Abstract
Microalgae are considered feedstock for biodiesel production due to their capability to accumulate triacylglycerols, which have a 99% conversion rate into biodiesel, under certain conditions. This study aims to evaluate thirty native microalgal strains as feedstock for biodiesel production based on their biomass and lipid productivities, and total lipid and triacylglycerol contents under nitrogen-sufficient and nitrogen starvation conditions. In addition, Chlamydomonas reinhardtii cw15 mutant strain was utilized as a reference strain for triacylglycerol accumulation. Among the eight potent strains, Chlorella vulgaris KP2 was considered as a most promising strain with the highest triacylglycerol content, highest total lipid content (28.56% of dry cell weight), and the highest lipid productivity (4.56 mg/L/day) under nitrogen starvation. Under nitrogen starvation, the major fatty acids in the triacylglycerol of Chlorella vulgaris KP2 were C18:1 (37.56%), C16:0 (23.16%), C18:0 (23.07), C18:2 (7.00%), and C18:3 (3.12%), and the percentages of saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids represented 49.26, 38.73, and 10.12% of the total fatty acids, respectively. Furthermore, the fatty acid methyl esters of triacylglycerol displayed remarkable biodiesel properties with a lower iodine value (59.00 gI2/100 g), higher oxidative stability (14.24 h) and higher cetane number (58.73) under nitrogen starvation. This study suggests that nitrogen-starved Chlorella vulgaris KP2 could be used as a feedstock for biodiesel production due to the considerable amounts of triacylglycerol and favorable biodiesel properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Efforts to develop renewable, sustainable, and environmental-friendly energy sources such as biodiesel have accelerated due to the increase in fossil fuel consumption, CO2 greenhouse gas emissions, and the global demand for energy in recent years. Microalgae are one of the promising organisms for third-generation biofuels due to their fast growth rates and higher lipid productivity compared to the best oil-producing plants [1]. Microalgae have other advantages, such as the ability to be cultivated in areas not suitable for agriculture using water sources such as brackish water, urban wastewater, and industrial wastewater [2]. In addition, many microalgae species can synthesize high amounts of triacylglycerol (TAG), which is the major form of storage lipids, and fatty acid compounds similar to vegetable oils [3].
The lipid content of microalgae is between 20 and 50% of dry weight on average, depending on the algal strain and growth conditions [4]. As an exception, the lipid content of Botryococcus braunii is 75% of its dry weight, but this strain has extremely slow growth kinetics [5]. Therefore, the selection of microalgae strains with high lipid productivity is an important criterion for sustainable biodiesel production. Chlorella vulgaris, Scenedesmus, Acutodesmus, Ankistrodesmus falcatus, and Mychonastes species have been reported as potential candidates for biodiesel production because of their high lipid productivities when grown under certain stress conditions [6,7,8,9,10].
Nitrogen starvation in culture medium is an ideal stress condition under which to promote the accumulation of high amounts of cellular lipids and TAGs, which are the raw materials of biodiesel [11]. Moreover, in the absence of nitrogen, the amount of monounsaturated fatty acids (MUFAs) improving biodiesel quality increases in many microalgae species (especially C18:1 or C16:1 fatty acids), whereas the amount of polyunsaturated fatty acids (PUFAs) negatively affecting the oxidation stability of the biodiesel decreases [12].
Turkey is in 36th place globally in biodiesel production, with a production of 1.3 thousand barrels per day [13]. Furthermore, considering that Turkey's external dependence on energy is approximately 75% [14], studies should be conducted to discover microalgae species in Turkey with promising biodiesel production potential. In this study, 30 microalgae were isolated from local water systems in Turkey and determined isolates having high TAG content and high lipid productivity under nitrogen starvation. Selected microalgae isolates were identified based on sequencing of the ITS1-5.8S-ITS2 region or 18S rRNA gene, and determined the fatty acid profile of the most potent isolate. The aim of the present study is to investigate the potential of using microalgae as sources of biodiesel production.
Materials and Methods
Microalgae Isolation and Cultivation
A total of thirty microalgae were isolated from several freshwater sources in Turkey. Water samples were collected from Koçpınar spring water in Aksaray Province, Salt Lake (Tuz gölü) in Middle Anatolia, Gölbaşı Lake in Adıyaman Province, Beşgöz River in Antalya Province, Beyşehir and Cihanbeyli Lakes in Konya Province, an industrial wastewater and Kızılırmak River in Nevşehir Province, and Saklıkent Canyon in Muğla Province. For the isolation process, 5 mL of freshwater samples were transferred to each 100 mL flasks containing 45 mL of sterilized BG11 medium [15], and 5 mL of saltwater samples were inoculated to Modified Johnson’s medium [16].
Each flask was incubated for grown of microalgae at room temperature and under continuous illumination (Philips, extreme cool daylight, 1050 lm) for 3–4 weeks. After incubation, 100 µL of growing microalgae mixed culture was streaked on to Petri plates containing the different culture media with 1.25% (w/v) of bacteriological agar, and the plates were kept at room temperature and under continuous light until microalgae colonies appeared. Single colonies were then transferred into a tube having suitable a growth medium and tubes were incubated under the conditions as described above. The purity of the culture was validated by repeated microscopic observation. In addition to the native isolates, the Chlamydomonas reinhardtii CC-400 (cw15 mt +) cell wall-less mutant strain, which is known as a good TAG accumulator under nitrogen starvation [17], was included in this study. The mutant strain was obtained from the Chlamydomonas Resource Center at the University of Minnesota, and it was grown in Tris–acetate-phosphate (TAP) medium under same conditions with the other microalgal isolates. Locations of water sources and cultivation media of 30 microalgae isolates are shown in Table 1. Among 30 isolates, eight isolates were selected based on their TAG and lipid contents, lipid and biomass productivities.
Microscopic Observation of Microalgae Isolates
Microscopic images of selected eight microalgae species were taken using a light microscope (Olympus BX53) attached to a digital camera (Olympus DP26). Cell images were collected at × 1000 magnification. Fluorescence microscope was used to visualization of lipid bodies in the cells of the most potent isolate. Cells were stained with Nile Red, then observed under green fluorescence using a fluorescence microscope (OPTO-EDU, model A16.0204) at × 1000 magnification.
DNA Extraction, Amplification and Sequencing
To perform genomic DNA extraction from selected eight microalgal isolates, algal cells (50 mL of culture) were collected, frozen in liquid nitrogen and pulverized with a mortar and a pestle. Subsequently, genomic DNA extraction was performed using a Genomic DNA Purification Kit (Thermo, K0512). For the identification of seven microalgae isolates, PCR amplification was performed using the ITS1-F (5′-AGGAGAAGTCGTAACAAGGT-3′) and ITS4-R (5′-TCCTCCGCTTATTGATATGC-3′) primers developed by [18]. For only one isolate (KPX4), PCR amplification was carried out using the following primers: 18S-F (5′-GCGGTAATTCCAGCTCCAATAGC-3) and 18S-R (5′-GACCATACTCCCCCCGGAACC-3′).
The 60 µL PCR reaction mix consisted of 45.75 µL of ultrapure water, 5 µL of 10 × PCR buffer, 2.5 µL of 2.5 mM dNTPs, 1.75 µL of 25 mM MgCl2, 1.25 µL of each primer (5 µM), 0.5 µL of Taq DNA polymerase (5 U/µL) and 2 µL of DNA template (50 ng/µL). The PCR thermal cycling profile was as follows: initial denaturation at 94 °C for 5 min; 40 cycles of 95 °C for 45 s, 59 °C for 45 s, and 72 °C for 45 s; and final extension at 72 °C for 5 min. PCR products were controlled by gel electrophoresis on 2% agarose gel in 0.5X TBE buffer stained with ethidium bromide and visualized by a UV transilluminator prior to sequencing. PCR products were then sent to Sentegen Biotech in Ankara Province, Turkey, for purification and sequencing.
Sequence Data Analysis and Phylogenetic Tree Construction
Sequences were analyzed using the NCBI/BLAST program for similarities to microalgae sequences in the NCBI DNA sequence database. A total of 48 nucleotide sequences showing the highest similarity scores to the query sequences were retrieved from GenBank sequence databases at NCBI. Multiple sequence alignment was performed among the retrieved and query sequences using ClustalW under default parameters using MEGA7 software. The dendrogram was constructed using the Maximum Likelihood method based on the Tamura-Nei model under default parameters using MEGA7 software. A phylogenetic test using the bootstrap method (1000 replicates) was used.
Nitrogen Starvation Treatment for Lipid Induction
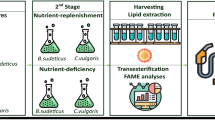
For nitrogen starvation, all nitrogen sources in cultivation media were completely removed. A two-stage cultivation was used to grow microalgae in a complete medium and a nitrogen-free medium. In the first stage, microalgae isolates were cultivated in complete medium for 12 − 15 days in a minitron incubator (Infors HT), at 23 °C, a 12:12 h light/dark photoperiod, and shaking at 90 rpm. In the second stage, 20 mL of cell suspension (OD680 = 1.0) was centrifuged to collect cells, the supernatant was discarded, and the cell pellet was then resuspended in either complete medium or nitrogen-free medium. Twenty mL of cell suspension was transferred into 80 mL each of nitrogen-free medium and complete medium, and 100 mL each of complete medium and nitrogen-free medium and were incubated for seven days in an incubator under the conditions as noted above. After incubation, cells were collected for further analysis and all experiments were performed in duplicate for each treatment.
Quantification of Total Lipid Content
Total lipid (TL) quantification was performed using the spectrophotometric sulfo-phospho-vanillin (SPV) method according to [19]. The SPV method was used for TL quantification due to the disadvantages of gravimetric methods, which are time-consuming and require the use of toxic chemicals and a large amount of dry biomass. One mL of cell culture was used for this analysis and the calibration was carried out simultaneously using canola oil (Sigma, 46961) as the standard. TL content was expressed as percentage of dry cell weight.
Determination of Biomass and Lipid Productivity
Biomass productivity was calculated based on Eq. (1),
where N1 and N2 are defined as the biomass concentration (mg/L) at time 1 (t1) and time 2 (t2), respectively. To maintain dry biomass, freshwater microalgae cells were harvested by centrifugation and supernatant discarded, and pellets were washed twice with distilled water, dried at 65 °C for 24 h, and the dry weight determined gravimetrically. Exceptionally, cell pellets of salt water microalgae isolates were washed twice with 0.5 M ammonium formate before drying. Lipid productivity was calculated via Eq. (2),
TAG and HPTLC Analysis
The Nile red staining method described by [20] was used to qualitatively analyze intracellular TAGs. For all isolates, 100 µL of cell culture were transferred into a black 96-well plate containing 50 µL of dimethyl sulfoxide (DMSO), 45 µL of phosphate buffered saline (PBS, 1X) and 5 µL of Nile red (Sigma, 72485) stock solution (0.04 mg/mL in acetone). The black 96-well plate was incubated at 40 °C in the dark for 15 min. After the cells were stained, intracellular TAGs were measured based on relative fluorescence units (RFU) using a spectrofluorometer (Spectramax Gemini XPS, Molecular Devices) at excitation/emission wavelengths of 520/580 nm with a 570 nm cut-off.
Separation of neutral lipids and TAG visualization were also carried out by high performance thin layer chromatography (HPTLC). For selected microalgal isolates, TAG were extracted using n-hexane:isopropanol (3:2, v/v) containing a 0.01% butylated hydroxytoluene solvent mixture [21]. Five µL of extract was loaded onto a silica 60 HPTLC plate (Merck, 113748) and neutral lipids were separated using n-hexane:diethyl ether:acetic acid (70:30:1, v/v/v). After separation, the plates were air-dried, dipped into a reagent (8% (w/v) H3PO4 containing 10% (w/v) CuSO4·5H2O) for 5 s and charred for 8 min at 180 °C. TAG bands were identified by co-migration with triolein (Sigma, T7140) used as a TAG standard.
Fatty Acid Analysis of Total Lipid and TAG
Fatty acid compositions of TL and TAG were determined for the most potent microalgal isolate. FAMEs were prepared from TL and TAG extracts using acid-catalyzed transesterification as reported by [22] with little modifications. TL was extracted according to [23]. For acidic transesterification of total lipid, TL extracts solved in 0.4 mL toluene was mixed with 1 mL of 98% H2SO4 in methanol (2.5%, v/v) in a glass screw cap tube. The tube was incubated at 50 °C for 24 h. Then water (0.5 mL) and n-hexane (0.7 mL) were added. The mixture was mixed for 2 − 3 min followed by a centrifugation and the upper hexane layer was collected to determine the profile of FAME.
For acidic transesterification of TAGs, TAG separation was performed by HPTLC as described in section TAG and HPTLC analysis. After separation, TAG bands were visualized using iodine vapor, recovered from the plate, and the fractions were transferred into glass tubes. Subsequently, transesterification process of TAG was performed by acidic transesterification as noted above. FAMEs were analyzed by an Agilent HP GC 6890 gas chromatograph equipped with a flame ionization detector (FID). Samples were loaded onto a 100 m × 0.25 mm diameter 0.20 μm film HP-88 capillary column with helium as the carrier gas. The initial temperature was 120 °C, ramped 10 °C min−1 to 175 °C where it held for 10 min, increased to 210 °C at a rate of 5 °C min−1 where it held for 15 min, and was lastly ramped to 230 °C at a rate of 5 °C min−1 and held for 5 min. FAMEs were identified by comparison to the retention times of authentic standards.
Estimation of Key Biodiesel Properties
In this study, the key biodiesel properties such as kinematic viscosity, specific gravity, cloud point, cetane number, iodine value, and higher heating value were calculated from FAME profiles using the Eqs. (4)−(9) described by [24] In addition, oxidation stability were predicted using the Eq. (10) described by [25]. The Average Degree of Unsaturation (ADU) was calculated based on Eq. (3):
where ADU is the average degree of unsaturation of microalgal oil; Yi is the mass fraction of each FA constituent; M is the number of carbon–carbon double bonds in each FA constituent.
where \(x\) is the ADU.
where X is the content of linoleic and linolenic acids (wt%) (\(0 < X < 100\)); and Y is the oxidation stability in hours.
Statistical Analysis
All experiments, except fatty acid and HPTLC analyses, were carried out in triplicate and data were saved as means with standard deviations. The unpaired two-tailed t tests were applied to determine statistical differences between the data collected from nitrogen- sufficient (N +) and nitrogen starvation (N −) conditions for each isolate using GraphPad software (v8.0). The significance level was set at P < 0.05.
Accession Numbers
Sequence data obtained in this study have been deposited in the NCBI database under accession numbers MT340971 to MT340978. The accession numbers are listed in Table 3.
Results
The TL contents, relative TAG contents, biomass productivities, and lipid productivities of 30 microalgal isolates were determined under N + and N − conditions (Table 2). The TL contents ranged between 6.84 and 31.96% under N − and ranged between 3.44 and 10.86% under N + conditions. The RFU values ranged from 0.57 to 138.43 under N − condition and ranged from 0.19 to 43.43 under N + conditions. In addition, the lipid productivities, of all isolates, ranged from 0.085 to 4.56 mg/L/day under N − conditions and ranged from 0.082 to 2.6 mg/L/day under N + conditions. Preliminary screening of the various microalgae isolates revealed that N − led to a significant increase in lipid productivity, TAG content, and TL content for most of the microalgae isolated. Meanwhile, of the 30 microalgae isolates, five (saltwater isolates) had significantly lower lipid productivity and the TL content compared with the most of the 25 freshwater isolates.
Among 30 isolates, only eight isolates having highest TAG content or highest lipid productivity were selected and identified. Eight selected isolates were identified based on the cell morphology using light microscopy (Fig. 1b). However, the microscopy method for the classification of microalgae species is not enough for phylogeny construction. In the present study, seven of eight selected microalgae were identified using the ITS1-5.8S-ITS2 region sequence. Merely, the strain KPX4 was identified based on the analysis of the 18S rRNA gene sequence. The phylogenetic tree was constructed to detect the taxonomic positions of all isolates. The NCBI-BLAST sequence analysis results are given in Table 3. Based on these results, the strains KP2 and KPX4 were identified as Chlorella vulgaris, S1 and EA7 were identified as Scenedesmus sp., EA25 was identified as Ankistrodesmus sp., M19 was identified as Ankistrodesmus falcatus, and B1 and EA22 were identified as Mychonastes sp. and Acutodesmus sp., respectively. These molecular-based identifications were confirmed by a phylogenetic tree (Fig. 1a).
a Phylogenetic tree for eight native microalgal strains was constructed using the Maximum Composite Likelihood method based on a dataset of 171 aligned positions of 48 nucleotide sequences. For the analysis, the GTR + G + I model was selected. The bootstrap values (1000 replicates) are displayed next to the branches. b Microscope images of eight microalgal strains. 1. Chlorella vulgaris KP2, 2. Acutodesmus sp. EA22, 3. Ankistrodesmus sp. M19, 4. Mychonastes sp. B1, 5. Scenedesmus sp. S1, 6. Chlorella vulgaris KPX4, 7. Ankistrodesmus falcatus EA25, 8. Scenedesmus sp. EA7
Total Lipid and Relative TAG Contents of Selected Microalgae Strains
At the end of the seven days of N − conditions, the TL content of strains had significantly (P < 0.05) increased compared to N + conditions (Fig. 2a). In comparison to the N + conditions, the TL contents of the C. vulgaris KPX4, C. vulgaris KP2, and C. reinhardtii cw15 strains under N − conditions had increased by approximately 511%, 320%, and 213%, respectively. The strain with the highest TL content under N − conditions was C. vulgaris, KP2 with an average of 28.56%, followed by the C. reinhardtii cw15 and the C. vulgaris KPX4 strains with an average of 21.05% and 20.96%, respectively. The Scenedesmus sp. S1 (13.15%) and Scenedesmus sp. EA7 (15.66%) strains had the lowest average TL content under N − conditions.
a Total lipid contents, b neutral lipid contents, c lipid productivities, and d biomass productivities of nine microalgae strains grown under nitrogen-sufficient and nitrogen starvation conditions for seven days. Cultivation in nitrogen-sufficient medium is represented by N + , and in nitrogen-free medium by N − . Data are presented as the mean ± standard deviation values of three replicate experiments (n = 3)
TAG contents of strains were determined qualitatively using the Nile red fluorescence method and HPTLC techniques. In this study, the strains with the highest TAG content based on the RFU values obtained under N − conditions were C. vulgaris KP2 (mean RFU: 138.43), C. reinhardtii cw15 (mean RFU: 82.05), and C. vulgaris KPX4 (mean RFU: 74.66) (Fig. 2b). The TAG contents of these strains were found to be significantly (P < 0.05) higher than that of other strains. Based on the RFU data, it was found that the TAG content of the C. vulgaris KP2 strain was significantly (P < 0.05) higher than those of the C. reinhardtii cw15 strain. However, the TAG content of the C. vulgaris KPX4 strain was almost the same as that of the C. reinhardtii cw15 strain.
HPTLC results indicated a correlation between the amount of TAG and RFU values (Fig. 3). In the HPTLC analysis, it was found that strains with low RFU had lower TAG bands, while strains with high RFU had higher TAG bands. Moreover, while all the NL bands other than TAGs were visible on the HPTLC for all strains placed under N + conditions, the TAG bands did not appear in any other strain. These results indicated that N − stress increases the amount of TAGs in neutral lipids.
HPTLC analysis of neutral lipids extracted from nine microalgae strains grown under nitrogen-sufficient and nitrogen starvation conditions for seven days. Cultivation in nitrogen-sufficient medium is represented by N + , and in nitrogen-free medium by N − . Triolein (20 µg and 40 µg) was used as a reference substance for triacylglycerol. The lipid bands comigrating with triolein represent triacylglycerols. For Acutodesmus sp. EA22 and Scenedesmus sp. EA7 strains, the N + and N − gels were constructed by merging two lanes coming from different runs. FFA free fatty acids, TAG triacylglycerol, DAG diacylglycerol, MAG monoacylglycerol, PL phospholipid
Lipid and Biomass Productivities of Selected Microalgae Strains
The lipid productivities of the microalgae strains are shown in Fig. 2c. The lipid productivities of the nine microalgae strains ranged from 0.86 to 8.07 mg/L/day. The highest lipid productivity under N + conditions was obtained for the cw15 (8.07 mg/L/day) strain. The C. reinhardtii cw15 (4.60 mg/L/day), C. vulgaris KP2 (4.56 mg/L/day) and Scenedesmus sp. EA7 (3.56 mg/L/day) strains had the highest lipid productivity under conditions. The lipid productivities of the C. reinhardtii cw15 strain under N − conditions significantly (P < 0.05) decreased compared to the N + conditions, while the lipid productivities of other strains had significantly (P < 0.05) increased. It was determined that the C. vulgaris KP2 and Scenedesmus strains (EA7 and S1) had higher lipid productivities than the other native strains. The maximum biomass productivity was obtained for the C. reinhardtii cw15 (82 mg/L/day) mutant strain under N + conditions. The minimum biomass productivity was found in the Mychonastes sp. B1 strain (10 mg/L/day) under N − conditions (Fig. 2d).
Among the selected eight native strains, C. vulgaris KP2 exhibited the potential for biodiesel production since it had high TAG content, high TL content, and high lipid productivity under N − conditions. Therefore, C. vulgaris KP2 was selected for further analysis.
Lipid Bodies in C. vulgaris KP2 Cells Grown Under N + and N − Conditions
Lipid bodies in C. vulgaris KP2 cells were observed by Nil red staining (Fig. 4a). In addition to HPTLC and spectrofluorimetric analysis, Nile red fluorescence microscopy analysis showed that C. vulgaris KP2 produced large amounts of TAGs in their cells.
a Nile red florescence of Chlorella vulgaris KP2 cells grown under nitrogen-sufficient and nitrogen starvation conditions for seven days. Lipid bodies in nitrogen-starved cells were visualized by the florescence microscope. Cultivation in nitrogen-sufficient medium is represented by N + , and in nitrogen-free medium by N − . b Percentages of SFA, MUFA, and PUFA of TAGs and total lipids in Chlorella vulgaris KP2 cells grown under nitrogen-sufficient and nitrogen starvation conditions for seven days. Cultivation in nitrogen-sufficient medium is represented by N + , and in nitrogen-free medium by N − . SFA saturated fatty acids, MUFA monounsaturated fatty acids, PUFA polyunsaturated fatty acids. TAG triacylglycerol, TL total lipid (Color figure online)
The FAME Profiles of Total Lipid and TAG in C. vulgaris KP2
The FAME profiles of TL and TAG in C. vulgaris KP2 under N + and N − conditions were determined (Table 4). Under N + conditions, C18:3 (39.07%), C18:2 (28.65%), and C16:0 (21.86%) fatty acids in total lipids were detected as the predominant fatty acids. Upon N − , a higher proportion of C18:1 (32.55%) and a lower proportion of C18:3 (23.81%) and C18:2 (15.66%) fatty acids in TL were detected. In addition, no changes in the proportions of C18:0 and C16:0 fatty acids in TL under N − were observed. Similar to the fatty acid profile of total lipid, the most abundant fatty acid in TAG under N − was C18:1, accounting for almost 37.56% of fatty acid content. The proportions of C16:0, C18:0, C18:2 and C18:3 fatty acids in TAG decreased under N − .
The relative percentage of saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) are shown in Table 4 and Fig. 4b. In FAMEs of total lipid, the proportion of MUFAs increased from 6.86% to 33.87% under N − while the proportion of PUFAs decreased from 67.72% to 39.47%. In contrast, SFAs remained at the same level in nitrogen-starved and N + cultures. In FAMEs of TAG, the proportion of MUFAs increased under N − meanwhile, the proportions of PUFAs and SFAs decreased.
The seven critical biodiesel properties; kinematic viscosity, specific gravity, cloud point, cetane number, iodine value, higher heating value, and oxidation stability were estimated based on the FAME profiles of TAG and TL in C. vulgaris KP2 (Table 5). Estimated biodiesel properties of FAME of TAG and TL in C. vulgaris KP2 grown under N − conditions were meet the biodiesel specifications as stated by the US (ASTM D6751) and/or Europe (EN 14214) standards. Some biodiesel properties of FAME of TAG were found much better FAME of TL under N − . On the other hand, FAME of TL in C. vulgaris KP2 grown under N + conditions did not meet the biodiesel specifications due to high iodine value (147.78 g I2/100 g).
Discussion
The manipulation of the nutritional and/or cultivation conditions is one of the most commonly used approaches to lipid induction in microalgae. It has been reported that the lipid content of microalgae is in the range of 2 − 77% of dry cell weight depending on the species and environments [26]. In this study, TL contents of 30 microalgae isolates ranged from 3.44 to 31.96%. Under N − conditions, TL contents of the most potent eight microalgae strains were comparable or even higher than those reported in literature. The TL content of Chlorella vulgaris KP2 under N − was 28.56%, which was much higher than the findings of previous studies under nitrogen depletion conditions (Table 6; [27, 28]). Furthermore, [29] showed that the TL contents of S. dimorphus SD16 and S. quadricauda SQ19 were 5% and 14%, respectively, when exposed to N − conditions and high salt stress. On the other hand, several studies have reported higher lipid content than our findings. A recent study noted that the lipid content of mixotrophic Chlorella vulgaris cells under N − reached as high as 62.6% [30]. Another study reported that the TL content of Scenedesmus sp. strain R-16 was 52.6% under nitrogen-depletion conditions [31]. Additionally, Ankistrodesmus sp. M19, Ankistrodesmus falcatus EA25, Acutodesmus sp. EA22 and Mychonastes sp. B1 strains had a lower lipid content compared to previous studies using similar species and stress conditions [32,33,34]. A literature review and our findings showed that the lipid content in microalgae is species-dependent under nitrogen-depletion conditions and can be influenced by cultivation conditions such as pH, different carbon and nitrogen sources in medium, temperature, CO2 levels, and light intensity.
As TAGs have a high percentage of fatty acids and a 99% conversion rate into biodiesel, they are an important indicator in the selection of potential strains for biodiesel production. Our results indicated that N − stress led to an increase in the TAG levels of many microalgae species. [12] reported that C. vulgaris, C. zofingiensis, N. oleoabundans, and S. obliquus accumulate TAGs up to more than 35% of their dry weight during N − . The present study indicated that the TAG content of the C. vulgaris KP2 native strain was much higher than those of the C. reinhardtii cw15 mutant strain, and these two strains under N − conditions accumulate TAGs more effectively than the Scenedesmus, Ankistrodesmus, Mychonastes, and Acutodesmus strains. Although N − is the most crucial stress condition for increasing the amount of TAG in microalgae, it causes a reduction in microalgal biomass by limiting protein synthesis, chlorophyll synthesis, and photosynthetic performance [35]. Furthermore, [22] demonstrated that a pronounced reduction, upon nitrogen depletion, in ribosomal polypeptides, tryptophan synthase β subunit, and some key enzymes for CO2 assimilation like ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco).
In this study, the biomass and lipid productivities of the microalgae strains were found to be higher than those reported by other studies. In these studies, it was demonstrated that the biomass productivities of various strains of Scenedesmus species under N + conditions were 0.0098 to 0.0157 g/L/day, and their lipid productivities ranged from 0.872 to 4.255 mg/L/day [36]. By contrast, [37] reported that the biomass productivities of 12 microalgae strains ranged from 0.08 to 0.73 g/L/day and that their lipid productivities ranged from 22.61 to 204.91 mg/L/day. Compared with the lipid productivity data of some studies conducted by Chlorella vulgaris, while lipid productivity of Chlorella vulgaris KP2 (4.56 mg/L/day) was higher than the findings reported by [38], it was considerably lower than most of the reports given in (Table 6) [39,40,41].
It has been reported that the carbon source and the carbon-to-nitrogen (C/N) ratio have a significant influence on biomass and lipid accumulation in microalgae [42]. A recent study noted that the maximum biomass productivity of 5.37 g/L/day was achieved in Chlorella sp. HS2 using optimal concentrations of glucose, sodium nitrate, and dipotassium phosphate [43]. In this context, optimizing the C/N ratio of the medium by supplementing microalgae culture with one of the various carbon sources and adding a nitrogen source at the minimal concentration may improve the lipid productivity of C. vulgaris and other microalgae species used in this study.
The fatty acid compositions of microalgal oils play a pivotal role in the production of high-quality biodiesel. The microalgal lipid, which is rich in MUFAs (C16-C18), is considered as suitable fatty acids for biodiesel production since it improves the cold flow property and oxidation stability of the biodiesel [44]. Under N − , the fatty acid profile of TL in C. vulgaris KP2 was consistent with the previous finding in C. vulgaris cultivated in a nitrogen-depleted medium, in which the predominant fatty acids were C18:1 (42.77%), C16:0 (19.70%), C18:3 (16.24%), and C18:2 (9.54%) [45]. Our findings indicated that N − stress had a strong effect on both increasing % of MUFA (C18:1) and decreasing % of PUFA (C18:2 and C18:3) in TAG and TL of C. vulgaris.
These findings are in agreement with previous studies reported that FAME profile of total lipids of C. vulgaris and other microalgae species contained a substantial amount of C18:1 and a modest amount of C18:2 and C18:3 fatty acids under N − conditions [46]. The reduction of PUFAs under N − may be a result of the oxidative damage or conversion of membrane lipids into TAGs [47].
The degree of unsaturation is one of the main features of FAME in determining biodiesel properties. CN, OS, and KV increase with a decreasing degree of unsaturation levels of FAME, while iodine value decreases [24]. This is in line with our findings that under N + conditions, the lowest KV, CN, and OS values were detected for the FAME obtained from total lipid, which had the highest degree of unsaturation levels.
As shown, under N − conditions, the estimated biodiesel properties of TAG and TL in C. vulgaris KP2 are meet the biodiesel specifications established by US (ASTM D6751) standard and/or European (EN 14214) standard. However, under N − conditions, the proportion of C18:3 fatty acid in FAME of total lipid is not meet the European (EN 14214) standard (C18:3 ≤ 12%). Biodiesel with higher CN and OS corresponds to better autoignition quality and storage time of any liquid fuel in large-scale production, respectively [48]. In addition, the low IV correlates to low NOx emission [49]. Under N − conditions, the estimated CN and OS of FAME obtained from TL were 53.75 and 5.58 h, and these values slightly exceed the minimum specification by ASTM D6751 (47, 3 h) biodiesel standard. Moreover, under N − conditions, the FAME produced from TAG has favorable biodiesel properties with a lower IV (59.00 gI2/100 g), higher OS (14.24 h), and higher CN (58.73) compared with the EN 14214 biodiesel standard (IV < 120 gI2/100 g, OS > 6 h, CN > 51). HHV is another important property of biodiesel concerning fuel consumption. It has been reported that HHVs of conventional biodiesels are typically in the range of 39 − 41 MJ/kg [50]. In this study, the estimated HHV of FAME produced from TL was 40.94 MJ/kg under N − conditions.
In conclusion, the present work showed that C. vulgaris KP2 tend to accumulate high ratios of lipids and TAGs inside their cells under N − conditions. In addition to high TAG content, the fatty acid profile of C. vulgaris KP2 strain has the desired characteristics for biodiesel production under N − conditions.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Schenk PM, Thomas-Hall SR, Stephens E et al (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. BioEnergy Res 1:20–43. https://doi.org/10.1007/s12155-008-9008-8
Draaisma RB, Wijffels RH, Slegers PME et al (2013) Food commodities from microalgae. Curr Opin Biotechnol 24:169–177. https://doi.org/10.1016/j.copbio.2012.09.012
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: A review. Renew Sustain energy Rev 14:217–232. https://doi.org/10.1016/j.rser.2009.07.020
Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2013) Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl Energy 103:444–467. https://doi.org/10.1016/j.apenergy.2012.10.004
Mandal S, Mallick N (2009) Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol 84:281–291. https://doi.org/10.1007/s00253-009-1935-6
Singh P, Guldhe A, Kumari S et al (2015) Investigation of combined effect of nitrogen, phosphorus and iron on lipid productivity of microalgae Ankistrodesmus falcatus KJ671624 using response surface methodology. Biochem Eng J 94:22–29. https://doi.org/10.1016/j.bej.2014.10.019
Sun Z, gang Zhou Z, Gerken H et al (2015) Screening and characterization of oleaginous Chlorella strains and exploration of photoautotrophic Chlorella protothecoides for oil production. Bioresour Technol 184:53–62. https://doi.org/10.1016/j.biortech.2014.09.054
Sun LY, Cui WJ, Chen KM (2018) Two Mychonastes isolated from freshwater bodies are novel potential feedstocks for biodiesel production. Energy Sources, Part A Recover Util Environ Eff 40:1452–1460. https://doi.org/10.1080/15567036.2018.1477869
Yun HS, Ji MK, Park YT et al (2016) Microalga, Acutodesmus obliquus KGE 30 as a potential candidate for CO2 mitigation and biodiesel production. Environ Sci Pollut Res 23:17831–17839. https://doi.org/10.1007/s11356-016-6971-z
Allen JW, DiRusso CC, Black PN (2015) Triacylglycerol synthesis during nitrogen stress involves the prokaryotic lipid synthesis pathway and acyl chain remodeling in the microalgae Coccomyxa subellipsoidea. Algal Res 10:110–120. https://doi.org/10.1016/j.algal.2015.04.019
Breuer G, Lamers PP, Martens DE et al (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour Technol 124:217–226. https://doi.org/10.1016/j.biortech.2012.08.003
EIA 2016 Intermational biodiesel production statistics (2016) The U.S. Energy Information Administration (EIA). https://www.eia.gov/beta/international/data/browser. Accessed 06 May 2019
Uysal D, Yilmaz KÇ, Taş T (2015) Enerji ithalatı ve cari açık ilişkisi Türkiye örneği. Anemon Muş Alparslan Üniversitesi Sos Bilim Derg. https://doi.org/10.18506/anemon.22254
Rippka R, Deruelles J, Waterbury JB (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61. https://doi.org/10.1099/00221287-111-1-1
Borowitzka MA (1988) Algal growth media and sources of cultures. In: Micro-algal Biotechnology. Cambridge University Press, pp 456–465
Siaut M, Cuine S, Cagnon C et al (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol 11:7. https://doi.org/10.1186/1472-6750-11-7
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc 18:315–322. https://doi.org/10.1016/b978-0-12-372180-8.50042-1
Mishra SK, Suh WI, Farooq W et al (2014) Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour Technol 155:330–333. https://doi.org/10.1016/j.biortech.2013.12.077
Chen W, Zhang C, Song L et al (2009) A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Methods 77:41–47. https://doi.org/10.1016/j.mimet.2009.01.001
Hara A, Radin NS (1978) Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 90:420–426. https://doi.org/10.1016/0003-2697(78)90046-5
Msanne J, Xu D, Konda AR et al (2012) Metabolic and gene expression changes triggered by nitrogen deprivation in the photoautotrophically grown microalgae Chlamydomonas reinhardtii and Coccomyxa sp. C-169. Phytochemistry 75:50–59. https://doi.org/10.1016/j.phytochem.2011.12.007
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/y59-099
Hoekman SK, Broch A, Robbins C et al (2012) Review of biodiesel composition, properties, and specifications. Renew Sustain energy Rev 16:143–169. https://doi.org/10.1016/j.rser.2011.07.143
Park JY, Kim DK, Lee JP et al (2008) Blending effects of biodiesels on oxidation stability and low temperature flow properties. Bioresour Technol 99:1196–1203. https://doi.org/10.1016/j.biortech.2007.02.017
Sajjadi B, Chen W-Y, Raman AAA, Ibrahim S (2018) Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew Sustain Energy Rev 97:200–232. https://doi.org/10.1016/j.rser.2018.07.050
Lv J-M, Cheng L-H, Xu X-H et al (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 101:6797–6804. https://doi.org/10.1016/j.biortech.2010.03.120
Wong YK, Ho YH, Ho KC, et al. (2017) Growth medium screening for Chlorella vulgaris growth and lipid production. J Aquac Mar Biol Doi: https://doi.org/10.15406/jamb.2017.06.00143
Sharma T, Gour RS, Kant A, Chauhan RS (2015) Lipid content in Scenedesmus species correlates with multiple genes of fatty acid and triacylglycerol biosynthetic pathways. Algal Res 12:341–349. https://doi.org/10.1016/j.algal.2015.09.006
Shen X-F, Qin Q-W, Yan S-K et al (2019) Biodiesel production from Chlorella vulgaris under nitrogen starvation in autotrophic, heterotrophic, and mixotrophic cultures. J Appl Phycol 31:1589–1596. https://doi.org/10.1007/s10811-019-01765-1
Ren HY, Liu BF, Ma C et al (2013) A new lipid-rich microalga Scenedesmus sp strain R-16 isolated using Nile red staining: effects of carbon and nitrogen sources and initial pH on the biomass and lipid production. Biotechnol Biofuels. https://doi.org/10.1186/1754-6834-6-143
Yuan C, Liu J, Fan Y et al (2011) Mychonastes afer HSO-3-1 as a potential new source of biodiesel. Biotechnol Biofuels 4:47. https://doi.org/10.1186/1754-6834-4-47
Ho SH, Chen CY, Chang JS (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252. https://doi.org/10.1016/j.biortech.2011.11.133
Álvarez-Díaz PD, Ruiz J, Arbib Z et al (2014) Lipid production of microalga Ankistrodesmus falcatus increased by nutrient and light starvation in a two-stage cultivation process. Appl Biochem Biotechnol 174:1471–1483. https://doi.org/10.1007/s12010-014-1126-5
Zhao L-S, Li K, Wang Q-M et al (2017) Nitrogen starvation impacts the photosynthetic performance of Porphyridium cruentum as revealed by chlorophyll a fluorescence. Sci Rep 7:1–11. https://doi.org/10.1038/s41598-017-08428-6
Gour RS, Chawla A, Singh H et al (2016) Characterization and screening of native Scenedesmus sp isolates suitable for biofuel feedstock. PLoS ONE. https://doi.org/10.1371/journal.pone.0155321
Nascimento IA, Marques SSI, Cabanelas ITD et al (2013) Screening microalgae strains for biodiesel production: Lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenergy Res 6:1–13. https://doi.org/10.1007/s12155-012-9222-2
Griffiths MJ, van Hille RP, Harrison STL (2014) The effect of nitrogen limitation on lipid productivity and cell composition in Chlorella vulgaris. Appl Microbiol Biotechnol 98:2345–2356. https://doi.org/10.1007/s00253-013-5442-4
Mujtaba G, Choi W, Lee CG, Lee K (2012) Lipid production by Chlorella vulgaris after a shift from nutrient-rich to nitrogen starvation conditions. Bioresour Technol 123:279–283. https://doi.org/10.1016/j.biortech.2012.07.057
Belotti G, Bravi M, de Caprariis B et al (2013) Effect of nitrogen and phosphorus starvations on Chlorella vulgarislipids productivity and quality under different trophic regimens for biodiesel production. Am J Plant Sci 04:44–51. https://doi.org/10.4236/ajps.2013.412a2006
Griffiths MJ, Van Hille RP, Harrison STL (2014) The effect of degree and timing of nitrogen limitation on lipid productivity in Chlorella vulgaris. Appl Microbiol Biotechnol 98:6147–6159. https://doi.org/10.1007/s00253-014-5757-9
Silaban A, Bai R, Gutierrez-Wing MT et al (2014) Effect of organic carbon, C: N ratio and light on the growth and lipid productivity of microalgae/cyanobacteria coculture. Eng Life Sci 14:47–56. https://doi.org/10.1002/elsc.201200219
Kim HS, Park W-K, Lee B et al (2019) Optimization of heterotrophic cultivation of Chlorella sp. HS2 using screening, statistical assessment, and validation. Sci Rep 9:1–13. https://doi.org/10.1038/s41598-019-55854-9
Knothe G (2009) Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ Sci 2:759–766. https://doi.org/10.1039/B903941D
Adamakis I-D, Lazaridis PA, Terzopoulou E et al (2018) Cultivation, characterization, and properties of Chlorella vulgaris microalgae with different lipid contents and effect on fast pyrolysis oil composition. Environ Sci Pollut Res 25:23018–23032. https://doi.org/10.1007/s11356-018-2368-5
Shen P-L, Wang H-T, Pan Y-F et al (2016) Identification of characteristic fatty acids to quantify triacylglycerols in microalgae. Front Plant Sci 7:162. https://doi.org/10.3389/fpls.2016.00162
Li X, Moellering ER, Liu B et al (2012) A galactoglycerolipid lipase is required for triacylglycerol accumulation and survival following nitrogen deprivation in Chlamydomonas reinhardtii. Plant Cell 24:4670–4686. https://doi.org/10.1105/tpc.112.105106
Azam MM, Waris A, Nahar NM (2005) Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenerg 29:293–302. https://doi.org/10.1016/j.biombioe.2005.05.001
Bart JCJ, Palmeri N, Cavallaro S (2010) Biodiesel science and technology from soil to oil. Elsevier, pp 226–284. https://doi.org/https://doi.org/10.1533/9781845697761.22650.
Demirbas A (2008) Biodiesel. Springer, pp 111−119.
Acknowledgements
The authors express their gratitude to TÜBİTAK (The Scientific and Technological Research Council of Turkey, 116Y345) and the University of Nevsehir Hacı Bektas Veli, Scientific Research Projects Unit (NEUBAP15F26), for their financial support.
Funding
This study was funded by TÜBİTAK (The Scientific and Technological Research Council of Turkey, 116Y345) and the University of Nevsehir Hacı Bektas Veli, Scientific Research Projects Unit (NEUBAP15F26).
Author information
Authors and Affiliations
Contributions
EEA performed the experiments, analyzed and interpreted the data, wrote the paper, and prepared figures and/or tables. SO and BA planned and designed the research, interpreted the data, and wrote and edited the paper.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andeden, E.E., Ozturk, S. & Aslim, B. Evaluation of Thirty Microalgal Isolates as Biodiesel Feedstocks Based on Lipid Productivity and Triacylglycerol (TAG) Content. Curr Microbiol 78, 775–788 (2021). https://doi.org/10.1007/s00284-020-02340-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02340-5