Abstract
Aromatic plants had been used since ancient times for their preservative and medicinal properties, and to impart aroma and flavor to food. Also their secondary metabolites are economically important as drugs, flavor and fragrances, pharmaceuticals, agrochemicals, dye, and pigments, pesticides, cosmetics, food additives, other industrially biochemical, and also play a major role in the adaptation of plants to their environment. Indole acetıc acid-producing rhizobacteria inoculations increase in stomatal density and level of secondary metabolite and have a synergistic effect on monoterpene biosynthesis. Bacterial inoculation significantly affected and increased the chemical composition of essential oil, citronellol, and geraniol content in rose-scented geranium; essential oil composition and total phenolic content in marigold; density, number, and size of glandular trichomes in sweet wormwood and peppermint essential oil components such as geranyl acetate, limonene, and β–pinene in coriander; oil yield and content in calendula; yield of the herb in hyssop; oxygenated compounds, essential oil content and yield, anethol and changing the chemical composition in fennel; growth, number of glandular trichomes and essential oil yield, root branching and length, and total amount of essential oil, production of monoterpenes such as pulegone, menthol, menthone, menthofuran, and terpineol content, biosynthesis of secondary metabolites in peppermint; growth and essential oil yield in marjoram; glandular hair abundance, essential oil yield, and monoterpene biosynthesis in basil; phellandrene, limonene, borneol, and campor in rosemary; carvacrol, thymol, linalool, and borneol in oregano; and α-thujene, α-pinene, α-terpinene, p-simen, β–pinene, and γ-terpinene contents and essential oil yield in summer savory. Inoculation with IAA-producing bacteria medicinal roots increased the valerenic acid in valerian, essential oil and quality in vetiver, curcumin content in turmeric alkaloid and ginsenoside content in ginseng, and inulin content in Jerusalem artichoke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant hormones are usually divided into five main groups namely; auxins, cytokinins, gibberellins, abscisic acid, and ethylene. In addition, the current discovered phytohormones belong to the group of strigolactones, brassinosteroids, jasmonic acid, salicylic acid, polyamines, and nitric oxide hormones [1,2,3]. Auxins, cytokinins, gibberellic acid, abcisic acid, and ethylene are the names of five main classes of plant hormones and they are known as the signal molecules required in many plant development processes [4,5,6], however, there are many prominent evidences are in hands that other compounds also regulate growth in plants. Auxins are the most important group of plant hormones that affect plant growth and development either alone or in combination with other plant hormones [7]. Moreover, the levels of auxin are affected by plant hormones namely; cytokines, ethylene, gibberellins, jasmonates, and brassinosteroids [6, 8,9,10].
Auxins are divided into two basic groups such as naturally occurring and synthetic auxin analogs. There are five naturally occurring auxins in plants include indole-3-acetic acid (IAA), 4-chloroindole-3-acetic acid (4-Cl-IAA), phenyl acetic acid (PAA), indole-3-butyric acid (IBA), and indole-3-propionic acid (IPA) [11, 12]. In addition to endogenous auxins, many synthetic compounds having auxinic activity, called synthetic auxin, that have been synthesized and developed. Some of the commonly known synthetic auxins are: 2,4-dichlorophenoxyacetic acid (2,4-D), α-naphthalene acetic acid (α-NAA), 2-methoxy-3,6-dichlorobenzoic acid (dicamba), 4-amino-3,5,6-trichloropicolinic acid (tordon or picloram), 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), and so on [13, 14]. Although many natural and synthetic compounds show auxin-like activity in bioassays, IAA is the best known and physiologically the most active natural auxin in the plant and is recognized as the key auxin in most plants. Indole-3-acetic acid, the major natural auxin found in plants is a signaling substance involved in almost every plant developmental process and plays a key role in the early stages of adventitious rooting [12, 15].
Indole-3-acetic acid (IAA) is one of the best-characterized auxin and the essential plant hormones that has the capacity to control plant growth. Many of bacteria have plant growth-promoting or phytopathogenic characteristics that can synthesize IAA. Indole-3-pyruvic acid, indole-3-acetamide, and indole-3-acetonitrile are the three basic ways for IAA synthesis [16]. For instance, it has been shown that the most important auxin, produced by the Azospirillum is the IAA [17, 18], while in some studies [19], indole-3-butyric acid (IBA) can be produced at a high rate and converted to IAA. Azospirillum can produce IAA at all stages of development [20]. It has been reported by Duca et al. [21] that the IBA is an important source and reserve for the IAA in Azospirillum strains, Azospirillum sp. can produce IAA in four different ways, three of them would be tryptophan-dependent indole-3-pyruvic acid, indole-3-acetamide and tryptamine, and the other as tryptophan-free way. The levels of IAA increase due to fluctuations in environmental factors, nutrient deficiencies, especially the limitation of nitrogen, carbon, and phosphorus [20]. The IAA is the most common endogenous auxin which plays a role in root development and elongation, and it is a common product of L-tryptophan metabolism by several microorganisms including plant growth-promoting bacteria (PGPB) [21,22,23,24,25]. IBA is an endogenous compound that appears to regulate both lateral and adventitious root formation [26]. Auxin production is considered as an important factor in the ability of rhizospheric bacteria to promote plant growth directly [27,28,29]. A significant proportion of the bacteria isolated from the rhizosphere and endosphere produced IAA, and the rhizosphere bacteria produced more indolic compounds than the other parts of rhizosphere found in soil [30,31,32,33,34]. The synthesis of indolic compounds in bacteria depends on the precursors in root secretions. Among the different root secretions, L-tryptophan has been identified as the main precursor of the synthesis of bacterial indolic compounds [35]. Microbial auxin production is not only responsible for strengthening the relationship between plant and microorganisms, but also is an important factor that positively promotes plant growth and development. In this way, bacterial auxin production potential is considered effective in reducing the hazardous effects of chemical fertilizers on the ecosystem and can be used to improve higher yields and growth [36]. Auxin production by PGPB activates biosynthetic signaling pathways [37]. It is thought that microbial auxin production by PGPB changes the auxin level and affects all physiological processes due to the critical effects of the level of auxins on plant growth and thus improving plant growth and yield [38].
Organic nitrogen sources stimulate IAA production in a better way than that of inorganic nitrogen sources [16, 20, 39]. The bacterial effect in the plant rhizosphere is largely related to the production of auxin phytohormone and indolic compounds such as IAA produced by many bacterial species play an important physiological role in rhizobacteria–plant interactions [40,41,42]. Jasim et al. [43] reported that, IAA synthesis by bacteria may have various regulatory effects in plant–bacterial interactions and significant effect on plant growth promotion. IAA-producing isolates can enhance and improve the compatibility of the plant–microorganism interaction [44, 45], and bacterial IAA stimulates the development of the root system of the host plant and allows more bacteria to be deployed in the rhizosphere by increasing root secretions [45, 46]. As in the defense mechanism, IAA is involved in almost all stages of growth and development of the plant. Bacterial IAA loosens plant cell walls, resulting in an increase in the amount of root exudations that provides additional nutrients to support the growth of rhizosphere bacteria [47, 48]. IAA stimulates excessive production of capillary roots hairs and lateral roots in the plant and promotes the release of saccharides from plant cell walls during the elongation process [49]. The IAA plant produced by the bacteria affects the plant physiological processes by changing the auxin pool. Moreover, bacterial IAA affects the morphology and development of root; then the root increases its surface area, volume, and length and it also allows the plant to reach the nutrients more easily in the soil. Consequently, PGPB indirectly promotes plant growth by increasing nutrient uptake [50] and also modify root functioning, improve plant nutrition, and influence the physiology of the whole plant [51, 52]. Inoculation with PGPB modulate root architecture due to increased IAA level, which allows plants to uptake more nutrients under salinity and drought stress [51,52,53,54,55].
Indole-3-acetic acid, an auxin compound, is produced by bacteria in different biosynthetic ways [4, 40]. Several microorganisms living in the plant rhizosphere can synthesize auxin as the secondary metabolite due to the rich substrates leaking from the roots as compared to the non-rhizosphere soil and it releases the auxin free in the environment [22, 56, 57]. A part of IAA secreted by the bacteria is taken up by plant cells and it induces cell division in the plant along with the plant's endogenous IAA content [58]. 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity can be increased by microbial auxin production because auxin stimulates the biosynthesis of ACC synthase enzyme in the plant which results an increase in ACC expression [59, 60]. As a result of the interaction between IAA and ACC, ACC deaminase is that by lowering plant ethylene levels, facilitates the stimulation of plant growth by IAA [17]. IAA secreted by bacteria can increase root growth by stimulating cell division or cell elongation in plant, furthermore, it can produce ACC deaminase indirectly [61]. In general, the bacterial IAA and ACC deaminase can increase the root surface area and length making the plant easier to access to nutrients and water in the soil [62]. On the other hand, PGPB, which simultaneously contains IAA and ACC deaminase, can lead to a better growth of plants as compared to IAA or ACC producer bacteria alone [63, 64].
Auxin production potential is not only limited to rhizospheric PGPBs, but also endophytic bacteria and plant growth-promoting fungi can produce auxins. Bacteria have been reported to produce cytokines and other plant hormones i.e., gibberellin, but the vast majority of plant hormone-producing bacteria can also produce IAA [65, 66]. An important part of rhizospheric bacteria can produce IAA. Erwinia herbicola, Agrobacterium tumefaciens, Agrobacterium rhizogenes, and Pseudomonas syringae like pathogenic bacteria and Azotobacter, Pseudomonas, Azospirillum, Bacillus, Paenibacillus, Burkholderia, Enterobacter, Aeromonas, Alcaligenes, Pantoea, Streptomyces, and Rhizobium like plant growth-promoting species can produce IAA [16, 23, 37, 38, 67,68,69,70,71,72,73]. Many bacteria species including soil, epiphytic, endophytic, marine, methylotrophs, and cyanobacteria have the ability to synthesize IAA [42, 74,75,76]. Plants provide suitable shelter and nutrient containing secretions to the millions of IAA-producing and mutually beneficial bacterial partners of these genera. Bacterial IAA increases the number and length of side and primary roots in the ideal concentration range by stimulating the formation of fibrous root, however, bacterial IAA inhibits primary root growth at high concentrations [49].
Among the various mechanisms involved in plant growth enhancement by PGPR is microbial auxin production, which has been reported to play major role in growth improvement of plants by PGPRs. The majority of bacteria cultured from plants are IAA producers, a fundamental substance in the plant life cycle. Auxins action and interaction regulate most of the physiological activities and growth in plants, and the production of indole acetic acid (IAA) is a vital feature of rhizobacteria that stimulate and facilitate plant growth and development [77]. In the production of many medicinal and aromatic plant species consumed without further processing, it is of great importance to avoid the use of chemical inputs and to develop growth-promoting strategies involving phytohormone-producing PGPR and bio-fertilizers. Therefore, this review focuses on the effect of effects of auxins, external IAA applications and IAA-producing bacterial inoculations on growth, yield, and essential oil content and components of medicinal and aromatic herbs, seeds, roots, tubers, bulbs and rhizomes.
Effects of Auxins on Plants
Generally, IAA hormone in plants plays an important role in cell division, proliferation, and differentiation, vascular tissue alteration, responses to light and gravity, general root and shoot architecture, seed and tuber germination, organ differentiation, peak predominance, ethylene synthesis, vegetative growth processes, fruit development and aging [16, 49, 78], initiation of lateral and floral organ and organogenesis [79], initiation of rooting, foliation and flowering [80], formation of lateral and adventitious roots [81, 82], and increasing the growth of cambium and size of xylem cells [83]. Auxin in particular is central to the establishment and maintenance of a root meristem. IAA also affects fluorescence and photosynthesis, pigment formation, biosynthesis of various metabolites, and resistance to stress conditions [52, 78]. Auxin is also effective in the growth and seed development of oilseed crops, subsequently increasing the production of oils from seeds. Among different plant hormones that play a role in regulating reproductive plant growth, auxins trigger flower and fruit development programs that are closely related to flower and fruit development [84]. Villacorta et al. [85] reported that IAA is the most abundant plant hormone and is associated with both vegetative and reproductive development in hop (Humulus lupulus) plants. Furthermore, previous reports suggest that the mechanism involved in indole-mediated signaling in plants leading to significant plant growth promotion [86].
The cells in the vicinity of the developing leaves often consume auxins, thus limiting the formation of new leaves that are very close to each other. An important part of auxins help the young leaves and fruits to remain intact, whereas, a decrease in auxin level leads to separation in the branch of the petiole or the stem of the fruit and causes the leaves and fruits to fall on ground. Studies show that inadequate IAA-producing plants lost their ability to avoid shade and showed a stunted growth as compared to normal plants [87]. In addition, the use of reactive oxygen species in combination with auxin may provide plants with a mechanism to optimize plant performance during stress [8]. It has been determined that IAA applications stimulate the number of branches, length of stem, number of nodes per plant, callus development, and formation of root and stem in Melissa officinalis [88].
The most common of hormones are auxins and IAA is one of the most active physiological auxins in plants. Auxins stimulate the root formation and initiation of lateral roots. Low concentrations of exogenous IAA can stimulate primary root elongation, whereas high IAA levels enhance formation of lateral roots and root hairs [61, 89]. However, auxins delay root development at higher concentrations but stimulate the growth of root at certain optimal levels [9, 36, 90]. The inhibitory effect of high auxin concentrations on the plant roots is the result of an increase in the stimulation of ethylene synthesis by increasing the auxin level of ACC synthesis which is the precursory substance of ethylene and the inhibition of root growth by auxins has been found to be reversed by the application of inhibitors of ethylene synthesis [10, 36]. Ethylene is necessary in the process of seed germination but high concentrations of ethylene can lead to the prevention of root growth after germination. On the other hand, it has been found that PGPBs inhibit the activity of ACC, the precursory substance of ethylene, reducing the ethylene synthesis which is responsible for inhibiting root growth [59, 91]. The observed plant response to auxins is cell growth, structure of root and stem, vascular growth and development in tissue culture, increased protein, and synthesis and polymerase activity of RNA [10, 90,91,92,93]. Auxins are critical to plants, and regulate and manage many developmental processes from embryogenesis to senescence. Of the various plant hormones, auxins acting as the master control mechanism can be considered directly and indirectly responsible for most of the plant development patterns as the most effective regulator of plant processes [94, 95]. Previous studies demonstrated that exogenous IAA influence root architecture, elongation and root surface area, play important roles on the regulation of C–N metabolism, and enhance the conversion of inorganic N to amino acids [96].
Effect of External IAA Applications on Medicinal and Aromatic Plants
Plant growth regulators can affect the biochemical pathways and physiological processes, and can also change the plant metabolism as well as essential oils biosynthesis. It affects the amount and the components of essential oil when applied externally. The effects of growth regulators on plant are likely to change the path of terpenoid biosynthesis and create a stress factor that stimulates defense responses. Auxins regulate most of the physiological activities and growth in plants [77]. On the other hand, IAA can also protect bacteria against environmental stresses [16]. It has been determined that IAA application increased nerol and geraniol in Melissa officinalis [97], aromadendrene, β-selinene, and α-humulene in Sambucus ebulus [98], the content of α-bisabolol oxide in Chamomile recutita [99], the content of linalool in Ocimum basilicum [100], the carvacrol and thymol content Lippia origanoides [101], and the yield of essential oil in Ocimum gratissimum [102]. It has been determined in some previous studies that plant growth regulators increased the herb yield of basil, fenugreek, and coriander plants [103, 104]; affected monoterpenes in Ocimum basilicum and Lavendula dentata plants [102, 105]. It has been reported that auxin and cytokinins increased certain components in Melissa oil [97], similarly, the application of NAA and IAA increased the essential oil in Mentha piperita [106]. In addition, it has been shown that cytokinins stimulated the metabolism and accumulation of essential oil, especially monoterpenes in Mentha piperita L. and Salvia officinalis L. plants [107]. Studies on the effect of plant tissue culture and plant growth regulators on the effect of essential oil profile, composition, and yield have been shown to affect the secondary metabolites [97, 108, 109]. In some other studies, it has been seen that the applications of external hormone affected the main components of essential oil [110, 111]. Roots of medicinal E. maritimum growing in auxin-supplemented media regulate in vitro morphogenesis and increase phenolic acid and triterpenoid saponin accumulation [112]. The applications of IAA increased the thymol content in Thymus vulgaris oil [110]; content of β–pinene, camphene, and caryophyllene in Alpinia zerumbet [111]; rooting, root length and soil surface overgrowth in Melaleuca alternifolia plant [113]; yield of essential oil in O. gratissimum [92] and in the aromatic grasses of Cymbopogon martinii and C. winterianus [114]; contents of neral and 1,8-cineole in Lippia citriodora [104], and number and weight of root in Salvia fruticosa plant have also been determined [115].
Effect of IAA-Producing Bacteria on Medicinal and Aromatic Herbs, Spices, and Seed Crops
Agricultural applications may change the amount of active substance and chemical composition in medicinal and aromatic plants. The plant growth-promoting bacteria were found to have a great potential for use as bio-inoculants to increase production in medicinal and aromatic plants. The production of plant growth hormones has been suggested as one of the mechanisms by which PGPB stimulate plant growth [23, 67]. The PGPB strains may increase the level of root hormone by exogenous production of IAA, cytokinin, and/or other plant hormones in the rhizosphere, which are then absorbed by the root. It is possible that PGPB strains affect root hormone levels by producing IAA and/or other plant hormones in the rhizosphere, which are then absorbed by the root [27]. The preincubation of the Pseudomonas sp. and Azotobacter sp. lead to the synthesis and release of IAA into the culture medium that could be readily assimilated by the plant. Indeed, when these are co-cultured with shoot cultures of medicinal herb Swertia chirayita under in vitro conditions have been reported to increase the number, length, vigor of shoots and roots, and then reduce the use of synthetic plant growth hormones [116]. On the other hand, the production of IAA by Azospirillum sp. has been accepted as the basic factor in stimulating the plant growth [117]. Plant growth-promoting effect of bacterial applications appeared to be related to phytohormone production and a positive relationship between the amount of IAA secreted and plant yield values was also observed for some of the other PGPB [24].
Martinez-Morales et al. [118] reported that Azospirillum brasilense strain produces IBA, a substance associated with auxin activity that regulates plant growth. It has been observed that the inoculation of A. brasilense stimulates the formation of lateral and adventitious roots, which stimulate the root system through the stimulation of capillary rooting. Bacterial-based microbial auxin production has been shown to play the most effective role on plant growth and development among PGPB action mechanisms. IAA is a secondary metabolite produced by bacteria and mostly affects the root system, and increases the number and size of adventitious roots [119]. It has been stated that IAA-producing bacteria are a very important tool in plant development [120], moreover, stimulate the lateral roots and root hairs, and increase the germination rate and the development of root and shoot [121,122,123].
In the studies conducted with microorganisms, inoculations of Glomus fasciculatum, Azotobacter chroococcum, and A. awamori have increased the essential oil content, weight of root and stem, and total biomass in Ocimum spp.[124], increased the growth of Ocimum basilicum with the inoculations of G. fasciculatum, P. fluorescens, and B. megaterium [125], increased the dry weight of root and stem, N, P, K, and essential oil content in Ocimum basilicum with the inoculations of P. putida and A. chroococcum similarly [126]. Similarly, in combination with single and combined PGPB applications or with mycorrhiza inoculations, synergistic effects have been observed in combination with the increase in growth and yield in Mentha piperita [127], Mentha arvensis [128], Salvia officinalis [129], Silybum marianum [130], Foeniculum vulgare [131], Ocimum basilicum [132], Withania somnifera [133], Catharanthus roseus [134, 135], Chrysanthemum cinerariifolium [136], Calendula officinalis [137], Hibiscus sabdariffa [138], Origanum majorana [139], and Phyllanthus amarus [140] medicinal and aromatic plants. In previous research carried out in tea plant (Camellia sinensis) with PGPB inoculations could be stimulated overall plant growth, including shoot development, plant height, trunk diameter, leaf yield, nutrient uptake, chlorophyll and anthocyanin content, leaf area, and activities of oxidative, catalytic, hydrolytic, and anti-oxidative enzymes [64, 141,142,143,144,145]. IAA-producing bacteria had significant effects on plant development and corm of Crocus sativus [146].
In previous studies, the inoculations of PGPB, also had a positive effect on the essential oil yield in Ocimum basilicum [147], Mentha piperita [148, 149], Anethum graveolens [150], Calendula officinalis [151], Foeniculum vulgare [152], Origanum onites [153], Origanum majorana [139], Origanum x majoricum [154], Pogostemon cablin [155], Salvia officinalis [156], Satureja hortensis [157], Tagetes minuta [158], and Pelargonium graveolens [159]. In addition, certain research findings showed that the applications of IAA could increase plant oil yield [102]. Development and yield of seed and essential oil could be increased with PGPB inoculations in medicinal and aromatic plants such as Pimpinella anisum [160, 161], Coriandrum sativum [162], Cymbopogon martini [163], Anethum graveolens [164], Borago officinalis and Nigella sativa [165, 166], Foeniculum vulgare [167,168,169], Ocimum basilicum [170], Ocimum sanctum [171], and Cuminum cyminum [172]. It has been reported that the IAA production from Bacillus subtilis and Serratia plymuthica produced the highest indole-3-acetic acid and increase for both the blossoms and the essential oil in chamomile [173].
In many studies conducted on different aromatic plant species, the use of PGPB has been found to have a strategy importance to increase yield and monoterpene production [122, 148, 149, 154, 158]. In addition, increase in the content of several alkaloid and terpenoid compounds of pharmaceutical relevance was demonstrated in medicinal plants following PGPB inoculation [51, 134, 174]. The strong positive relationship between the menthol content and the IAA-producing bacteria showed that auxin production increased the monoterpene accumulation to the mint with high commercial importance [149]. The use of PGPB increases the yield, essential oil components, monoterpene production and accumulation [104, 120, 122, 139, 150, 154, 167, 175], and it affects the biosynthesis of secondary metabolite such as phenolic and flavonoid, and increases the main components of essential oil [156].
It has been determined that the inoculation of plant growth stimulator namely, P. fluorescence increased alkaloid content and biomass yield in Catharanthus roseus [134]; phosphate solubilizing bacteria increased the grain yield in the medicinal species of Phyllanthus amarus [176]; Azotobacter and Azospirillum bacteria increased plant height, fresh, and dry weight of root in Salvia officinalis [177]; Azotobacter inoculation increased essential oil ratio in Rosmarinus officinalis [178], and herbage and oil yield in Mentha piperita [179]; single and combination of Pseudomonas pseudoalcaligenes and Bacillus pumilus enhanced photosynthetic leaf area, chlorophyll and carotenoid content, leaf-stem ratio and oil yield, menthol, and the total phenolic content under normal condition in Hyptis suaveolens [180]; the combined inoculation of mycorrhizal Azotobacter fungi with Azospirillum and Bacillus bacteria increased the total biomass production in Cymbopogon martinii species [163]; inoculation with Serratia liquefaciens enhanced carvacrol in Origanum syriacum subsp. sinaicum [181]; rhizobacteria interactions may alter the alkaloid composition in Datura stramonium shoot [182] and total ginsenoside content in Panax ginseng [183] and biological fertilizer applications significantly increased the development of Thymus vulgaris species [184]. PGPR inoculation has been reported to have a positive effect on essential oil yield and phellandrene main compounds of essential oils in Rosmarinus officinalis under normal and salinity conditions [185].
Inoculation with P. fluorescens increased the main components in M. piperita oil such as the amount of pulegone, menton, menthol, and menthofuran [148]. The full interaction of bacteria with plants stimulates the secondary metabolite response [139, 154]. Inoculation of the Origanum majorana plant with P. fluorescens could increase the yield of essential oil. Bacteria are encouraged to stimulate the plant growth hormones, produce essential organic compounds, dissolve phosphate, oxidize the sulfur, increase nitrate intake and root permeability, and also combine plant growth with bacterial volatiles [148].
In the studies conducted on Ocimum basilicum, it has been shown that the efficiency of the glandular hair and essential oil yield can be increased by PGPB applications [147], and the content of α-terpineol and eugenol can also be increased from the oil components along with the yield of essential oil [122]. It has been noted that the biological fertilizer caused an increase in components of α-pinene, β-pinene, limonene, 1,8-cineole, linalool, camphor, β-terpineol, borneol, terpinene-4-ol, carvone, thymol, carvacrol, linalyl acetate, geranyl acetate, β-caryophyllene, and caryophyllene oxide in Rosmarinus officinalis plant [178]. The inoculations of Origanum x majoricum with P. fluorescens, B. subtilis and A. brasilense have been found to increase the yield of essential oil and the biosynthesis of the components of essential oil such as cis- and trans-sabinene hydrate, γ-terpinen, carvacrol, and thymol [154]. Essential oil, dry herbage and leaf yield, chlorophyll content, and biosynthesis of major essential oil components such as carvacrol, thymol, linalool, and borneol of Origanum onites were significantly affected by inoculation with effective IAA-producing, N2-fixing, and P solubilizing PGPR species such as P. fluorescens, P. putida, B. subtilis, and P. polymyxa [153]. It has been determined that the biological fertilization increases the quality as well as the yield in Anethum graveolens [150] and in Chrysanthemum cinerariifolium [136].
Santoro et al. [148] reported that the plant hormone-producing bacterial inoculations (P. fluorescens, B. subtilis, A. brasilense) stimulate the biosynthesis of secondary metabolites as well as influencing the flow and specific stages of the path of monoterpene metabolism in Mentha piperita plant. In Coriandrum sativum, PGPB inoculations were determined to increase geranyl acetate, limonene, and beta pinene content in essential oil [186]; and bacterial biological fertilizers were determined to increase the leaf area index, total chlorophyll content, and the weight of fresh stem of Sideritis montana [187]. In Tagetes minuta aromatic plant, the inoculations of P. fluorescens and A. brasilense were found to increase essential oil yield and total phenolic content, and also were important in the accumulation of secondary metabolites [158] and P. monteilii PsF84 and P. plecoglossicida PsF610 increased the dry biomass of stem, dry root weight, yield of essential oil and chlorophyll content; changed oil composition; and increased the content of citronellol and geraniol of Pelargonium graveolens cv. Bourbon [159].
The applications of bacteria were significantly affected the plant growth, oil content and yield in Calendula officinalis [151]; in Mentha piperita, the root and stem biomasses, leaf area, number of nodes, and densities of hair were increased, and significant quantitative and qualitative changes have been shown in monoterpenes, and the components (pulegone, menton, menthol, 1,8-cineole, and linaol) of essential oil [175]. The increased monoterpene content observed in inoculated plants in these studies may result from growth-promoting substances secreted by PGPB that affect plant metabolic processes. The fresh stem weight, number of glandular hair, dry weight, yield of essential oil, and the biosynthesis of main components in Mentha piperita were increased with PGPB inoculations, a positive correlation has been seen between bacterial IAA production and menthol content [149] and root and stem biomass, P content of leaf, yield of essential oil, total phenolic and flavonoid content; and cis-thujene, camphor, and 1,8-cineole from the main components of essential oil have been increased in Salvia officinalis [156]. It has been found that the density of glandular trichomes in peppermint inoculated with IAA-producing bacteria is higher and that volatile oils of aromatic plants are generally associated with the total number and distribution of glandular trichomes where the oil components are synthesized and stored [149, 188]. On the other hand, the number and diameter of glandular varices have been reported to affect the oil yield of Mentha piperita [179]. PGPB-hosting plants are correlated with nutritional status, and factors that increase dry matter production, affect the interrelationship between primary and secondary metabolism, and leading to increased biosynthesis of secondary products. In addition, increased secondary metabolite production may be directly related to improved nutritional status and primary metabolism of plants after PGPR inoculation.
Effect of IAA-Producing Bacteria on Medicinal Roots, Tubers, Bulbs, and Rhizomes
Bacterial IAA plays a major role in the development of the host plant root system. Auxins have an effect on the whole plant; in particular, IAA is synthesized by microbe mostly affects the root system, increases the size and number of adventurous roots, and leads to a significant increase in the number of branches and hence the surface area in soil contact [119, 189]. Inoculations with the phytohormone-producing bacteria produced the highest root weights and total root numbers and encouraged adventitious root formation. Root growth is a key auxin-regulated process for plant development. Patten and Glick [61] reported that the IAA, produced from Pseudomonas putida by indole pyruvic acid pathway, had a direct positive effect on the root development. Bacterial IAA can attract more rhizosphere bacteria by increasing more amount of root exudation, stimulates the development of the host plant root system, modify the architecture of the root system, and plays a very important role in bacteria-plant interactions.
Bacterial phytohormone production, especially IAA production, is widely distributed among plant-associated bacteria and is still considered the primary mechanism that enhances the growth and yield of plants [190]. Therefore, a number of IAA-producing microorganisms such as Bacillus, Paenibacillus, Lysinibacillus, Burkholderia, Acetobacter, Acinetobacter, Aeromonas, Azotobacter, Agrobacterium, Alcaligenes, Chryseobacterium, Herbaspirillum, Micrococcus, Microbacterium, Klebsiella, Lactobacillus, Enterobacter, Flavobacterium, Pantoea, Rhizobium, Rhodobacter, Serratia, Stenotrophomonas, and Pseudomonas have been isolated from the various medicinal roots, tubers, and rhizomes plants including turmeric, ginger, shallots, ashwagandha, picrorhiza, Jerusalem artichoke, ginseng, and valerian [34, 75, 76, 190,191,192,193,194,195,196,197,198,199,200].
IAA production from Pseudomonas has been reported to significantly increase the amount of valerenic acid in the root extract of Valeriana officinalis [201]. Endophytic IAA-producing bacterial strain Pseudomonas sp. enhanced the nutrient level in soil and growth of medicinal Withania somnifera [195]. On the other hand, the co-inoculation of salt-tolerant Mesorhizobium sp. with the IAA-producing Pseudomonas extremorientalis TSAU20 strains have been reported to alleviate the salt stress of Chinese liquorice (Glycyrrhiza uralensis), and increase plant growth, shoot and root weights [202]. It has been reported that the best IAA-producing Enterobacter sp. N10 performed best in both root and shoot dry mass growth in Jerusalem artichoke [203]. Auxin production of bacterial isolate is also potential to promote shallot (Allium ascalonicum) growth, bulb dry weight and bulb dry biomass weight, leaf number, and the number of bulbs tillers [204]. IAA-producing Pseudomonas poae inoculation enhanced Astragalus mongholicus seedling root biomass and root shoot ratio, and accumulations of calycosin-7-O-glucoside and ononin in the medicinal part root under drought stress conditions [205].
Inoculation of Curcuma longa with IAA-producing Bacillus spp.[196, 206], and with P. fluorescens [194] increased plant growth, the fresh rhizome biomass, morphological yield, turmeric production, and its major bioactive component, curcumin. Combined inoculation of Azospirillum, Azotobacter, Pseudomonas, and Bacillus increased plant height, root length, and alkaloid content in Withania somnifera [133]. Similarly, inoculation with PGPR showed significantly improved growth and yield in Withania somnifera [207]; and increased not only plant height, root length and weight, but also root activity and the content of total ginsenoside a standard on ginseng quality in Panax ginseng [183]. Due to the close relationship of root-associated bacteria with root cells, it has the potential to contribute directly to the composition and quality of vetiver (Chrysopogon zizanioides) oil, which is the ability to produce essential oil in its roots [208]. Furthermore, Vollú et al. [209] demonstrated that PGPR might contribute to plant growth promotion and improvement of the production of essential oil in vetiver. Inoculation with IAA-producing bacteria increased the shoot and root dry weight [198], tuber numbers and weight, and inulin content in tuber in Helianthus tuberosus [210], and could promote the growth in ginger plant [190].
Conclusion
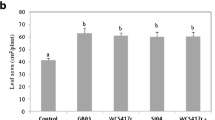
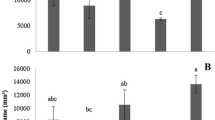
In recent years, the importance of secondary metabolites has become an important area of interest, especially in the production of bioactive and commercially valuable plant metabolites used in particularly, medicinal compounds, agrochemical, pharmaceutical, flavor and fragrances, dye and pigments, and food additives. Although, such studies are still in their initial stage, inoculation with PGPB produced a certain increase in stomatal density and also in the levels of secondary metabolites in several aromatic plant species. Biological nitrogen fixation and microbial production of phytohormones were observed to be the major factors responsible for plant growth improvement by PGPB, which help in the development of efficient root system for enhancing soil nutrients and water uptake. Inoculating medicinal and aromatic plants with root-associated bacteria enhances plant growth, development, and secondary metabolite production through increased nutrient and moisture availability. One of the most prominent features of plants inoculated with auxin-producing PGPBs is the modification in the root morphology and development. Bacterial IAA play central regulatory role in cell elongation, stimulate rooting and root formations, increasing the number of roots and root hairs, also number and development distribution patterns of glandular trichomes, part of the biosynthetic machinery that rapidly and efficiently converts imported carbohydrates into essential oils. Thus, plant growth-promoting bacteria promote root growth by increasing root surface area, which in turn promotes nutrient uptake thereby indirectly stimulating plant growth positively.
Inoculation of IAA-producing PGPB increased growth, biomass, photosynthesis and their pigments, nutrients, root branching and length, and also the total amount of essential oil and its yield, the production monoterpenes and biosynthesis of major essential oil components, and production of volatile secondary metabolites such as thymol, geraniol, α-terpineol, caryophyllene, phenols, ortho-dihydroxy phenols, tannins, flavonoids, and alkaloids; induce secondary metabolites responses, influence pathway flux of specific steps of monoterpene metabolism and phenolic compound pathways. The use of growth-promoting bacteria reduces the need for chemical fertilizers and pesticides applied to cultivated medicinal and aromatic plant species. Only a limited number of commercial rhizospheric microorganisms are currently marked for medicinal and aromatic plants. Bacterial inoculants are an efficient biotechnological tool for stimulating plant growth parameters and secondary metabolism in aromatic plants and future studies of their activities will increase our understanding of certain adaptive processes that are poorly understood at present era.
The action of IAA- producing PGPR on the growth and essential oil and their components in medicinal aromatic plants remain a focus area for future research. Although there is increasing interest in IAA-producing PGPB inoculations due to some exciting new findings, much less is known about the potential to associate PGPB with other economically important medicinal and aromatic plants, and only a few studies are found in literatures. Bacterial inoculants are an effective biotechnological tool for stimulating secondary metabolism in plants, but much less is known about the biosynthesis, regulation, and localization of terpenes synthesized in roots and whole plant. Microbial strategy is an attractive way for medicinal herbs and roots crops, but little is known about the potential and capabilities of PGPR to produce and increase plant secondary metabolites, and processes affecting the accumulation of monoterpenes and phenolic compounds.
In several medicinal and aromatic plant species consumed without further processing, the absence of any synthetic compounds in the harvested product and the use of chemical inputs in the production are of great importance in the food and pharmaceutical industries, so developing growth-promoting strategies involving PGPR and bio-fertilizers is the most ideal strategy. Further studies are needed to investigate possible mechanisms, by which bacteria increase phytochemical components in medicinal plants at the tissue, cell or molecular level, and to determine and regulate the main molecular mechanisms that drive the increase in essential oil after PGPB inoculation. In research, it may be desirable to isolate and screen new, valuable, environmentally adapted and natural strains to understand the mechanisms of various plant–microbe interactions that are able to trigger the biosynthetic pathways of bioactive compounds to enhance plant growth and secondary metabolite. Additionally, the modification, proliferation, and extraction of the production of medicinally important volatile constituents and secondary metabolites in medicinal and aromatic plants through plant cell and tissue culture technology can be used as an alternative and useful production system in the future. Further research is needed to clarify the role of other ecological factors, such as competition between root colonization sites and competition between PGPB strains and indigenous soil microflora, and characterize the mode of actions of these bacterial isolates in the metabolic pathway of essential oil production, and evaluate their field scale.
References
Santner A, Estelle M (2009) Recent advances and emerging trends in plant hormone signalling. Nature 459:1071–1078
Bulgarelli D, Schlaeppi K, Spaepen S, Loren V, van Themaat E, Schulze-Lefert P (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838
Spaepen S (2015) Plant hormones produced by microbes. In: Lugtenberg B (ed) Principles of plant-microbe interactions. Springer, Switzerland, pp 247–256
Wu CH, Bernard SM, Andersen GL, Chen W (2009) Developing microbe–plant interactions for applications in plant-growth promotion and disease control, production of useful compounds, remediation and carbon sequestration. Microb Biotechnol 2:428–440
Jamil M, Charnikhova T, Houshyani B, Van Ast A, Bouwmeester HJ (2012) Genetic variation in strigolactone production and tillering in rice and its effect on Striga hermonthica infection. Planta 235:473–484
Robles LM, Deslauriers SD, Alvarez AA, Larsen PB (2012) A loss-of-function mutation in the nucleoporin AtNUP160 indicates that normal auxin signalling is required for a proper ethylene response in Arabidopsis. J Exp Bot 63:2231–2241
Miransari M, Abrishamchi A, Khoshbakht K, Niknam V (2014) Plant hormones as signals in arbuscular mycorrhizal symbiosis. Crit Rev Biotechnol 34:123–133
Blomster T, Salojärvi J, Sipari N, Brosche M, Ahlfors R, Keinänen M, Overmyer K, Kangasjärvi J (2011) Apoplastic reactive oxygen species transiently decrease auxin signaling and cause stress-induced morphogenic response in Arabidopsis. Plant Physiol 157:1866–1883
Facella P, Daddiego L, Giuliano G, Perrotta G (2012) Gibberellin and auxin influence the diurnal transcription pattern of photoreceptor genes via CRY1a in tomato. PLoS ONE 7:1–10
Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17:181–195
Simon S, Petrášek J (2011) Why plants need more than one type of auxin. Plant Sci 180:454–460
Ludwig-Müller J (2011) Auxin conjugates: their role for plant development and in the evolution of land plants. J Exp Bot 62:1757–1773
Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot 95:707–735
De Rybel B, Audenaert D, Beeckman T, Kepinski S (2009) The past, present, and future of chemical biology in auxin research. ACS Chem Biol 4:987–998
Tivendale ND, Cohen JD (2015) Analytical history of auxin. J Plant Growth Regul 34:708–722
Duca D, Lorv J, Patten CL, Rose D, Glick BR (2014) Indole-3-acetic acid in plant–microbe interactions. Antonie Van Leeuwenhoek 106:85–125
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169:30–39
Mehnaz S (2015) Azospirillum a biofertilizer for every crop. In: Arora NK (ed) Plant microbes symbiosis: applied facets. Springer, India, pp 297–314
Couillerot O, Ramírez-Trujillo A, Walker V, Von Felten A, Jansa J, Maurhofer M, Défago G, Prigent-Combaret C, Comte G, Caballero-Mellado J, Moënne-Loccoz Y (2013) Comparison of prominent Azospirillum strains in Azospirillum-Pseudomonas-Glomus consortia for promotion of maize growth. Appl Microbiol Biotechnol 97:4639–4649
Malhotra M, Srivastava S (2009) Stress-responsive indole-3-acetic acid biosynthesis by Azospirillum brasilense SM and its ability to modulate plant growth. Eur J Soil Biol 45:73–80
Frankenberger WT, Brunner W (1983) Methods of detection of auxin-indole acetic acid in soil by high performance liquid chromatography. Soil Sci Soc Am J 47:237–241
Ahmad F, Ahmad I, Khan MS (2005) Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk J Biol 29:29–34
Aslantaş R, Çakmakçı R, Şahin F (2007) Effect of plant growth promoting rhizobacteria on young apple tree growth and fruit yield under orchard conditions. Sci Hortic 111:371–377
Çakmakçı R, Erat M, Oral B, Erdogan Ü, Şahin F (2009) Enzyme activities and growth promotion of spinach by indole-3-acetic acid-producing rhizobacteria. J Hortic Sci Botech 84:375–380
Barnawal D, Bharti N, Tripathi A, Pandey SS, Chanotiya CS, Kalra A (2016) ACC-deaminase-producing endophyte Brachybacterium paraconglomeratum strain SMR20 ameliorates Chlorophytum salinity stress via altering phytohormone generation. J Plant Growth Regul 35:553–564
Kreiser M, Giblin C, Murphy R, Fiesel P, Braun L, Johnson G, Wyse D, Cohen JD, (2016) Conversion of indole-3-butyric acid to indole-3-acetic acid in shoot tissue of hazelnut (Corylus) and elm (Ulmus). J Plant Growth Regul 35:710–721
Çakmakçı R, Erat M, Erdoğan Ü, Dönmez F (2007) The influence of plant growth-promoting rhizobacteria on growth and enzyme activities in wheat and spinach plants. J Plant Nutr Soil Sci 170:288–295
Egamberdieva D (2011) Indole-acetic acid production by root associated bacteria and its role in plant growth and development. In: Keller AH, Fallon MD (eds) Auxins: structure, biosynthesis and functions. Nova Science Publishers, USA, pp 1–14
Tewari S, Arora NK (2014) Multifunctional exopolysaccharides from Pseudomonas aeruginosa PF23 involved in plant growth stimulation, biocontrol and stress amelioration in sunflower under saline conditions. Curr Microbiol 69:484–494
Costa MH, Souza-Filho JDC, Ribeiro A (2004) Comments on “The regional evapotranspiration of the Amazon”. J Hydrometeorol 5:1279–1280
Khalid A, Tahir S, Arshad M, Zahir ZA (2004) Relative efficiency of rhizobacteria for auxin biosynthesis in rhizosphere and non-rhizosphere soils. Soil Res 42:921–926
Lin L, Xu X (2013) Indole-3-acetic acid production by endophytic Streptomyces sp. En-1 isolated from medicinal plants. Curr Microbiol 67:209–217
Souza R, Beneduzi A, Ambrosini A, Costa PB, Meyer J, Vargas LK, Schoenfeld R, Passaglia LMP (2013) The effect of plant growth-promoting rhizobacteria on the growth of rice (Oryza sativa L.) cropped in southern Brazilian fields. Plant Soil 366:585–603
Rohini S, Aswani R, Kannan M, Sylas VP, Radhakrishnan EK (2018) Culturable endophytic bacteria of ginger rhizome and their remarkable multi-trait plant growth-promoting features. Curr Microbiol 75:505–511
Souza R, Ambrosini A, Passaglia LMP (2015) Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol 38:401–419
Ahmed A, Hasanian S (2014) Auxins as one of the factors of plant growth improvement by plant growth promoting rhizobacteria. Pol J Microbiol 63:261–266
Roy BD, Deb B, Sharma GD (2010) Role of acetic acid bacteria in biological nitrogen fixation. Biofrontiers 2:47–57
Khan Z, Doty SL (2009) Characterization of bacterial endophytes of sweet potato plants. Plant Soil 322:197–207
Narayana KJ, Peddikotla P, Krishna PSJ, Yenamandra V, Muvva V (2009) Indole-3-acetic acid production by Streptomyces albidoflavus. J Biol Res 11:49–55
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-aceticacid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Spaepen S, Vanderleyden J (2011) Auxin and plant-microbe interactions. Cold Spring Harb Perspect Biol 3:a001438
Etesami H, Hosseini HM, Alikhani HA, Mohammadi L (2014) Bacterial biosynthesis of 1-aminocyclopropane-1-carboxylate (ACC) deaminase and indole-3-acetic acid (IAA) as endophytic preferential selection traits by rice plant seedlings. J Plant Growth Regul 33:654–670
Jasim B, Geethu PR, Mathew J, Radhakrishnan EK (2015) Effect of endophytic Bacillus sp. from selected medicinal plants on growth promotion and diosgenin production in Trigonella foenum-graecum. Plant Cell Tiss Org 122:565–572
Brandl MT, Lindow SE (1998) Contribution of indole-3-acetic acid production to the epiphytic fitness of Erwinia herbicola. Appl Environ Microbiol 64:3256–3263
De Salamone IEG, Hynes RK, Nelson LM (2005) Role of cytokinins in plant growth promotion by rhizosphere bacteria. In: Siddiqui ZA (ed) PGPR: biocontrol and biofertilization. Springer, Netherlands, pp 173–195a
Etesami H, Alikhani HA, Mirseyed Hosseini H (2015) Indol-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice promoting agents. MethodsX 2:72–78
James EK, Gyaneshwar P, Mathan N, Barraquio WL, Reddy PM, Iannetta PP, Olivares FL, Ladha JK (2002) Infection and colonization of rice seedlings by the plant growth-promoting bacterium Herbaspirillum seropedicae Z67. Mol Plant Microbe In 15:894–906
Chi F, Shen SH, Cheng HP, Jing YX, Yanni YG, Dazzo FB (2005) Ascending migration of endophytic rhizobia, from roots to leaves inside rice plants and assessment of benefits to rice growth physiology. Appl Environ Microbiol 71:7271–7278
Davies PJ (2010) The plant hormones: their nature, occurrence and functions. In: Davies PJ (ed) Plant hormones. Springer, Dordrecht, pp 1–15
Egorshina AA, Khairullin RM, Sakhabutdinova AR, Luk'yantsev MA, (2012) Involvement of phytohormones in the development of interaction between wheat seedlings and endophytic Bacillus subtilis strain 11BM. Russ J Plant Physiol 59:134–140
Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moënne-Loccoz Y, Muller D, Lagendre L, Wisniewski-Dyé F, Combaret CP (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 4:356
Parray JA, Jan S, Kamili AN, Qadri RA, Egamberdieva D, Ahmad P (2016) Current perspectives on plant growth-promoting rhizobacteria. J Plant Growth Regul 35:877–902
Barnawal A, Maji D, Bharti N, Chanotiya CS, Kalra A (2013) ACC deaminase-containing Bacillus subtilis reduces stress ethylene-ınduced damage and improves mycorrhizal colonization and rhizobial nodulation in Trigonella foenum-graecum under drought stress. J Plant Growth Regul 32:809–822
Goswami D, Dhandhukia P, Patel P, Thakker JN (2014) Screening of PGPR from saline desert of Kutch: growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol Res 169:66–75
Erdoğan U, Çakmakçı R, Varmazyari A, Turan M, Erdoğan Y, Kıtır N (2016) Role of inoculation with multi-trait rhizobacteria on strawberries under water deficit stress. Zemdirbyste 103:67–76
Kampert M, Strzelczyk E, Pokojska A (1975) Production of auxins by bacteria isolated from the roots of pine seedlings (Pinus silvestris L.). Acta Microbiol Pol B 7:135–143
Strzelczyk E, Pokojska-Burdziej A (1984) Production of auxins and gibberellin like substances by mycorrhizal fungi, bacteria and actinomycetes isolated from soil and mycorhizosphere of pine (Pinus silvestris L.). Plant Soil 81:185–194
Glick BR, Todorovic B, Czarny J, Cheng Z, Duan J, McConkey B (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26:227–242
Glick BR (2005) Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol Lett 251:1–7
Argueso CT, Hansen M, Kieber JJ (2007) Regulation of ethylene biosynthesis. J Plant Growth Regul 26:92–105
Patten CL, Glick BR (2002) Role of Pseudomonas putida indole-acetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Belimov AA (2012) Interactions between associative bacteria and plants: the role of biotic and abiotic factors. Palmarium Acad Publ, Moscow
Çakmakçı R (2016) Screening of multi-trait rhizobacteria for improving the growth, enzyme activities, and nutrient uptake of tea (Camellia sinensis). Commun Soil Sci Plan 47:1680–1690
Ortiz-Castro R, Martinez-Trujillo M, Lopez-Bucio J (2008) N-acyl-L-homoserine lactones: a class of bacterial quorum-sensing signals alter post-embryonic root development in Arabidopsis thaliana. Plant Cell Environ 31:1497–1509
Morrone D, Chambers J, Lowry L, Kim G, Anterola A, Bender K, Peters RJ (2009) Gibberellin biosynthesis in bacteria: Separate ent-copalyl diphosphate and ent-kaurene synthases in Bradyrhizobium japonicum. FEBS Lett 583:475–480
Çakmakçı R, Dönmez MF, Erdoğan Ü (2007) The effect of plant growth promoting rhizobacteria on barley seedling growth, nutrient uptake, some soil properties, and bacterial counts. Turk J Agric For 31:189–199
Swain MR, Naskar SK, Ray RC (2007) Indole 3-acetic acid production and effect on sprouting of yam (Dioscorea rotundata L) minisetts by Bacillus subtilis isolated from culturable cowdung microflora. Pol J Microbiol 56:103–110
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181
Hariprasad P, Niranjana SR (2009) Isolation and characterization of phosphate solubilizing rhizobacteria to improve plant health of tomato. Plant Soil 16:13–24
Merzaeva OV, Shirokikh IG (2010) The production of auxins by the endophytic bacteria of winter rye. Appl Biochem Microbiol 46:44–50
Apine OA, Jadhav JP (2011) Optimization of medium for indole-3-acetic acid production using Pantoea agglomerans strain PVM. J Appl Microbiol 110:1235–1244
Celloto VR, Oliveira AJB, Gonçalves JE, Watanabe CSF, Matioli G, Gonçalves RAC (2012) Biosynthesis of indole-3-acetic acid by new Klebsiella oxytoca free and immobilized cells on inorganic matrices. Sci World J 2012:1–7
Sergeeva E, Liaimer A, Bergman B (2002) Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 215:229–238
Vendan RT, Yu YJ, Lee SH, Rhee YH (2010) Diversity of endophytic bacteria in ginseng and their potential for plant growth promotion. J Microbiol 48:559–565
Shakeela S, Padder SA, Bhat ZA (2017) Isolation and characterization of plant growth promoting rhizobacteria associated with medicinal plant Picrorhiza kurroa. J Pharmacogn Phytochem 6:157–168
Mitra D, Sharma K, Uniyal N, Chauhan A, Sarkar P (2016) Study on plant hormone (indole-3-acetic acid) producing level and other plant growth promotion ability (PGPA) by Asparagus racemosus (L.) rhizobacteria. J Chem Pharm Res 8:995–1002
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. JKSUS 26:1–20
Chandler JW (2011) The hormonal regulation of flower development. J Plant Growth Regul 30:242–254
Phillips KA, Skirpan AL, Liu X, Christensen A, Slewinski TL, Hudson C, Barazesh S, Cohen JD, Malcomber S, McSteen P (2011) Vanishing tassel2 encodes a grass-specific tryptophan aminotransferase required for vegetative and reproductive development in maize. Plant Cell 23:550–566
McSteen P (2010) Auxin and monocot development. CSH Perspect Biol 2:1–27
Bellini C, Pacurar D, Perrone I (2014) Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol 65:639–666
Uggla C, Moritz T, Sandberg G, Sundberg B (1996) Auxin as a positional signal in pattern formation in plants. P Nat Acad Sci USA 93:9282–9286
Brcko A, Pĕnčík A, Magnus V, Prebeg T, Mlinarić S, Antunović J, Lepeduš H, Cesar V, Strnad M, Rolčík J, Salopek-Sondi B (2012) Endogenous auxin profile in the christmas rose (Helleborus niger L.) flower and fruit: free and amide conjugated IAA. J Plant Growth Regul 31:63–78
Villacorta NF, Fernández H, Prinsen E, Bernad PL, Revilla MA (2008) Endogenous hormonal profiles in hop development. J Plant Growth Regul 27:93–98
Bhattacharyya D, Garladinne M, Lee YH (2015) Volatile ındole produced by rhizobacterium Proteus vulgaris JBLS202 stimulates growth of Arabidopsis thaliana through auxin, cytokinin, and brassinosteroid pathways. J Plant Growth Regul 34:158–168
Tao Y, Ferrer J, Ljung K, Pojer F, Hong F, Long JA, Moreno JE, Bowman ME, Ivans LJ, Cheng Y, Lim J, Zhao Y, Ballare CL, Sandberg G, Noel JP, Chory J (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133:164–176
Mohebalipour N, Aharizad S, Mohammadi SA, Motallebiazar AR, Maddah Arefi H (2012) Effect of plant growth regulators BAP and IAA on micropropagation of Iranian lemon balm (Melissa officinalis L.) landraces. JFAE 10:280–286
Spaepen S, Versees W, Gocke D, Pohl M, Steyaert J, Vanderleyden J (2007) Characterization of phenylpyruvate decarboxylase, involved in auxin production of Azospirillum brasilense. J Bacteriol 18:7626–7633
Taiz L, Zeiger E (2010) Plant Physiology, 5th edn. Massachusetts, USA
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth promoting bacteria. J Theor Biol 190:63–68
Hagen G (1990) The Control of gene expression by auxin. In: Davies PJ (ed) Plant hormones and their role in plant growth and development. Kluwer Academic Publishers, Netherlands, pp 149–163
Niklas KJ, Kutschera U (2012) Plant development, auxin, and the subsystem incompleteness theorem. Front Plant Sci 3:1–11
Tanimoto E (2005) Regulation of root growth by plant hormones: roles for auxin and gibberellin. Crit Rev Plant Sci 24:249–265
Wu H, Hazak O, Cheung AY, Yalovsky S (2011) RAC/ROP GTPases and auxin signaling. Plant Cell 23:1208–1218
Lang D, Lyu D, Zhu Z, Qin S, (2019) Exogenous glucose mediates the regulation of root morphology and carbon–nitrogen metabolism by ındole-3-acetic acid (IAA) in Malus baccata (L.) Borkh. in soil with low organic carbon content. J Plant Growth Regul 38:1–18
Silva S, Sato A, Lage CLS, San Gil RAS, Azevedo DA, Esquibel MA (2005) Essential oil composition of Mellisa officinalis L. in vitro produced under the influence of growth regulators. J Brazil Chem Soc 16:1387–1390
Feizbakhsh A, Pazoki H, Mohammadrezaei V, Ebrahimzadeh MA (2014) Effect of phytohormones on the composition of Sambucus ebulus leaf essential oil. Trop J Pharm Res 13:581–586
Reda F, Abd El-Wahed MSA, Gamal El-Din KM (2010) Effect of indole acetic acid, gibberellic acid and kinetin on vegetative growth, flowering and essential oil pattern of chamomile plant. WJAS 6:595–600
Monfort LEF, Bertolucci SKV, Lima AF, de Carvalho AA, Mohammed A, Blank AF, Pinto JEBP (2018) Effects of plant growth regulators, different culture media and strength MS on production of volatile fraction composition in shoot cultures of Ocimum basilicum. Ind Crops Prod 116:231–239
Castilho CVV, Leitão SG, Silva VD, Miranda CdO, C, Santos MCdS, Bizzo HR, da Silva NCB, (2019) In vitro propagation of a carvacrol-producing type of Lippia origanoides Kunth: a promising oregano-like herb. Ind Crops Prod 130:491–498
Hazzoumi Z, Moustakime Y, Joutei KA (2014) Effect of gibberellic acid (GA3), indole acetic acid (IAA) and benzylaminopurine (BAP) on the synthesis of essential oils and the isomerization of methyl chavicol and trans-anethole in Ocimum gratissimum L. Springerplus 3:1–7
Rohamare Y, Nikam TD, Dhumal KN (2013) Effect of foliar application of plant growth regulators on growth, yield and essential oil components of Ajwain (Trachyspermum ammi L.). Int J Seed Spices 3:34–41
Nourafcan H, Sefidkon F, Ahmad Khalighi A, Mousavi A, Sharifi M (2014) Effects of IAA and BAP on chemical composition and essential oil content of lemon verbena (Lippia citriodora H.B.K). J Herb Med 5:25–32
Li Z, Wang X, Chen F, Kim HJ (2007) Chemical changes and overexpressed genes in sweet basil (Ocimum basilicum L.) upon methyl jasmonate treatment. J Agr Food Chem 55:706–713
Koseva-kovacheva D, Stanev D (1978) Effect of some growth regulators and hydrogen peroxide on the content and quality of peppermint oil. Resteniednidni u Nauki 15:21–25
El-Keltawi NE, Croteau R (1987) Influence of foliar applied cytokinins on growth and essential oil content of several members of the Lamiaceae. Phytochemistry 26:891–895
Sudriá C, Palazón J, Cusidó R, Bonfill M, Piñol MT, Morales C (2001) Effect of benzyladenine and indolebutyric acid on ultrastructure, glands formation, and essential oil accumulation in Lavandula dentata plantlets. Biol Plantarum 44:1–6
Affonso VR, Bizzo HR, Lima SS, Esquibel MA, Sato A (2007) Solid phase microextraction (SPME) analysis of volatile compounds produced by in vitro shoots of Lantana camara L. under the influence of auxins and sytokinins. J Brazil Chem Soc 18:1504–1508
Affonso VR, Bizzo HR, Lage CLS, Sato A (2009) Influence of growth regulators in biomass production and volatile profile of in vitro plantlets of Thymus vulgaris L. J Agr Food Chem 57:6392–6395
Victório CP, Kuster RM, Lage CLS (2011) Leaf and root volatiles produced by tissue cultures of Alpinia zerumbet (pers.) Burtt & Smith under the influence of different plant growth regulators. Quím Nova 34:430–433
Kikowska M, Thiem B, Sliwinska E, Rewers M, Kowalczyk M, Stochmal A, Oleszek W (2014) The effect of nutritional factors and plant growth regulators on micropropagation and production of phenolic acids and saponins from plantlets and adventitious root cultures of Eryngium maritimum L. J Plant Growth Regul 33:809–819
De Oliveira Y, Pinto F., Da Silva ALL, Guedes I, Biasi LA, Quoirin M (2010) An efficient protocol for micropropagation of Melaleuca alternifolia Cheel. In Vitro Cell Dev–Pl 46:192–197
Farooqi AHA, Fatima S, Khan A, Sharma S (2005) Ameliorative effect of chlormequat chloride and IAA on drought stressed plants of Cymbopogon martinii and C. winterianus. Plant Growth Regul 46:277–284
Sağlam AC, Yaver S, Başer İ, Cinkılıç L (2014) The effects of different hormones and their doses on rooting of stem cuttings in Anatolian sage (Salvia fruticosa Mill.). APCBEE Procedia 8:348–353
Sharma V, Kamal B, Srivastava N, Negi Y, Dobriyal AK, Jadon VS (2015) Enhancement of in vitro growth of Swertia chirayita Roxb. Ex Fleming co-cultured with plant growth promoting rhizobacteria. Plant Cell Tiss Org 121:215–225
Mehry A, Akbar M, Giti E (2008) Colonization and nitrogenase activity of Triticum aestivum (cv. Baccross and Mahdavi) to the dual inoculation with Azospirillum brasilense and Rhizobium meliloti plus 2,4-D. PJBS 11:1541–1550
Martinez-Morales LJ, Soto-Urzua L, Baca BE, Sanchez-Ahedo JA (2003) Indole-3-butyric acid (IBA) production in culture medium by wild strain Azospirillum brasilense. FEMS Microbiol Lett 228:167–173
Ribeiro CM, Cardoso EJBN (2012) Isolation, selection and characterization of root-associated growth promoting bacteria in Brazil pine (Araucaria angustifolia). Microbiol Res 167:69–78
Wahyudi AT, Astuti RP, Widyawati A, Meryandini A, Nawangsih AA (2011) Characterization of Bacillus sp. strains isolated from rhizosphere of soybean plants for their use as potential plant growth for promoting rhizobacteria. J Microbiol Antimicrob 3:34–40
Fatima Z, Saleemi M, Zia M, Sultan T, Aslam M, Rehman R, Chaudhary MF (2009) Antifungal activity of plant growth-promoting rhizobacteria isolates against Rhizoctonia solani in wheat. Afr J Biotechnol 8:219–225
Banchio E, Xie X, Zhang H, Paré PW (2009) Soil bacteria elevate essential oil accumulation and emissions in sweet basil. J Agr Food Chem 57:653–657
Mohite B (2013) Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J Soil Sci Plant Nut 13:638–649
Vinutha T (2005) Biochemical studies on Ocimum species inoculated with microbial inoculants. MSc. thesis, University of Agricultural Sciences, Bangalore, India
Hemavathi VN, Shvakumar BS, Suresh CK, Earanna N (2006) Effect of Glomus fasciculatum and plant growth promoting rhizobacteria on growth and yield of Ocimum basilicum. Karnataka J Agri Sci 19:17–20
Ordookhani K, Sharafzadeh S, Zare M (2011) Influence of PGPR on growth, essential oil and nutrients uptake of sweet basil. Adv Environ Biol 5:672–677
Kaymak HÇ, Dönmez MF, Çakmakçı R (2013) N2-fixing plant growth-promoting rhizobacteria: potential to increase yield, growth and element contents of Mentha piperita L. leaves. Eur J Plant Sci Biotechnol 1:38–42
Singh S, Tripathi A, Maji D, Awasthi A, Vajpayee P, Kalra A (2019) Evaluating the potential of combined inoculation of Trichoderma harzianum and Brevibacterium halotolerans for increased growth and oil yield in Mentha arvensis under greenhouse and field conditions. Ind Crops Prod 131:173–181
Samani MR, Pirbalouti AG, Moattar F, Golparvar AR (2019) L-Phenylalanine and bio-fertilizers interaction effects on growth, yield and chemical compositions and content of essential oil from the sage (Salvia officinalis L.) leaves. Ind Crops Prod 137:1–8
Egamberdieva D, Jabborova D, Mamadalieva N (2013) Salt-tolerant Pseudomonas extremorientalis able to stimulate growth of Silybum marianum under salt stress. Med Aromat Plant Sci Biotechnol 7:7–10
Darzi MT (2012) Effect of biofertilizers application on quantitative and qualitative yield of fennel (Foeniculum vulgare) in a sustainable production system. IJACS 4:187–192
Heidari M, Mousavinik SM, Golpayegani A (2011) Plant growth promoting rhizobacteria (PGPR) effect on physiological parameters and mineral uptake in basil (Ociumum basilicm L.) under water stress. ARPN JABS 6:6–11
Rajasekar S, Elango R (2011) Effect of microbial consortium on plant growth and improvement of alkaloid content in Withania somnifera (Ashwagandha). Curr Bot 2:27–30
Jaleel CA, Manivannan P, Sankar B, KishorekumarA GR, Somasundaram R, Panneerselvam R (2007) Pseudomonas fluorescens enhances biomass yield and ajmalicine production in Catharanthus roseus under water deficit stress. Colloids Surface B 60:7–11
Karthikeyan B, Joe MM, Jaleel CA, Deiveekasundaram M (2010) Effect of root inoculation with plant growth promoting rhizobacteria (PGPR) on plant growth, alkaloid content and nutrient control of Catharanthus roseus (L.) G. Don Nat Croat 19:205–212
Mishra RK, Pra O, Alam M, Dikshit A (2010) Influence of plant growth promoting rhizobacteria (PGPR) on the productivity of Pelargonium graveolens L. herit. Recent Res Sci Technol 2:53–57
Hosseinzadah F, Satei A, Ramezanpour MR (2011) Effects of mycorrhiza and plant growth promoting rhizobacteria on growth, nutrient uptake and physiological characteristics in Calendula officinalis L. Middle East J Sci Res 8:947–953
Hassan F (2009) Response of Hibiscus sabdariffa L. plant to some biofertilization treatments. AOAS 54:437–446
Banchio E, Bogino PC, Zygadlo J, Giordano W (2008) Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem Syst Ecol 36:766–771
Earanna N, Bagyaraj DJ (2004) Influence of AM fungi and growth promoting rhizomicroorganisms on growth and herbage yield of Phyllanthus amarus Schum. and Thom. Geobios 31:117–120
Çakmakçı R, Ertürk Y, Dönmez F, Erat M, Haznedar A, Sekban R (2012) Tea growth and yield in relation to mixed cultures of N2-fixing and phosphate solubilizing bacteria. J Ege University Faculty Agric Special Issue 1:17–21
Çakmakçı R, Ertürk Y, Sekban R, Haznedar A, Varmazyari A (2013) The Effect of single and mixed cultures of plant growth promoting bacteria and mineral fertilizers on tea (Camellia sinensis) growth, yield and nutrient uptake. Soil Water Journal Special Issue for AGRICASIA 2:653–662
140. Çakmakçı R, Ertürk Y, Varmazyari A, Atasever A, Kotan R, Erat M, Türkyılmaz K, Sekban R, Haznedar A (2015) The effect of mixed cultures of plant growth promoting bacteria and mineral fertilizers on tea (Camellia sinensis L.) growth, yield, nutrient uptake, and enzyme activities. Inter Soil Sci Cong, 19–23 October, Sochi, Russia, pp 67–71
Varmazyari A, Çakmakçı R, Ertürk Y, Atasever A (2014) Diversity and plant growth promoting properties of rhizobacteria isolated from acidic soils of tea. Inter Mezsopotamia Agric Congres, 22–25 September, Diyarbakır, Turkey, pp 49–51
Çakmakçı R (2014) Mikrobiyal gübre olarak kullanılabilecek mikroorganizmaların etki mekanizmaları ve özellikleri. Soil, fertilizer and water resources Central Research Institute Publication, Turkey, pp 5–17
Parlakova Karagöz F, Dursun A, Kotan R, Ekinci M, Yıldırım E, Mohammadi P (2016) Assessment of the effects of some bacterial ısolates and hormones on corm formation and some plant properties in saffron (Crocus sativus L.). JAS 22:500–511
Copetta A, Lingua G, Berta G (2006) Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 16:485–494
Santoro MV, Zygadlo J, Giordano W, Banchio E (2011) Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita). Plant Physiol Bioch 49:1177–1182
Santoro MV, Cappellari LR, Giordano W, Banchio E (2015) Plant growth-promoting effects of native Pseudomonas strains on Mentha piperita (peppermint): an in vitro study. Plant Biol 17:1218–1226
Hellal FA, Mahfouz SA, Hassan FAS (2011) Partial substitution of mineral nitrogen fertilizer by bio-fertilizer on (Anethum graveolens L.) plant. ABJNA 2:652–660
Kammak FD, Dahmardeh M, Khammari I, Rahimian AR (2015) The effect of application type and composition of growth stimulating bacteria on quantitative and qualitative characteristics of medicinal plant calendula (Calendula officinallis L.). INDJSRT 8:1–9
Mahfouz SA, Sharaf Eldin MA (2007) Effect of mineral vs. biofertilizer on growth, yield, and essential oil content of fennel (Foeniculum vulgare Mill). Int Agrophys 21:361–366
Kutlu M, Çakmakçı R, Hosseinpour A, Karagöz H (2019) The use of plant growth promoting rhizobacteria (PGPR)’s effect on essential oil rate, essential oil content, some morphological parameters and nutrient uptake of Turkish oregano. Appl Ecol Env Res 17:641–1653
Banchio E, Bogino PC, Santoro M, Torres L, Zygadlo J, Giordano W (2010) Systemic induction of monoterpene biosynthesis in Origanum majoricum by soil bacteria. J Agr Food Chem 58:650–654
Arpana J, Bagyaraj DJ, Rao EVSP, Parameswaran TN, Rahiman BA (2009) Effect arbuscular mycorrhizal fungus and plant growth promoting rhizomicroorganisms on the growth, nutrition and essential oil content of patchouli (Pogostemon cablin). JMAPS 31:118–123
Ghorbanpour M, Hatami M, Kariman K, Abbaszadeh Dahaji P (2016) Phytochemical variations and enhanced efficiency of antioxidant and antimicrobial ingredients in Salvia officinalis as inoculated with different rhizobacteria. Chem Biodivers 13:319–330
Sharafzadeh S, Sabahi A, Ordookhani K, Zare M (2013) Growth and active substances of summer savory as affected by PGPR. JNAS 2:997–1000
Cappellari LR, Santoro MV, Nievas F, Giordano W, Banchio E (2013) Increase of secondary metabolite content in marigold by inoculation promoting rhizobacteria. Appl Soil Ecol 70:16–22
Dharni S, Srivastava AK, Samad A, Patra DD (2014) Impact of plant growth promoting Pseudomonas monteilii PsF84 and Pseudomonas plecoglossicida PsF610 on metal uptake and production of secondary metabolite (monoterpenes) by rose-scented geranium (Pelargonium graveolens cv. Bourbon) grown on tannery sludge amended soil. Chemosphere 117:433–439
Gomaa AO, Abou-Aly HE (2001) Efficiency of bioferitlization in the presence of both inorganic and organic fertilizers on growth, yield and chemical constituents of anise plant (Pimpinella anisum L.). Proc fifth Arabian Hortic Conf, Ismailia, Egypt, March 24–28. vol 11, pp 49–61
Zand A, Darzi MT, Hadi MRHS (2013) Effects of phosphate solubilizing microorganisms and plant density on seed yield and essential oil content of anise (Pimpinella anisum). Middle-East J Sci Res 14:940–946
Mishra BK, Dubey PN, Aishwath OP, Kant K, Sharma YK, Vishal MK (2017) Effect of plant growth promoting rhizobacteria on coriander (Coriandrum sativum) growth and yield under semi-arid condition of India. Indian J Agric Sci 87:607–612
Ratti N, Kumar S, Verma HN, Gautam SP (2001) Improvement in bioavailability of tricalcium phosphate to Cymbopogon martinii var. motia by rhizobacteria. AMF and Azospirillum inoculation. Microbiol Res 156:145–149
Kandeel YM, Menesy FA, Khalafalla MM, Gad WM (2004) Effect of some commercial biofertilizers on growth, seed, volatile oil yield and chemical composition of Anethum graveolens L. J Agric Res Tanta Univ 30:705–720
Shaalan MN (2005) Effect of compost and different sources of biofertilizers, on borage plants (Borago officinalis L.). Egyptian J Agric Res 83:271–284
Shaalan MN (2005) Influence of biofertilizers and chicken manure on growth, yield and seeds quality of Nigella sativa L. plants. Egyptian J Agric Res 83:811–828
Darzi MT, Ghalavand A, Rejali F, Sephidkon F (2007) Effects of biofertilizers application on yield and yield components in fennel (Foeniculum vulgare Mill.). IJMAPR 22:276–292
Moradi R, Mahallati MN, Moghaddam PR, Lakzian A, Nezhadali A (2011) The effect of application of organic and biological fertilizers on quantity and quality essential oil of Foeniculum vulgare Mill. (fennel). J Hortic Sci 25:25–33
Mishra BK, Meena KK, Dubey PN, Aishwath OP, Kant K, Sorty AM, Bitla U (2016) Influence on yield and quality of fennel (Foeniculum vulgare Mill.) grown under semi-arid saline soil, due to application of nativephosphate solubilizing rhizobacterial isolates. Ecol Eng 97:327–333
Rashmi KR, Earanna N, Vasundhara M (2008) Influence of biofertilizers on growth, biomass and biochemical constituents of Ocimum gratissimum L. Biomed J 3:123–130
Tiwari R, Kalra A, Darokar MP, Chandra M, Aggarwal N, Singh AK, Khanuja SPS (2010) Endophytic bacteria from Ocimum sanctum and their yield enhancing capabilities. Curr Microbiol 60:167–171
Saeid Nejad AH, Rezvani Moghaddam P (2011) Evaluation of compost, vermicompost and cattle manure application on yield, yield components and essential oil percent in cumin (Cuminum cyminum). J Hortic Sci Botech 24:142–148
Mostafa A, Khalafallah M, Sedera SA, Fathy H, Higazy A (2019) Different methods of bacterial inoculation on the yield of chamomile blossoms and essential oil. Global J Environ Sci Manage 5:237–248
Bharti N, Yadav D, Barnawal D, Maji D, Kalra A, (2013) Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. World J Microb Biot 29:379–387
Cappellari LR, Santoro MV, Reinoso H, Travaglia C, Giordano W, Banchio E (2015) Anatomical, morphological, and phytochemical effects of inoculation with plant growth promoting rhizobacteria on peppermint (Mentha piperita). J Chem Ecol 41:149–158
Annamalai A, Lakshmi PTV, Lalithakumari D, Murugesan K (2004) Optimization of biofertilizers on growth, biomass and seed yield of Phyllanthus amarus (Bhumyamalaki) in sandy loam soil. J Med Arom Plant Sci 26:717–720
Vande Broek A, Lambrecht M, Eggermont K, Vanderleyden J (1999) Auxin upregulate expression of the indol-3-pyruvate decarboxylase gene in Azospirillum brasilense. J Bacteriol 181:1338–1342
Leithy S, El-Meseiry TA, Abdallah EF (2006) Effect of biofertilizer, cell stabilizer and irrigation regime on rosemary herbage oil yield and quality. TJASR 2:773–779
Hend MFS, Sakr WRA, Sabh AZ, Ragab AA (2007) Effect of some chemical and bio-fertilizers on peppermint plants grown in sandy soil: 2. Effect on essential oil production, chemical composition and anatomical features. Annals Agric Sci 52:465–484
Jha Y, Subramanian RB (2016) Rhizobacteria enhance oil content and physiological status of Hyptis suaveolens under salinity stress. Rhizosphere 1:33–35
Alraey DA, Haroun SA, Omar MN, Abd-El Gawad AM, El-Shobaky AM, Mowafy AM (2019) Fluctuation of essential oil constituents in Origanum syriacum subsp. sinaicum in response to plant growth promoting bacteria. J Essent Oil-Bear Plants 22:1022–1033
Rahmoune B, Morsli A, Khelifi-Slaoui M, Khelifi L, Strueh B, Erban A, Kopka J, Prell J, van Dongen JT (2017) Isolation and characterization of three new PGPR and their effects on the growth of Arabidopsis and Datura plants. J Plant Interact 12:1–6
Ji WX, Leng X, Jin ZX, Li H (2019) Plant growth promoting bacteria increases biomass, effective constituent, and modifies rhizosphere bacterial communities of Panax ginseng. Acta Agric Scand B 69:135–146
Youssef AA, Edris AE, Gomaa AM (2004) A comparative study between some plant growth regulators and certain growth hormones producing microorganisms on growth and essential oil composition of Salvia officinalis L. AOAS 49:299–311
Bidgoli RD, Azarnezhad N, Akhbari M, Ghorbani M (2019) Salinity stress and PGPR effects on essential oil changes in Rosmarinus officinalis L. Agric Food Secur 8:1–7
Darzi MT, Hadi MHS (2012) Effects of organic manure and nitrogen fixing bacteria on some essential oil components of coriander (Coriandrum sativum). IJACS 4:787–792
Dadaşoğlu E, Dadaşoğlu F, Varmazyari A, Kotan R, Çakmakçı R (2013) Effect of vermicompost, solid and liquid organic fertilizer and biological fertilizer applications on the growth and chlorophyll contents of mountain tea (Sideritis montana L.). Mediterr Symp Medicinal Aromatic Plants (MESMAP), Famagusta, Northern Cyprus, pp 97
Lange BM, Turner GW (2013) Terpenoid biosynthesis in trichomes-current status and future opportunities. Plant Biotechnol J 11:2–22
Goswami D, Thakker JN, Dhandhukia PC (2016) Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food Agric 2:1127500
Chen T, Chen Z, Ma GH, Du BH, Shen B, Ding YQ, Xu K (2014) Diversity and potential application of endophytic bacteria in ginger. Genet Mol Res 13:4918–4931
Aswathy AJ, Jasim B, Jyothis M, Radhakrishnan EK (2012) Identification of two strains of Paenibacillus sp. as indole 3 acetic acid-producing rhizome-associated endophytic bacteria from Curcuma longa. Biotech 3:219–224
Behnoushsadat G, Masoud A, Mohsen S, Pari BM, Rahim TF (2013) Isolation and characterization of rhizobacteria and their effects on root extracts of Valeriana officinalis. Aust J Crop Sci 7:338–344
Hussein KA, Joo JH (2015) Isolation and characterization of rhizomicrobial isolates for phosphate solubilization and indole acetic acid production. J Korean Soc Appl Biol Chem 58:847–855
Kumar A, Vandana SM, Singh PP, Singh SK, Singh PK, Pandey KD (2016) Isolation of plant growth promoting rhizobacteria and their impact on growth and curcumin content in Curcuma longa L. Biocatal Agric Biotechnol 8:1–7
Singh R, Arora NV (2016) Growth enhancement of medicinal plant Withania somnifera using phosphate solubilizing endophytic bacteria Pseudomonas sp. as bioinoculant. IJSTS 2:13–18
Chauhan AK, Maheshwari DK, Dheeman S, Bajpai VK (2017) Termitarium-inhabiting Bacillus spp. enhanced plant growth and bioactive component in turmeric (Curcuma longa L.). Curr Microbiol 74:184–192
Resti Z, Reflin R, Gani S (2017) Antagonistic and plant growth promoting potentials of indigenous endophytic bacteria of shallots. IJAST 2:42–49
Sritongon K, Mongkolthanaruk W, Boonlue S, Jogloy S, Puangbut D, Riddech N (2017) Rhizobacterial candidates isolated from jerusalem artichoke (Helianthus tuberosus L.) rhizosphere for host plant growth promotion. Chiang Mai J Sci 44:83–93
Sundaram NM, Murali SR (2018) Isolation and characterization of bacteria from rhizospheric soils of Curcuma longa for different plant growth promotion (PGPR) activities. World J Pharm Res 7:692–700
Bijitha PK, Bhai RS (2019) Burkholderia cepacia strain IISRCLRB5, a promising bioagent fort he management of rhizome rot of turmeric (Curcuma longa L.). IJASR 9:1–12
Ghodsalavi B, Ahmadzadeh M, Soleimani M, Madloo PB, Taghizad-Farid R (2013) Isolation and characterization of rhizobacteria and their effects on root extracts of Valeriana officinalis. Aust J Crop Sci 7:338–344
Egamberdieva D, Li L, Lindström K, Räsänen LA (2016) A synergistic interaction between salt-tolerant Pseudomonas and Mesorhizobium strains improves growth and symbiotic performance of liquorice (Glycyrrhiza uralensis Fish.) under salt stress. Appl Microbiol Biotechnol 100:2829–2841
Liu X, Li X, Li Y, Li R, Xie Z (2016) Plant growth promotion properties of bacterial strains isolated from the rhizosphere of the Jerusalem artichoke (Helianthus tuberosus L.) adapted to saline–alkaline soils and their effect on wheat growth. Can J Microbiol 63:228–327
Kafrawi N, Zahraeni K, Baharuddin B (2017) Comparison of IAA production by shallot rhizosphere ısolated bacteria in solid and liquid media and their effect on shallot plant growth. J Microb Biochem Technol 9:266–269
Sun HF, Kong L, Du H, Chai HZ, Gao JP, Cao QF (2019) Benefits of Pseudomonas poae S61 on Astragalus mongholicus growth and bioactive compound accumulation under drought stress. J Plant Interact 14:205–212
Chauhan AK, Maheshwari DK, Kim K, Bajpai VK (2016) Termitarium-inhabiting Bacillus endophyticus TSH42 and Bacillus cereus TSH77 colonizing Curcuma longa L.: isolation, characterization, and evaluation of their biocontrol and plant-growth-promoting activities. Can J Microbiol 62:880–892
Anuroopa N, Bagyaraj DJ (2017) Selection of an efficient plant growth promoting rhizobacteria for inoculating Withania somnifera. JSIR 76:244–248
Del Giudice L, Massardo DR, Pontieri P, Bertea CM, Mombello D, Carata E, Tredici SM, Talà A, Mucciarelli M, Groudeva VI, De Stefano M, Vigliotta G, Maffei ME, Alifano P (2008) The microbial community of Vetiver root and its involvement into essential oil biogenesis. Environ Microbiol 10:2824–2841
Vollú RE, Blank AF, Seldin L, Coelho MRR (2012) Molecular diversity of nitrogen-fixing bacteria associated with Chrysopogon zizanioides (L.) Roberty (vetiver), an essential oil producer plant. Plant Soil 356:101–111
Namwongsa J, Jogloy S, Vorasoot N, Boonlue S, Riddech N, Mongkolthanaruk W (2019) Endophytic bacteria improve root traits, biomass and yield of Helianthus tuberosus L. under normal and deficit water conditions. J Microbiol Biotechnol 29:1777–1789
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Çakmakçı, R., Mosber, G., Milton, A.H. et al. The Effect of Auxin and Auxin-Producing Bacteria on the Growth, Essential Oil Yield, and Composition in Medicinal and Aromatic Plants. Curr Microbiol 77, 564–577 (2020). https://doi.org/10.1007/s00284-020-01917-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-01917-4