemiological dynamics of system (83) tend to a disease free or endemic equilibrium. The disease free equilibrium is given by \((N,0,0)\).
In the case where the epidemiological dynamics of system (83) are fast and the evolutionary dynamics are slow, the evolutionary dynamics of system (83) in the slow evolutionary time scale are
where \(S^{*},\,I^{*}\), and \(R^{*}\) are the endemic equilibrium values of the host classes for the pathogen with trait \(\theta \). Values of \(\theta \) that make the right hand side of Eq. (84) zero are evolutionary equilibria. Because the trait dynamics are one dimensional, evolutionary equilibria are attracting if they are local fitness maxima and repelling if they are local fitness minima. We are interested in the conditions on the functional forms of \(\mathcal F _I\) and \(\mathcal V _I\) that result in evolution maximizing the basic reproductive number of the pathogen.
The basic reproductive number, \(\mathcal R _0(\theta )\), for a pathogen in system (83) with trait \(\theta \) is
The derivative of \(\mathcal R _0(\theta )\) with respect to \(\theta \) is
We are interested in the conditions on \(\mathcal F _I\) and \(\mathcal V _I^{-}\) that result in Eqs. (84) and (86) having the same sign for all values of \(\theta \). When the two equations have the same sign for all values of \(\theta \), the local maxima of the two are be the same and evolution will maximize \(\mathcal R _0(\theta )\) locally. Note that because the trait dynamics in system (83) are driven by the fitness gradient, the trait cannot cross a fitness valley. Consequently, evolution may end up a selecting for a local maximum of \(\mathcal R _0(\theta )\) that is not the global maximum. If one wanted to ensure that evolution always selected for the strain with the global maximum value of \(\mathcal R _0(\theta )\), then it is necessary to assume that \(\mathcal R _0(\theta )\) is a unimodal function with a single local (and hence global) maximum.
Equations (84) and (86) have the same sign for all values of \(\theta \) under the following conditions
-
(o1)
\(\mathcal F _I = If(S,I,R)\eta (\theta )\)
-
(o2)
\(\mathcal V _I^{-} = Iv(S,I,R)\nu (\theta )\).
Conditions (i1) and (i2) are a special case of the following conditions
-
(p1)
\(\mathcal F _I = I f_1(S,I,R)\eta (\theta ) + I f_2(S,I,R)\nu (\theta )\)
-
(p2)
\(\mathcal V _I^{-} = I v_1(S,I,R)\eta (\theta )+ I v_2(S,I,R)\nu (\theta )\)
-
(p3)
\((f_1 v_2 - f_2 v_1)|_{(N,0,0,\theta )}\) has the same sign as \((f_1 v_2 - f_2 v_1)|_{[S^*(\theta ),I^*(\theta ),R^*(\theta ), \theta ]}\) for all values of \(\theta \).
Under conditions (o1) and (o2), Eqs. (84) and (86) have the same sign and evolution maximizes \(\mathcal R _0(\theta )\). The algebra justifying this statement is presented at the end of this subsection. Note that condition (p3) is satisfied when \(f_2 =0\) or \(v_1=0\).
Biologically, conditions (p1) and (p2) imply that the trait has the same effect on different aspects of pathogen transmission and virulence. This seems unlikely to occur in nature. Thus, we focus on the interpretation of conditions (o1) and (o2). Conditions (o1) and (o2) have the same interpretation as conditions (A1) and (A2) from the main text except that there are no genotype-by-environment interactions. Thus, these conditions require that the effects of the environment and the pathogen trait are independent for evolution to maximize \(\mathcal R _0(\theta )\) in frequency independent selection gradient models.
The proof of conditions (p1) through (p3) is as follows. Under conditions (p1) and (p2), \(\mathcal R _0(\theta )\) for the pathogen in system (84) is
Differentiating with respect to \(\theta \) yields,
where \(\eta ^{\prime } = d\eta /d\theta \) and \(\nu ^{\prime } = d\nu /d\theta \).
Under the same conditions, Eq. (86) becomes
Since \(\frac{dI}{dt}(S^*(\theta ), I^*(\theta ), R^*(\theta ), \theta ) =0\) by definition, we have
Equation (90) has the same sign as Eq. (88) when condition (c3) is met.
1.2 G.2 Frequency dependent selection
Following the notation in Appendix F, the gradient dynamic model for system with a single infectious class where selection is frequency dependent is
where \(\theta \) is the trait value of the resident. Note that when selection is frequency dependent the fitness gradient in the \(d\theta /dt\) equation is calculated by differentiating the per capita fitness of a pathogen with respect to the invader’s trait, \(\theta _i\). We assume that for any fixed value of the trait, the epidemiological dynamics of system (91) tend to a disease free or endemic equilibrium. The disease free equilibrium is given by \((N,0,0)\).
In the limit where evolutionary dynamics are much slower than the epidemiological dynamics of system (91), the evolutionary dynamics of system (91) in the slow evolutionary time scale are given by
where \(S^{*},\,I^{*}\), and \(R^{*}\) are the endemic equilibrium densities of the host classes for the pathogen with trait \(\theta \). We are interested in the conditions on the functional forms of \(\mathcal F _I\) and \(\mathcal V _I^{-}\) that result in evolution maximizing the basic reproductive number of the pathogen.
The basic reproductive number for a pathogen with trait \(\theta \) in system (91) is
The gradient of \(\mathcal R _0(\theta )\) with respect to the trait is
Equations (92) and (94) have the same sign for all values of \(\theta \) under the following conditions
-
(q1)
\(\mathcal F _I(S,I,R,\theta I,\theta _i) = f(S,I,R,\theta )\eta (\theta _i)\)
-
(q2)
\(\mathcal V _I^{-}(S,I,R,\theta I,\theta _i) = v(S,I,R,\theta )\nu (\theta _i)\).
-
(q3)
\(f(S,0,0,\theta _1) = f(S,0,0,\theta _1)\) and \(v(S,0,0,\theta _1) = v(S,0,0,\theta _2)\) for all \(\theta _1\) and \(\theta _2\).
Conditions (q1) through (q3) fall under the more general conditions
-
(r1)
\(\mathcal F _I(S,I,R,\theta I,\theta _i) = If_1(S,I,R,\theta )\eta (\theta _i)+If_2(S,I,R,\theta )\nu (\theta _i)\)
-
(r2)
\(\mathcal V _I^{-}(S,I,R,\theta I,\theta _i) = Iv_1(S,I,R,\theta )\eta (\theta _i)+Iv_2(S,I,R,\theta )\nu (\theta _i)\).
-
(r3)
\((f_1 v_2 - f_2 v_1)|_{(N,0,0,\theta )}\) has the same sign as \((f_1 v_2 - f_2 v_1)|_{[S^*(\theta ),I^*(\theta ),R^*(\theta ), \theta ]}\) for all values of \(\theta \).
-
(r4)
\(f_j(S,0,0,\theta _1) = f_j(S,0,0,\theta _2)\) and \(v_j(S,0,0,\theta _1) = v_j(S,0,0,\theta _2)\) for \(j =1,2\) and for all \(\theta _1\) and \(\theta _2\).
The algebra showing that conditions (r1) through (r4) yield \(\mathcal R _0\) maximization is included at the end of this subsection. Note that condition (r4) is satisfied when \(f_2 =0\) or \(v_1=0\). An example of a function that satisfies condition (q1) and (q3) is
Biologically, conditions (r1) and (r2) imply that the trait has the same effect on different aspects of pathogen transmission and virulence. This seems unlikely to occur in nature, thus we focus on the interpretation of conditions (q1) through (q3). The interpretation of conditions (q1) and (q2) is analogous to that of the conditions for the frequency independent case. The difference here is that the term representing the effects of the environment also includes the effects due to the resident trait. Hence, there are no genotype-by-genotype interactions. Condition (q3) implies that effects of the pathogen trait and the host density are independent in completely susceptible populations.
The proof for conditions (r1) through (r4) is as follows. Under conditions (r1) and (r2), we have
Differentiating with respect to \(\theta \) yields
where \(\eta ^{\prime } = d\eta /d\theta \) and \(\nu ^{\prime } = d\nu /d\theta \).
Under the same conditions, Eq. (94) becomes
Since \(\frac{dI}{dt}(S^*, I^*, R^*, \theta ) =0\), where \(\theta \) is the resident pathogen’s trait, we have
Equation (100) has the same sign as Eq. (97) when condition (r4) is met.
Rights and permissions
About this article
Cite this article
Cortez, M.H. When does pathogen evolution maximize the basic reproductive number in well-mixed host–pathogen systems?. J. Math. Biol. 67, 1533–1585 (2013). https://doi.org/10.1007/s00285-012-0601-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-012-0601-2