Abstract
Electronic waste (E-Waste) is consumed at high speed in the world. These residues contain metals that increase their price each year, generating new research on the ability of microorganisms to recover the metals from these wastes. Therefore, this work evaluated the biologic lixiviation of Cu, Ag and Au from printed circuit boards (PCB) of mobile phones by three strains of Aspergillus niger, Candida orthopsilosis, Sphingomonas sp. and their respective consortia, in addition to leaching with citric acid. The microorganisms were cultured in mineral media with 0.5 g of PCB, and the treatments with 1M citric acid were added the same amount of PCB. All treatments were incubated for 35 days at room temperature. The results showed that Sphingomonas sp. MXB8 and the consortium of C. orthopsilosis MXL20 and A. niger MXPE6 can increase their dry biomass by 147% and 126%, respectively, in the presence of PCB. In the bioleaching of metals, the inoculation of A. niger MXPE6, the consortium of Sphingomonas sp. MXB8/C. orthopsilosis MXL20 and Sphingomonas sp. MXB8 leached 54%, 44.2% and 35.8% of Ag. The consortium of A. niger MX5 and A. niger MXPE6 showed a leaching of 0.53% of Au. A. niger MX5 leaching 2.8% Cu. Citric acid increased Cu leaching by 280% compared to treatments inoculated with microorganisms. Although further research is required, A. niger MXPE6 and the consortium of Sphingomonas sp. MXB8/C. orthopsilosis MXL20 could be an alternative to recover Ag from PCB of mobile phones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Electrical and Electronic Equipment (EEE) has been consumed at high speed in the world, shortening the life of such devices [14, 20] and generated a lot of Waste Electrical and Electronic Equipment (WEEE). Within this rubbish we find the mobile telephones which are in greater abundance due to the technological advance [33]. An important component of obsolete mobile phones is the printed circuit board (PCB), which represents 3 to 6% of the equipment and consists of non-metallic parts such as polymers and ceramics, as well as metal parts such as copper (Cu), silver (Ag) and gold (Au) [28, 39]. Within PCB, Cu is 13%, while Ag is 0.134% and Au is 0.0035% [29]. These metals are obtained from sources considered as non-renewable resources [9, 25] and in recent years it has increased its price due to the low mining production and the increase in the demand of important consumers like China and India. These countries acquire large tons of Cu, Ag and Au for the production of electronic equipment [22, 38]. These metals are also used in the automotive and aeronautical industries because of their high electrical and thermal conductivity [1]. Within the electronics industry, Cu is used as a conductive material in electrical cables, current bars and coil wires, whereas Au and Ag are used as contact surfaces in PCB boards [6, 43].
The extraction of these metals in the mining industry involves the removal of large amounts of soil and the use of hydrometallurgical and pyrometallurgical techniques that generate pollutant residues that damage the environment [40]. Secondary sources are currently being sought for the recovery of these metals, such as electronic waste, but the techniques used for their extraction are hydrometallurgical and pyrometallurgical on an industrial scale or by primitive techniques [17]. Hydrometallurgical processes consist mainly of acid or alkaline leaching with H2SO4, H2O2, HNO3, NaOH and HCl of WEEE [41]. Moreover, pyrometallurgical processes consist of the incineration and casting of electronic waste at more than 1200 °C, this processing has become in the last two decades a traditional method for the recovery of metals [10].
Therefore, investigations have been initiated to discover the ability of microorganisms to recover metals from electronic waste, finding that bacteria such as Thiobacillus thiooxidans and ferrooxidans, Acidithiobacillus thiooxidans and Sulfobacillus thermosulfidooxidans in consortium with acidophilic bacteria are able to leach Cu in a 90, 89 and 60% from PCB [7, 16, 18]. In the case of filamentous fungi, few investigations have been reported, some of the most important are Aspergillus niger and Penicillium simplicissimum reported for their ability to leach 65% Cu from WEEE. In the case of Aspergillus niger, its leaching capacity is attributed to the production of organic acids (citric acid, gluconic acid and oxalic acid) [7, 32]. While for Au, Acidithiobacillus ferrivorans, Acidithiobacillus thiooxidans, Pseudomonas fluorescens and Pseudomonas putida were able to leach 44% of this metal from PCB of mobile phones and computers [19]. With regard to Ag, there are few reports of the ability of microorganisms to leach this metal from PCB of electronic waste. On the other hand, the strains of A. niger MXPE6 and MX7 have been reported as tolerant to AuCl3 and their consortium has recovered 87% of Au from PCB of cell phones in a culture medium with 50 g L−1 of glucose and pH 4.4 [23]. Unpublished data show that A. niger MX5 and C. orthopsilosis MXL20 have been tolerant to AuCl3, and A. niger MX5, MXPE6, MX7 and Sphingomonas sp. MXB8 have shown tolerance to Ag2SO4. Highlighting the capacity of Sphingomonas sp. MXB8 to form an Ag mirror when grown with Ag2SO4 and the ability of C. orthopsilosis MXL20 to form precipitates when grown with AuCl3. Due to the above, the objective of this work is to evaluate the biological lixiviation of Cu, Au and Ag from mobile phone’s PCB by three strains of Aspergillus niger MX5, MXPE6 and MX7, Sphingomonas sp., Candida orthopsilosis, microbial consortia and the leaching of citric acid.
Materials and Methods
Collection and Acid Digestion of PCB of Mobile’s Phone
For this investigation, the obsolete mobile phones were collected in Xalapa City (Veracruz State, México). After, were dismantled for the removal of the printed circuit boards. These cards of PCB were dismantled, cut and milled in a blender (Waring ®) obtaining a particle size of 0.594 mm (59.4 µm). The PCB powder (0.5 g) was digested with 20 mL of concentrated HNO3 (J.T. Baker, 66.2%) in heating plates at 83 °C for 2 h to quantify Cu and Ag. While the Au digests were made with 20 mL of aqua regia (3:1 HCl/HNO3) to 108 °C for 2 h. In both procedures, each digested solution was filtered through Whatman No. 50 filter paper, and raised to 50 mL with deionized water in volumetric flasks. Finally, dissolved samples were analyzed in an Optical Emission Spectrometer ICP-OES (Varian ® Mod. 725-ES). The analysis of Cu, Au and Ag in the optical emission spectrometer, calibration curves were made with standards for ICP-OES (Sigma-Aldrich®). Samples were read at 213.59 nm for Cu, 242.795 nm for Au and 328.068 nm for Ag.
Microorganisms
Sphingomonas sp. MXB8, Candida orthopsilosis MXL20, Aspergillus niger MX7 and MX5 were isolated from metal-contaminated soil around a landfill located at Tronconal, Xalapa, Veracruz, México, where the deposition of battery waste, computers and printed circuit boards was evidenced. While A. niger MXPE6 was isolated from an electronic board from the same soil at the same location.
Identification of Microorganisms
The extraction of DNA from the microbial strains was carried out using the method of Wilson et al. [35] modified. The integrity of genetic material was observed on a 1% agarose gel, in which 10 µL of each sample plus 4 µL of Green-DNA dye ™ from Biobasic were loaded. The gel was run at 75 V for 30 min and the 100 bp ladder was used. For the PCR reaction of the bacteria, primers 3A and 5B (16S rDNA) were used, obtaining a good quality of amplicons, visualized on a 1.5% agarose gel in which 5 µL of sample was loaded along with 1 µL of Green-DNA dye ™ from Biobasic. While for the case of fungi, the ITS1 and ITS4 primers were used, obtaining a good quality of amplicons. The PCR products were purified with the phenol–chloroform technique [42]. The purified products (40 ng) were sent for sequencing to the Microbial Genetic Resources Laboratory (CNRG-INIFAP) in Guadalajara, México. The sequences were edited with different bioinformatics tools (BioEdit, ClustalX, Seaview, MEGA6) and on the BLAST platform for the assignment of identities where percentages were considered higher than 97% identity for a species level and 95% to 96% for gender.
Culture Conditions
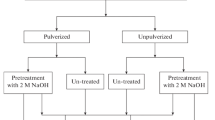
Three filamentous fungi: Aspergillus niger MX5, Aspergillus niger MXPE6 and Aspergillus niger MX7 which were cultivated in Petri dishes with Potato Dextrose Agar (DB Bioxon®). After 5 days of growth, the fungi were cut into 6 mm discs, then 3 discs of the corresponding fungus were inoculated into each respective tube with 25 mL of a mineral medium containing 20 g of Dextrose, 1.5 g of (NH4)2SO4, 0.5 g KH2PO4, 0.025 g MgCl2, and 0.1 g CaCl2 with a pH of 5.0. Sphingomonas sp. MXB8 and yeast Candida orthopsilosis MXL20 were grown on Nutrition Agar (DB Bioxon®) and after 5 days of growth a microbial suspension with a concentration of 1 × 108 colony forming units (CFU) was prepared according to the Mcfarland scale. Subsequently, 2 mL of each suspension was inoculated into each tube with 25 mL of mineral medium containing 20 g of Dextrose, 1.5 g of (NH4)2SO4, 0.5 of NaH2PO4, 0.1 g of MgCl2, 0.1 g of CaCl2, with a pH of 5.8. In the case of the microbial consortiums mentioned in Table 1, 25 mL of the mineral medium used for fungi with pH = 5.0 was used. A disc of each respective fungus was inoculated for fungal consortia, while for bacteria/fungus consortia, 1 mL of the bacteria or yeast and a disc of the filamentous fungus were inoculated. To the treatments with electronic residue 0.5 g of PCB powder of mobile phones were added and inoculated with the respective microorganism. While the biotic controls were not added PCB powder and inoculated with the corresponding microorganisms, the abiotic controls were added 0.5 g of PCB powder and were not inoculated. All treatments had three replicates and were incubated for 35 days at room temperature (17 to 24 °C).
Leaching with Citric Acid
To 50 mL plastic tubes were added 25 mL of 1 M citric acid, 0.5 g of PCB mobile phone powder was added to the treatments with electronic residue and controls of these experiments were not added to PCB powder. All treatments remained static for 35 days at room temperature (17 to 24 °C).
Evaluated Parameters
After the incubation period, the biomass of Sphingomonas sp. MXB8 and Candida orthopsilosis MXL20, were centrifuged at 3200 rpm for 10 min. Subsequently, the supernatant was removed and the biomass was dried at 50 °C for 72 h. On the other hand, treatments inoculated with Aspergillus niger MX5, Aspergillus niger MXPE6 and Aspergillus niger MX7 were filtered under vacuum to separate the mycelium from the fungi and the procedure described above was followed for the drying of the biomass. The microbial consortiums were filtered under vacuum and then centrifuged at 3200 rpm for 10 min, the biomass obtained was dried at 50 °C for 72 h. The supernatants obtained from each treatment were used to determine the pH and quantify Cu, Au and Ag in an ICP-OES optical emission spectrometer (Varian ® Mod. 725-ES).
Experimental Design and Statistical Analysis
In the analysis of metals were used a 17 × 3 factorial experiment was set in a completely randomized design, including seventeen levels of treatments and three levels of metals. The resulting fifty one treatments had three replicates. While for dry biomass and pH, it was used a 16 × 2 factorial experiment was set in a completely randomized design, including sixteen levels of microbial treatments and two levels with PCB and without PCB. The resulting thirty two treatments had three replicates. Data were analyzed using an analysis of variance, and the mean comparison test (Tukey, α = 0.05) using the SAS statistical program [37].
Results
According to the results obtained in the total quantification of the study metals, a higher amount of Cu (Table 2) was observed in printed circuit boards of mobile phones. On the other hand, the statistical analyzes of the treatments inoculated with microorganisms presented significant differences (P ≤ 0.001) in their dry biomass with and without PCB. Most treatments inoculated with microorganisms increased their biomass at 35 days of exposure to mobile phone PCB dust. Sphingomonas sp. MXB8 in mineral medium with PCB increased its biomass by 147%, the consortium of C. orthopsilosis MXL20/A. niger MXPE6, as well as the consortium of C. orthopsilosis MXL20/A. niger MXP5 in the presence of PCB increased their dry biomass by 126 and 96%, respectively, when comparing this biomass with their corresponding treatments without PCB (biotic control). However, the presence of the electronic residue in the treatments inoculated with the consortium of A. niger MX5/A. niger MX7 and the consortium of C. orthopsilosis MXL20/A. niger MX7 decreased their dry biomass to 69 and 52%, respectively, comparing it with their respective biotic controls (Fig. 1).
Regarding pH, all treatments remained at pH below 7.0. However, the presence of the microorganisms acidified the mineral medium in treatments with and without PCB compared to abiotic control (treatments not inoculated with microorganisms). The inoculation of A. niger MX7 acidified the mineral medium by up to 61% in both PCB containing treatments and those without PCB (biotic control). On the other hand, the consortium of C. orthopsilosis MXL20/A. niger MXPE6 in the presence of electronic waste alkalized the medium by 8.8% compared to the abiotic control that did not exceed pH = 6.5 (Fig. 2).
Regarding the bioleaching of metals, the results showed significant differences (P ≤ 0.001) between treatments inoculated with the study microorganisms and their consortium. The best results were presented for Ag, in which the inoculation of A. niger MXPE6, leached 54% of said metal. On the other hand, with the inoculation of the consortium of Sphingomonas sp. MXB8/C. orthopsilosis MXL20 an Ag leaching of 44.2% was observed. Meanwhile the PCB powder was in contact with Sphingomonas sp. MXB8, this bacterium had the ability to leach 35.8% Ag (Fig. 3).
For the case of the Au, it is important to mention that individual inoculation and in consortia of A. niger strains showed a higher leaching of this precious metal. Emphasizing that the presence of the consortia of A. niger MX5/A. niger MXPE6, A. niger MXPE6/A. niger MX7 and the single inoculation of A. niger MXPE6 showed the highest Au leaching in percentages of 0.53, 0.43 and 0.43%, respectively. Finally the results obtained for the Cu indicate that the presence of A. niger MX5 in the culture medium with PCB mobile phone powder was able to leach 2.8%, this indicates that the inoculation of this fungus increased 6.5 times the Cu leaching with respect to abiotic control. While the consortium of Sphingomonas sp. MXB8/C. orthopsilosis MXL20 and A. niger MX5/A. niger MXPE6 had a leaching of 1 and 0.9%, respectively. The inoculation of these consortia to the culture medium with PCB powder increased the leaching 2.5-fold with respect to their abiotic control (Fig. 4).
The treatment with citric acid and PCB powder presented significant differences (P ≤ 0.001), with respect to the treatments inoculated with microorganisms and PCB powder, finding that the presence of the citric acid leached 8% Cu and 16% Ag (Fig. 5). The treatment with citric acid and PCB powder presented a pH of 3.6, while the treatment without PCB powder showed a pH of 3.4.
Discussion
With respect to the results of the amount of metals in the printed circuit board of mobile phones, several authors have reported that Cu is one of the metals found in most of these devices, while Au and Ag are found in small amounts [10, 29, 42]. On the other hand, it has been reported that some microorganisms affect their growth when grown with WEEE, due to the variability of metals and components present in these residues [7]. It has been reported that metals such as Ga, Ge, V, Sc, La, Eu and Yb have a negative effect on the growth of some strains of A. niger, which decreases the production of biomass [31].The decrease of dry biomass in the A. niger MX7 strain in consortium with A. niger MX5 and C. orthopsilosis MXL20 respectively has already been reported by [23]. On the other hand, this same author reports an increase of the dry biomass of A. niger MXPE6 exposed to PCB of mobile phones, which is in agreement with the results obtained in this research for the consortium of A. niger MXPE6/C. orthopsilosis MXL20. In relation to the increase of the dry biomass of Sphingomonas sp. MXB8 [18]. observed that the bacteria Sulfobacillus thermosulfidooxidans and acidophilus bacteria (A1TSB) increased their biomass and improved their bioleaching when grown with PCB.
For the case of the consortia of C. orthopsilosis MXL20 with the strain of A. niger MXPE6 and A. niger MX7 increased the pH of the mineral medium as compared to the control. Some reports mention that Chromobacterium violaceum presents this same mechanism, in which the pH of the mineral medium is increased when being cultivated at pulp densities of electronic residues of 0.5% since they generate extracellular alkaline metabolites. However, at higher pulp densities of electronic waste (4%) the pH decreases because of the toxic and inhibitory effect on the microorganism [27], as observed in most treatments.
With regard to the recovery of Ag from electronic waste the reports are very scarce and as was observed in this work A. niger MX5, the consortium of Sphingomonas sp. MXB8/C. orthopsilosis MXL20 and Sphingomonas sp. MXB8 presented the highest leaching of this metal. A. niger has the ability to leach up to 98% of Ag and other metals from solder residues for 60 h, because it produces citric acid [15]. In the case of bacteria, it has been reported that low concentrations of Ag inhibit its growth, however, Pseudomonas plecoglossicida can leach 5% Ag from waste jewelry (286 mg L−1) [8]. Another Pseudomonas able to leach Ag (33%) from PCB pulp (10 g L−1) is P. balearica SAE1 grown in medium with glycine (5 g L−1), pH 9.0 and at a temperature of 30 °C, to induce the production of cyanide [21]. The bioleaching obtained for Sphingomonas sp. MXB8 in this investigation it is a relevant fact, because it has been reported that 15 µg L−1 of Ag inhibits the growth of this bacterial genus [13]. In addition, the effect of WEEE on Sphingomonas sp. MXB8 and its potential to leach metals from these residues has not been reported, but the tolerance and accumulation of metals (Cu, Ni, Pb and Zn) from mining waste by some bacteria of this genus has been reported [2]. In addition, it has been reported that Sphingomonas sp. MXB8 it is able to degrade a great variety of aromatic hydrocarbons, which shows its ability to grow and adapt in contaminated environments [5]. In the case of Au, it was found that the strains of A. niger are the ones that show the greatest recovery of this metal, in comparison with the microorganisms and consortiums tested. This coincides with that reported for A. niger MXPE6 in in PCB of cellular telephones, where a bioleaching of 40% of Au is obtained [23]. While the low efficiency in bioleaching by C. orthopsilosis MXL20 and Sphingomonas sp. MXB8 is not widely discussed because of the limited information available. Among the microorganisms capable of recovering Au from electronic waste is C. violaceum, a bacterium capable of producing cyanide when grown in culture media with glycine, which allows it to form complexes with this metal, recovering up to 63% Au from PCB of cell phones [26]. Another bacterium with the capacity to leach Au from PCB of computers pre-treated is Bacillus megaterium, which recovers up to 63% Au [4].
The efficiency of bioleaching of Cu depends on the concentration of electronic waste, microorganisms and pH, an example of how these factors affect, is reported for the bacterium C. violaceum, which presents a low Cu leaching when it is cultivated with high concentrations of PCB and pH between 6 and 7 [26, 27]. The production of organic acids metabolized by microorganisms also influences Cu leaching. For example, heterotrophic microorganisms (fungi) at an optimum pH (2.0) are more effective for Cu leaching present in WEEE compared to leaching of sulfuric acid generated by the bacterium Acidithiobacillus [34]. It has also been reported that A. niger is one of the filamentous fungi that presents high percentages (56 to 97%) of Cu bioleaching, and mention that this fungus produces organic acids (oxalic or citric) that helps to leach the metals [3, 24, 32]. It has been reported that to obtain higher percentages of Cu from PCB, two bioleaching treatments are required [30].
On the other hand, the leaching obtained by the citric acid for Ag was lower in comparison with the bioleaching obtained by the microorganisms. It has been documented that the leaching of Ag, Sn and Cu with NaOH, NaCl and H2S in solder residues is lower compared to the bioleaching of A. niger and Acidithiobacillus ferrooxidans [15]. In the case of Cu the leaching increased 280% using citric acid compared to the bioleaching by A. niger MX5. The use of acids is widely reported for the leaching of metals, in the case of PCB it is mentioned that [Bmim] HSO4 (1-Butyl-3-methylimidazolium Hydrogen Sulfate) recovers 100% of Cu and the efficiency of leaching of Cu depends of the concentration that is used of this substance. Particle size plays an important role, since the smaller the particle size, the less Cu leaching [11]. In addition, citric acid in combination with H2O2 has the capacity to recover metals from secondary sources, since it has been observed that it can leach up to 96.4% of V present in spent catalysts [12]. It has also been reported that for Co and Zn, the citric acid produced by Aspergillus niger presents higher percentages of leaching than commercial citric acid [36].The above shows that the efficiency in the recovery of metals between chemical leaching and biological leaching depends on the metal, the metal matrix and the study conditions.
Conclusions
The excessive generation of electronic waste makes it necessary to search for environmentally friendly technologies to recover metals from WEEE, so knowing the response of microorganisms and microbial consortiums grown with WEEE is increasingly important for to be able to develop useful technologies that can solve this problem. Each contribution provides information for future research, in this context the present research shows that the majority of the microbial consortiums employed did not show favorable results (high percentages of recovery) for the bioleaching of Ag, Au and Cu from the PCB, under the tested conditions. Highlighting the bioleaching of the consortiums formed by Sphingomonas sp. MXB8/C. ortopsilosis MXL20 for Ag, A. niger MX5 + A. niger MX7 for Au and A. niger MX5 + A. niger MXPE6 + A. niger MX7 for Cu. Also, Sphingomonas sp. MXB8 shows an excellent potential for the bioleaching of Ag. While citric acid showed higher bioleaching for Cu. Which indicates that the chemical leaching as well as the biological leaching for PCB of cellular telephones depends on physicochemical factors (metallic species, amount of electronic waste, type of electronic waste, pH, temperature, among others). Finally, the bioleaching of metals from PCB by the microorganisms studied was carried out without agitation and at room temperature, which involves a lower energy consumption than that of other reports. Although more research is required, microorganisms and their consortia can undoubtedly be an alternative for the bioleaching of Ag, Au and Cu from electronic waste.
References
Águeda E, Garcia JL (2005) Automoción: elementos amovibles y fijos no estructurales. Thomson Paraninfo, Madrid
Andreazza R, Okeke BC, Pieniz S, Bortolon L, Lambais MR, Camargo FAO (2012) Effects of stimulation of sopper bioleaching on microbial community in vineyard soil and copper mining waste. Biol Trace Elem Res 146(1):124–133. https://doi.org/10.1007/s12011-011-9213-8
Anjum F, Bhatti HN, Asgher M, Shahid M (2010) Leaching of metal ions from black shale by organic acids produced by Aspergillus niger. Appl Clay Sci 47(3):356–361. https://doi.org/10.1016/j.clay.2009.11.052
Arshadi M, Mousavi SM (2015) Enhancement of simultaneous gold and copper extraction from computer printed circuit boards using Bacillus megaterium. Bioresour Technol 175:315–324. https://doi.org/10.1016/j.biortech.2014.10.083
Ban YH, Ahn JI, Sekhon SS, Cho SJ, Kim YH, Kim YC (2016) Identification of inducible proteins in the phenanthrene degrader Sphingobium chungbukense DJ77 by 2-dimentional electrophoresis and liquid chromatography/tandem mass spectrometry. Genes Genom 38(5):397–405. https://doi.org/10.1007/s13258-015-0374-2
Bastian P, Eichler W, Huber F, Jaufmann N, Manderla J, Spielvogel O, Springer G, Stricker F, Tkbtz K (2001) Electrotecnia, vol 1. Ediciones Akal, Madrid
Brandl H, Bosshard R, Wegmann M (2001) Computer-munching microbes: metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy 59(2):319–326. https://doi.org/10.1016/S0304-386X(00)00188-2
Brandl H, Lehmann S, Faramarzi MA, Martinelli D (2008) Biomobilization of silver, gold, and platinum from solid waste materials by HCN-forming microorganisms. Hydrometallurgy 94(1):14–17. https://doi.org/10.1016/j.hydromet.2008.05.016
Common MS, Stagl S (2008) Introducción a la economía ecológica. Reverté Barcelona, España;
Cui J, Zhang L (2008) Metallurgical recovery of metals from electronic waste: a review. J Hazard Mater 158(2):228–256. https://doi.org/10.1016/j.jhazmat.2008.02.001
Chen M, Huang J, Ogunseitan OA, Zhu N, Wang Y (2015) Comparative study on copper leaching from waste printed circuit boards by typical ionic liquid acids. Waste Manage 41:142–147. https://doi.org/10.1016/j.wasman.2015.03.037
Erust C, Akcil A, Bedelova Z, Anarbekov K, Baikonurova A, Tuncuk A (2016) Recovery of vanadium from spent catalysts of sulfuric acid plant by using inorganic and organic acids: laboratory and semi-pilot tests. Waste Manage 49:455–461. https://doi.org/10.1016/j.wasman.2015.12.002
Han DW, Lee MH, Lee MH, Uzawa M, Park JC (2005) The use of silver-coated ceramic beads for sterilization of Sphingomonas sp. in drinking mineral water. World J Microbiol Biotechnol 21(6):921–924. https://doi.org/10.1007/s11274-004-6721-0
He W, Li G, Ma X, Wang H, Huang J, Xu M, Huang C (2006) WEEE recovery strategies and the WEEE treatment status in China. J Hazard Mater 136(3):502–512. https://doi.org/10.1016/j.jhazmat.2006.04.060
Hocheng H, Hong T, Jadhav U (2014) Microbial leaching of waste solder for recovery of metal. Appl Biochem Biotechnol 173(1):193–204. https://doi.org/10.1007/s12010-014-0833-2
Hong Y, Valix M (2014) Bioleaching of electronic waste using acidophilic sulfur oxidising bacteria. J Clean Prod 65:465–472. https://doi.org/10.1016/j.jclepro.2013.08.043
Hoque ME, Philip OJ (2011) Biotechnological recovery of heavy metals from secondary sources—An overview. Mater Sci Eng 31(2):57–66
Ilyas S, Anwar MA, Niazi SB, Ghauri MA (2007) Bioleaching of metals from electronic scrap by moderately thermophilic acidophilic bacteria. Hydrometallurgy 88(1–4):180–188. https://doi.org/10.1016/j.hydromet.2007.04.007
Işıldar A, Van de Vossenberg J, Rene ER, van Hullebusch ED, Lens PNL (2016) Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB). Waste Manage 57:149–157. https://doi.org/10.1016/j.wasman.2015.11.033
Kang HY, Schoenung JM (2005) Electronic waste recycling: a review of US infrastructure and technology options. Resour Conserv Recy 45(4):368–400
Kumar A, Saini HS, Kumar S (2018) Bioleaching of gold and silver from waste printed circuit boards by Pseudomonas balearica SAE1 isolated from an e-waste recycling facility. Curr Microbiol 75(2):194–201. https://doi.org/10.1007/s00284-017-1365-0
Lardé J, Ciudad JC, Rebolledo A, Picozzi A (2005) Situación y tendencias recientes del mercado del cobre. Recursos Naturales e Infraestructura
Madrigal-Arias JE, Argumedo-Delira R, Alarcón A, Mendoza-López MR, García-Barradas O, Cruz-Sánchez JS, Ferrera-Cerrato R, Jiménez-Fernández M (2015) Bioleaching of gold, copper and nickel from waste cellular phone PCBs and computer goldfinger motherboards by two Aspergillus niger strains. Braz J Microbiol 46(3):707–713. https://doi.org/10.1590/S1517-838246320140256
Mehta KD, Das C, Pandey BD (2010) Leaching of copper, nickel and cobalt from Indian Ocean manganese nodules by Aspergillus niger. Hydrometallurgy 105(1):89–95. https://doi.org/10.1016/j.hydromet.2010.08.002
Moustafa ESI (1999) Nonrenewable resources. In: Environmental geology. Encyclopedia of earth science. Springer, New York, pp 436–438
Natarajan G, Ting YP (2014) Pretreatment of e-waste and mutation of alkali-tolerant cyanogenic bacteria promote gold biorecovery. Bioresour Technol 152:80–85. https://doi.org/10.1016/j.biortech.2013.10.108
Natarajan G, Ting YP (2015) Gold biorecovery from e-waste: an improved strategy through spent medium leaching with pH modification. Chemosphere 136:232–238. https://doi.org/10.1016/j.chemosphere.2015.05.046
Ongondo FO, Williams ID, Cherrett TJ (2011) How are WEEE doing? A global review of the management of electrical and electronic wastes. Waste Manage 31(4):714–730. https://doi.org/10.1016/j.wasman.2010.10.023
Pant D, Joshi D, Upreti MK, Kotnala RK (2012) Chemical and biological extraction of metals present in E waste: a hybrid technology. Waste Manage 32(5):979–990. https://doi.org/10.1016/j.wasman.2011.12.002
Pradhan JK, Kumar S (2012) Metals bioleaching from electronic waste by Chromobacterium violaceum and Pseudomonads sp. Waste Manag Res 30(11):1151–1159. https://doi.org/10.1177/0734242x12437565
Qu Y, Li H, Tian W, Wang X, Wang X, Jia X, Shi B, Song G, Tang Y (2015) Leaching of valuable metals from red mud via batch and continuous processes by using fungi. Miner Eng 81:1–4. https://doi.org/10.1016/j.mineng.2015.07.022
Ren WX, Li PJ, Geng Y, Li XJ (2009) Biological leaching of heavy metals from a contaminated soil by Aspergillus niger. J Hazard Mater 167(1):164–169. https://doi.org/10.1016/j.jhazmat.2008.12.104
Robinson BH (2009) E-waste: an assessment of global production and environmental impacts. Sci Total Environ 408(2):183–191. https://doi.org/10.1016/j.scitotenv.2009.09.044
Saidan M, Brown B, Valix M (2012) Leaching of electronic waste using biometabolised acids. Chin J Chem Eng 20(3):530–534. https://doi.org/10.1016/S1004-9541(11)60215-2
Sambrook J, Rusell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York pp. 7.4–7.8
Sayer JA, Gadd GM (2000) Binding of cobalt and zinc by organic acids and culture filtrates of Aspergillus niger grown in the absence or presence of insoluble cobalt or zinc phosphate. Mycol Res 105(10):1261–1267. https://doi.org/10.1017/S0953756201004749
SAS II (2017) The SAS system for windows, ver. 9.4. SAS Institute Inc, Cary
Vats MC, Singh SK (2015) Assessment of gold and silver in assorted mobile phone printed circuit boards (PCBs): Original article. Waste Manage 45:280–288. https://doi.org/10.1016/j.wasman.2015.06.002
Veit HM, Bernardes AM (2015) Electronic waste: generation and management. In: Viet HMB (ed) Electronic waste, recycling techniques. Springer, New York, pp 3–12. https://doi.org/10.1007/978-3-319-15714-6_2
Villas-Bôas RC, Sánchez M (2006) Tecnologías limpias en las industrias extractivas minero-metalúrgica y petrolera. Rio de Janeiro: CETEM/MCT/CNPq/CYTED/AECI:17–31
Weidenhamer JD, Clement ML (2007) Leaded electronic waste is a possible source material for lead-contaminated jewelry. Chemosphere 69(7):1111–1115. https://doi.org/10.1016/j.chemosphere.2007.04.023
Wilson KH, Blitchington RB, Greene RC (1990) Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction. J Clin Microbiol 28(9):1942–1946
Wu BY, Chan YC, Middendorf A, Gu X, Zhong HW (2008) Assessment of toxicity potential of metallic elements in discarded electronics: a case study of mobile phones in China. J Environ Sci 20(11):1403–1408. https://doi.org/10.1016/S1001-0742(08)62240-8
Acknowledgements
Authors thank CONACYT for financial support provided by the research Grant CB-239601.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Díaz-Martínez, M.E., Argumedo-Delira, R., Sánchez-Viveros, G. et al. Microbial Bioleaching of Ag, Au and Cu from Printed Circuit Boards of Mobile Phones. Curr Microbiol 76, 536–544 (2019). https://doi.org/10.1007/s00284-019-01646-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-019-01646-3