Abstract
The recovery of metals from electronic waste was investigated by using fungal strain Aspergillus fumigatus A2DS, isolated from the mining industry wastewater. Fifty-seven percent of copper and 32% of nickel were leached (analyzed by Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-OES)) by the organism after one-step leaching at a temperature of 30 °C (shaking condition for 7 days). Maximum % of copper and nickel were obtained at a pH of 6 (58.7% Cu and 32% Ni), the temperature of 40 °C (61.8% Cu and 27.07% Ni), a pulp density of 0.5% (62% Cu and 42.37% Ni), and inoculums of 1% (58% Cu and 32.29% Ni). The XRD pattern of PCB showed 77.6% of copper containing compounds. XRD analysis of the leachate residue showed only 23.2% Euchorite (ASCu2H7O8) and 9.4% other copper containing compounds, indicating the leaching property of the fungus. HPLC analysis of the spent medium showed the presence of different acids like citric, succinic, and fumaric acid. The FTIR spectrum showed a decrease in carboxylic stretching in the leachate produced after bioleaching using spent medium. ICPOES of the leachate obtained using spent medium showed that 61% of the copper and 35% of nickel were leached out after seven days of incubation at shaking condition and 57% of copper and 32.8% of nickel at static condition confirming acidolysis property of the strain. A. fumigatus A2DS metal absorption and adsorption ability were observed using transmission electron microscopy (TEM) and scanning electron microscopy energy dispersive X-ray (SEM-EDX) respectively. The results thus indicate that bioleaching of Cu and Ni is bioleached by A. fumigatus A2DS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing amount of e-waste is mainly fueled by higher consumption rates of EEE (electrical and electronic equipment), short life cycles, and few repair options. Asia generated the highest quantity of e-waste in 2019 at 24.9 Mt, followed by America (13.1 Mt) and Europe (12 Mt), while Africa and Oceania generated 2.9 Mt and 0.7 Mt, respectively. Europe ranked first worldwide in terms of e-waste generation per capita, with 16.2 kg per capita. Oceania was second (16.1 kg per capita), followed by America (13.3 kg per capita), while Asia and Africa generated just 5.6 and 2.5 kg per capita, respectively (Vanessa [33]. Most printed circuit boards (PCBs) of e-waste contain various kinds of metals [35]. Copper (19.91%), aluminum (7.06%), nickel (5.35%), iron (3.56%), tin (2.03%), lead (1.01%), and also precious metals like silver and gold are present in PCB [38]. Landfilling, burning, and incineration are common methods used for the disposal of e-waste. Pyrometallurgy, hydrometallurgy, and pyro-hydrometallurgy are used to extract heavy metals from ores. The major disadvantage of these methods is that large amounts of hazardous wastes are generated at the end. The bioleaching method generates very little quantity of waste. Four different stages like (i) acidolysis, (ii) complexolysis, (iii) redoxolysis, and (iv) bioaccumulation [8] are involved in bioleaching. There are certain approaches in bioleaching of metals using fungi,one step, two step, and spent medium are the main procedures which are reported by several researchers [1, 18]. In a one-step approach, the WPCBs are added into the medium at the first moment of incubation,in a two-step approach, they are added when a sudden drop in pH is detected (3rd–5th days), and eventually, in spent medium approach, leaching experiments took place after 14 days of fungal activation in fungus free acidic filtrates. Each of the approaches has its own benefits and may lead to different outputs. Many reports are available on the bioleaching ability of bacteria from mobile phone printed circuit board (PCB) [24, 25]. A few researchers have studied the impact of fungi on the recycling of metals from cellular phone PCBs. Solubilization through fungi is by the formation of organic or inorganic acids. There is various advantage of using fungi for bioleaching. The major advantage include (i) they can to grow under harsh environmental condition (ii) leaching manner with a shorter lag phase,and (iii) secondary metabolites (organic acids) produced by fungi, interact with metal ions and form complexes with metal ions, thus lowering the toxicity of the metabolites to the biomass [7]. Studies on fungi like Aspergillus niger and Penicillium simplicissimum on the bioleaching ability were carried out by Brandl et al. [5] and by Faraji et al. [10]. However, no reviews are available in the capacity of Aspergillus fumigatus to leach metals from cellular PCBs. The main aim of the present work is to study the capacity of Aspergillus fumigatus A2DS to leach out metals by acidolysis and also the ability of fungal mycelium to adsorb and absorb metals present inside the PCB of a cellular phone.
Materials and methods
Chemicals
All the chemicals used for the study were of analytical grade. Sucrose, NaNO3, KH2PO4, MgSO4·7H2O, and KCl were procured from S.D. Fine Chem. Limited (Mumbai, India) and yeast extract from Hi Media, Mumbai, India.
Collection and processing of PCB of mobile phone
Mobile phones for the present study were obtained from e-waste recyclers and the students of Kadi Sarva Vishwavidyalaya (KSV). The PCBs of mobile phones were separated from other parts of mobile phones carefully. The PCBs were made into a fine powder using an instrument called File (it is an instrument used for grinding) at the Department of Mechanical Engineering, KSV Gandhinagar, Gujarat. The larger particles were separated using a sieve (pore size 100μm). The fine powder (Fig. 1A) thus obtained was used for studying the bioleaching ability of fungi.
Analysis of the particle size of PCB powder
Wet dispersion unit SUCELL and the compact laser diffraction sensor HELOS/BR (carried out at SCICART, Gujarat) were used for analyzing the particle size.
Acid digestion and ICP-OES analysis of PCB (mobile phone)
Forty-milliliter aqua regia (3 parts hydrochloric acid to 1 part nitric acid) was used for digesting 1 g of PCB [10]. Using ICP-OES (Perkin Elmer, USA, Optima 3300 RL), the metal concentration of digested samples was then analyzed (Carried out at SCICART, Gujarat).
Collection of metal containing effluent samples
Metal resistant organisms were isolated from different effluent/soil samples. The selection of area was based on the assumption that these samples contain heavy metals and thus heavy metal resistant organisms. The area included mining, textile effluent, motor garages soil sample, and steel store soil samples. Textile effluent was from Tadkeshwar district, Surat. Mine effluent from Rajparadi district Bharuch, motor garages soil sample and steel store soil sample from Gandhinagar (Samples were collected before the wastewater treatment and contain metals like copper and nickel less than 10 mgl−1). Collected samples were transported to the laboratory and were used for the study.
Isolation of metal resistant organisms from contaminated soil and water samples
Sucrose broth medium consisted of (g/L) the following: sucrose (100), NaNO3 (1.5), KH2PO4 (0.5), MgSO4·7H2O (0.025), KCl (0.025), and yeast extract (1.6) [4] that was used for the isolation of the fungi. Sucrose media with different concentrations of PCB (1, 2, and 3%) were prepared followed by the addition of 10 mL of soil suspension/effluent sample (10% w/v). For the growth of fungi, the flasks were kept at 30 °C at 120 rpm for 7 days [5]. To obtain individual colonies, the above enriched samples were spread onto the sucrose agar medium (agar added in sucrose broth medium with composition same as above) and incubated at 30 °C. The plates were observed for colonies after 48–72 h of incubation. The isolated colonies were purified by sub-culturing on to sucrose agar medium supplemented with 1% PCB.
Phenotypic and Genotypic Identification of fungal strain
Lacto phenol cotton blue mounting technique was used for observing the morphology of the isolated fungi. Cultural characteristics and the microscopic view were used for phenotypic identification. Internal Transcribed Space Sequencing (ITS) of 18 srRNA gene (rDNA) was used for genotypic identification (performed at Xcelris Labs Ltd, Ahmedabad, and Gujarat). The phylogenetic tree was constructed using the Mega software.
Study on bioleaching ability of A. fumigatus A2DS by one-step bioleaching
A suspension of A. fumigatus A2DS (106 spores/mL) was prepared. One milliliter of the suspension was added into two sets (n=3) of 100 mL sucrose broth containing 1% PCB of mobile phone in 250-mL Erlenmeyer flasks. Flasks (medium+culture+PCB) were incubated in an orbital shaker at 120 rpm at 30 °C. One set of the flask was incubated for 7 days [37], and the other set of flask was kept for 30 days [14]. After incubation, the flasks were kept idle for 5 min, and 5–8 mL of leachate was collected separately from each flask. The larger particles present in the leachate was filtered using Whatman No 1 filter paper (pore size: 11μm). The fungal spores/hyphae present in leachate were also removed by centrifugation at 10,000×g for 10 min. The metal present in the leachate was analyzed using ICP-OES method (Perkin Elmer, USA; Optima-3300 RL). To nullify the effect of evaporation on bioleaching, a control set without any inoculum (PCB+medium) was also used. The above procedure was repeated for static conditions. The following formula was used to find out the % of metal leached by the strain (using the mean value obtained (n=3)), where c1 is the concentration of metal in the leachate and c0 is the initial concentration of metal in PCB.
Optimization of various factors involved in one-step bioleaching
The bioleaching ability of the fungus is affected by different factors like pH, temperature, percentage inoculums, and percentage pulp density. Using the same procedure mentioned above, the effects of pH (4, 6, 7, 8), temperature (20, 30, 40 °C), inoculum size (spore suspension of 0.5, 1, 2% (v/v)), and the pulp density (0.5, 1, 2% (w/v)) on bioleaching ability of A. fumigatus were also studied. For studying all factors mentioned above, the flasks were incubated at 120 rpm for 7 days. Sucrose medium containing PCB without fungal inoculum was kept as a control in all studies. The percentage of metal leached was found out by using the formula mentioned above.
XRD spectrum of bioleached residue and PCB of mobile phone
X-ray diffraction (XRD) analysis was used to study the structural changes in PCB. The bioleached residues obtained after one-step bioleaching experiment were treated with deionized water. After removing the moisture content, the pellet was made into a fine powder using mortar and pestle and analyzed using XRD (carried out at SCICART, Gujarat) (model: Xpert MPD; Make, Philips, Holland). XRD analysis of the PCB powder was also carried out (control). The variation in the diffraction pattern (between test and control) was observed using Match 3 Software.
Study on the organic acid production by A. fumigatus A2DS
The ability of A. fumigatus to produce organic acid was studied by inoculating 1 mL of spore suspension (106 spores/mL) of A. fumigatus A2DS into 100 mL sucrose broth (without PCB) containing flasks. The flasks were incubated at 30 °C at 120 rpm. The changes in pH and biomass were noted till 20 days. Variations in pH and biomass were noted throughout the 20 days (data not shown). pH decreased up to the sixth day, and thereafter the pH remained constant. To confirm the production of acids, after the sixth day of incubation in sucrose broth, the content was filtered through the Whatman No 1 filter paper (pore size: 11μm) and centrifuged (Remi 2-24L) at 10,000 g for 10 min to remove fungal pellets. The presence of citric acid and oxalic acid in the sucrose medium without e-waste was estimated using titrimetric and spectrophotometric methods respectively. The presence of organic acid in the filtrate (spent medium) was also analyzed using FTIR (Spectrum GX Perkin Elmer, USA) (Outsourced at SCICART, Gujarat) and HPLC (Agilent, Model 1100 series, outsourced at AUM Research Laboratories). HPLC was carried out by using a C18, 5 micron column (250-mm length and 4.6-mm internal diameter), with a mobile phase of 25 mM K2HPO4, adjusted to pH 2.5. A 1.5 mL/min of the sample was injected for 15 min, and elutes were detected at λ 210 nm.
Role of acids in leaching of metals by A. fumigatus A2DS
The ability of acids to leach the metal was carried after confirmation of organic acid secretion by A. fumigatus A2DS strain. Five milliliters of the spent medium (obtained after growing fungus in sucrose broth) was added to the sucrose broth with PCB (1%) and kept for incubation at 30 °C for 7 days in shaking (120 rpm) and static condition. After incubation, the medium was centrifuged at 10,000xg for 10 min, and the medium was filtered through Whatman No 1 filter paper (pore size: 11μm), and the filtrate was analyzed for the presence of metal using ICP-OES. Organic acids present in the leachate (shaking condition) were analyzed using FTIR.
Metal absorption and adsorption ability of A. fumigatus A2DS analyzed using TEM and SEM-EDX
TEM (Tecnai 20, Philips, and Holland) and SEM-EDX (ESEM EDX XL-30, Philips, the Netherlands) analysis were used to establish the ability of fungi to absorb and adsorb metals respectively. The fungal culture of A. fumigatus A2DS was inoculated into a sucrose broth medium containing 1% PCB of mobile phone and incubated at 30 °C for 7 days. To obtain the biomass, the fungal containing medium was passed through a Whatman No 1 filter paper. The resulting biomass was washed with 0.02 phosphate buffer and dehydrated in a graded ethanol series for 5 min each. The fungal biomass was observed under TEM and SEM-EDX (carried out at SCICART, Gujarat).
Statistical analysis
Three sets of readings were taken for all the experiments. Data points in the figures represent means with error bars shown (±SD). Two-way ANOVA was used to analyze the significance of experimental results (with a 95% level of confidence, α=0.05) (Sundar and Richard, 2012).
Results
Particle size analysis using SUCELL and the compact laser diffraction sensor HELOS/BR
Particles with X 90 of 114.77 μm and X10 of 3.45 μm were used for all studies. A reduction in the size of the particles after bioleaching (data not shown) was observed.
ICP-OES analysis of metals present in PCB of mobile phone
The ICP-OES analysis of the acid digested PCB mobile phone showed 242.3 mg.g−1 of copper, 3.05 mg.g−1 of lead, and 7.10 mg.g−1 of nickel. Bhumika et al. [3] studies also showed similar results.
Isolation and identification of metal leaching organisms
Metal resistant individual colonies were observed on sucrose agar medium supplemented with 1% and 2% PCB (Fig. 1A) and not in 3% PCB. No growth was observed on media plated with soil samples. The isolated organisms were purified and maintained on a sucrose agar medium (1% PCB). On sucrose agar medium, the culture showed bluish grey-colored conidial spores (Fig. 1B). Fungi formed balls in shaking conditions in sucrose broth medium (Fig. 1C). Lacto phenol cotton blue mounted, fungal slides were observed under light microscope (×40). The macroscopic and microscopic view showed morphological characteristics similar to Aspergillus. Genotypic identification was carried out using ITS sequencing of 18 srRNA. Sequences were deposited into the Genbank (NCBI) DNA sequence database (accession no. MK424485) and also analyzed by BLAST. BLAST analysis showed 99% similarity with Aspergillus fumigatus and 1% different from reported Aspergillus fumigatus. So the isolate was named as Aspergillus fumigatus A2DS. Phylogenetic trees were constructed, and evolutionary history was inferred by using the maximum likelihood method and Kimura 2-parameter model [21]. The bootstrap consensus tree inferred from 500 replicates was taken to represent the evolutionary history of the taxa analyzed [11]. Phylogenetic analysis was conducted using MEGA X software. The phylogenetic tree was constructed using Mega software (Fig. 2).
ICP-OES analysis of the leachate after one-step bioleaching and optimization of various factors influencing bioleaching of PCB
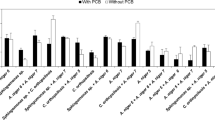
The bioleaching ability of the isolated organism was analyzed using ICP-OES, and the results are presented in Table 1. Fifty-seven percent of copper and 32% of nickel were leached by the organism after the one-step leaching at a temperature of 30 °C (shaking condition (120 RPM) for 7 days) (Fig. 3A). Increasing the period of incubation of the strain along with PCB powder showed a slight variation in the biomass (data not shown). Incubating for 30 days also did not show much difference in the percentage of metals in the leachate (Fig. 3A); this might be due to the depletion of sucrose and thereby decrease in biomass production and acid production. Various factors influencing bioleaching were also analyzed. Effects of various factors like pH, temperature, inoculum %, and pulp density were also analyzed. Maximum % of copper and nickel were obtained at a pH of 6 (58.7% Cu and 32% Ni), inoculum of 1% (58% Cu and 32.29% Ni), the temperature of 40 °C (61.8% Cu and 27.07% Ni), and a pulp density of 0.5% (62% Cu and 42.37% Ni) (Fig. 3 B, C, D, and E, respectively). In the present study, maximum leaching was obtained at pH 6 (A. fumigatus A2DS showed maximum oxalic production (spectrophotometrically) at pH 6 (data not included) indicating the role of oxalic acid in bioleaching.
Role of various factors in bioleaching (one-step bioleaching) from PCB of mobile phone by A. fumigatus A2DS strain. A Shaking and static conditions at varying time period (after 7 days and after 30 days) (P value < 0.0001; all values were significant when compared to control and also between groups). B Varying pH like 4, 6, 7, and 8 (P value < 0.0001; all values were significant when compared to control and also between groups). C Varying % inoculums like 0.5%, 1%, and 2% (P value < 0.0005; all values were significant when compared to control and also between groups). D At different temperatures (P value < 0.0001; all values were significant when compared to control and also between groups). E Varying pulp density (P value < 0.0001; all values were significant when compared to control and also between groups). The data presented in this figure presents the mean of values obtained from triplicate experiments at a significant level (P < 0.05) based on two-way ANOVA analysis
XRD spectrum of PCB and bioleached residue after bioleaching
X-ray diffraction pattern of the bioleached (Fig. 4A) and non-bioleached (Fig. 4B) (control: without fungal inoculation) residues were varying. The XRD spectrum of PCB of mobile phone powder and the residue obtained after bioleaching showed 100% intensity at an angle of 43.444°. Figure 4B shows 77.6% of copper containing compounds and in Fig. 4A shows only around 23.2% Euchorite and 9.4% other copper containing compounds.
TEM and SEM–EDX analysis of leached residue
The metals absorbed/adsorbed by the cell were visualized by TEM and SEM respectively. The EDX analysis of the printed circuit board (control) showed maximum copper (13%), followed by aluminum (10.76%) (Fig. 5A). The % weight of the metal in the EDAX spectrum was calculated using eZAF algorithms. The EDX analysis of the residue after fungal bioleaching showed copper (1.38%) and aluminum (2.47%) were adsorbed onto the surface of mycelium (Fig. 5B). SEM analysis of leached residue was carried out to find out the ability of metal ions to be adsorbed onto the surface of fungal mycelium (Fig. 5C). The length of the metal absorbed on fungal mycelium as observed using TEM is represented in yellow color (Fig. 5D). The absorption and adsorption of the metal can be also confirmed by other methods also.
TEM and SEM- EDX analysis of the PCB of the mobile phone powder and the residue obtained after leaching. A EDX spectrum of PCB of mobile phone powder. B EDX spectrum of the metal adsorbed on A. fumigatus A2DS mycelium. C SEM view (scale bar = 20 nm) of the A. fumigatus A2DS mycelium. D TEM (scale bar = 500 nm) view of A. fumigatus A2DS mycelium with metal absorbed (yellow color)
FTIR and HPLC analysis of spent medium (fungus grown on sucrose broth) and filtrate obtained after bioleaching using spent medium (medium+1% PCB+spent medium)
The production of organic acid was further confirmed using HPLC and FTIR. The FTIR spectrum of the spent medium (A. fumigatus A2DS+sucrose broth) showed stretching corresponding to acid groups (FTIR 1401.3 Ft (cm−1)). The FTIR spectrum of spent medium (Fig. 6A and Table 2) (without any PCB) was compared with the FTIR of the leachate (spent medium + PCB) obtained. The stretches corresponding to active functional acid groups were present in the spent medium, but the height of the peak decreased in the leachate, showing utilization of acids in bioleaching of PCB (Fig. 6B and Table 2). HPLC chromatogram showed the presence of different acids like citric, succinic, and fumaric acid in the spent medium (Fig. 6C). To confirm the acidolysis of the metal by the fungal metabolites, the ICP-OES analysis of the leachate obtained after bioleaching using spent medium was carried out. Sixty-one percent of copper and thirty-five percent of nickel in the leachate after 7 days of incubation at shaking condition and 57% of copper and 32.8% of nickel at static condition were obtained confirming the role of fungal metabolites in the leaching process (Fig. 7).
Discussion
Young and Veasey [39] have reported that “particle size has a negative correlation with the metal removal efficiency and high particle sizes may lead to poor metal extraction efficiency.” Particles with X 90 of 114.77 μm and X10 of 3.45 μm were used for all studies, as the surface area increases with decreasing particle size, the contact between the metal-bearing waste particles and the leachate in the leaching medium increases, thus leading to higher metal extraction efficiency [29]. A reduction in the size of the particles after bioleaching (data not shown) was observed. Similar outcomes were obtained by Faraji et al. [10] using Aspergillus niger. The metallic parts of the PCBs were dissolved during the bioleaching experiment while the non-metallic parts remained undissolved [10]. The ICP-OES analysis of the acid digested PCB mobile phone showed 242.3 mg.g-1 of copper, 3.05 mg.g-1 of lead, and 7.10 mg.g−1 of nickel. Bhumika et al.’s [3] studies also showed similar results. Brandl et al. [5] had reported the role of microbes to mobilize metals from electronic waste materials. But there are limited data available on bioleaching studies using a mobile phone.
Primary ores are less complex in nature, whereas electronic waste is in a complex and concentrated form [28]. The PCB added effluent, favored only the growth of the metal resistant organisms. Lacto phenol cotton blue mounted, fungal slides were observed under the light microscope (×40); sequencing techniques and bioinformatics tool confirmed that the organism is Aspergillus fumigatus A2DS. The bioleaching ability of the isolated organism was analyzed using ICP-OES. Fifty-seven percent of copper and 32% of nickel were leached by the organism after a one-step leaching at a temperature of 30 °C (shaking condition (120 RPM) for 7 days. Studies on Aspergillus niger MXPE6 by Jorge et al. [20] showed that 24 and 5% of Cu was bioleached from gold-plated finger integrated circuits found in computer motherboards (GFICMs) and cellular phone printed circuit boards (PCBs), respectively. When compared to Aspergillus niger MXPE6, Aspergillus fumigatus A2DS has more bioleaching ability. The effects of various factors like pH, temperature, inoculum %, and pulp density were also analyzed. Maximum % of copper and nickel were obtained at a pH of 6 (58.7% Cu and 32% Ni), inoculum of 1% (58% Cu and 32.29% Ni), the temperature of 40 °C (61.8% Cu and 27.07% Ni), and a pulp density of 0.5% (62% Cu and 42.37% Ni). The results are in accordance with studies carried out by Nwagu and Okolo [27]. The studies of Walaszczyk et al. [34] on Aspergillus niger showed that oxalic production is maximum at pH 6. The released organic acids such as oxalic and citric acids which in turn reduced the host metal oxides/hydroxides to their lower valence states, and thus dissolving the base metals following the indirect mechanism. In acidolysis, the protonation of oxygen atoms covering the surface of metallic compounds. The protons and oxygen are associated with water; thus, the metal is detached from the surface (Eq. (1)). In complexolysis, metal complexes and chelates are formed, leading to the solubilization of the metal ions (due to the complex capacity of a molecule). For example, a complex of oxalic acid with aluminum, iron and magnesium, a complex of citric acid with magnesium and calcium and a complex of tartaric acid with iron, calcium, silica, magnesium, and aluminum. Equation (2) is an example of complexolysis reaction that produces nickel citrate (Asghari et al. 2012):
Furthermore, metal ions solubilized into solution by acidolysis are stabilized in complexolysis [7]. In redoxolysis, the oxidation-reduction processes help the fungal leaching. In the present study, maximum leaching was obtained at pH 6 (A. fumigatus A2DS showed maximum oxalic production (spectrophotometrically) at pH 6: data not included) indicating the role of oxalic acid in bioleaching. Maximum citric acid (5.76 gm %) production by A. fumigatus A2DS was at a pH of 7, at a temperature of 50 °C, and in the medium containing sucrose (10%) (data not shown). A. fumigatus A2DS was able to produce maximum oxalic acid at a pH of 8, temperature of 30 °C, and at static condition (0.071 mg/mL) (data not shown). The production of organic acid was further confirmed using HPLC and FTIR. The stretches corresponding to active functional acid groups were present in the spent medium, but the height of the peak decreased in the leachate, showing utilization of acids in bioleaching of PCB. Similar results were reported by Bullen et al. [6], Fischer et al. [12], Grube et al. [15], Smidt and Schwanninger [30], and Willner [36]. HPLC chromatogram showed the presence of different acids like citric, succinic, and fumaric acid in the spent medium. This finding is in agreement with those of Burgstaller and Schinner [7] and Bosshard et al. [4]. The role of citric, fumaric, succinic, and malic acid in bioleaching has been reported by Vanessa et al. [32]. To confirm the acidolysis of the metal by the fungal metabolites, the ICP-OES analysis of the leachate obtained after bioleaching using spent medium was carried out. Sixty-one percent of copper and thirty-five percent of nickel in the leachate after 7 days of incubation at shaking condition and 57% of copper and 32.8% of nickel at static condition were obtained confirming the role of fungal metabolites in the leaching process.
X-ray diffraction pattern of the bioleached and non-bioleached (control: without fungal inoculum) residues were varying. In addition, comparing the diffraction patterns, after bioleaching experiments the patterns have become much noisier and the intensities of peaks have been decreased. [17]. The structural pattern was changed due to the leaching of metal ions. Similar results were obtained by Faraji et al. [10] using Aspergillus niger.
Kuyucak and Volesky [23] and Kratochvil and Volesky [22] had stated that “chemi-sorption mechanisms include complexation, chelation, micro precipitation, and microbial reduction, while physical sorption mechanisms generally involve electrostatic forces and ion exchange.” Biological processes like biosorption, bioreduction, bio-mineralization, and bio-precipitation are used for the recovery of metals [16]. Metal absorption and adsorption are the property of microbes like bacteria, eukaryotic cells of the phyla algae, fungi, and yeast [9]. It is typically carried out by inactive biomass. The positive charges of the amine group present on the cell wall attract ions and get adsorbed on the surface [26]. The metals absorbed/adsorbed by the cell were visualized by TEM and SEM respectively. (The property of absorption/adsorption has to be again confirmed by other methods). Fifty-seven percent of copper and 32.8% of nickel were leached by the organism, and rest of the metal was absorbed/adsorbed by the fungal mycelium.
Conclusion
Fifty-seven percent of copper and thirty-two percent of nickel were leached by the organism after one-step leaching at a temperature of 30 °C (static condition for 7 days). Maximum % of copper and nickel were obtained at a pH of 6 (58.7% Cu and 32% Ni), the temperature of 40 °C (61.8% Cu and 27.07% Ni), a pulp density of 0.5% (62% Cu and 42.37% Ni), and inoculums of 1% (58% Cu and 32.29% Ni). The XRD peak patterns of leachate residue and e-waste show much difference indicating the solubilization of copper from PCB. The ability of the fungus to absorb and adsorb metals was carried out by TEM and SEM-EDX respectively. The SEM-EDX analysis of the printed circuit board (control) showed maximum copper (13%), followed by aluminum (10.76%). The EDX analysis of the residue after fungal bioleaching showed copper (1.38%) and aluminum (2.47%) adsorbed on to mycelium. FTIR of the leachate (sucrose medium+A. fumigatus A2DS) also indicated the use of acid metabolites in the bioleaching of PCB. HPLC chromatogram showed the presence of different acids like citric, succinic, and fumaric acid in the spent medium. ICP_OES analysis of leachate obtained using spent medium showed that 61% of copper and 35% of nickel after 7 days of incubation at shaking condition and 57% of copper and 32.8% of nickel at the static condition also confirmed the role of fungal metabolites in the leaching process. The results thus showed that the strain A. fumigatus A2DS can leach copper and nickel from the PCB of the mobile phone as well as absorb and adsorb metals.
References
Amiri F, MousaviSM , Yaghmae iS, Bara M 2012 Bioleaching kinetics of a spent refinery catalyst using Aspergillus niger at optimal conditions Bioch Biochem Eng J 67 208 217
Asghari I,Mousavi SM, Amiri F Tavassoli S 2013 Bioleaching of spent refinery catalysts: a review J Ind Eng Chem 19 069 1081
Bhumika R, Khatri Asha B, Sodha Monal B, Shah Devayani R Tipre, Shailesh R, Dave 2018 Chemical and microbial leaching of base metals from obsolete cell-phone printed circuit boards. Sustain Environ Res 333–339.
Bosshard P, Bachofen R, Brandl H 1996 Metal leaching of fly ash from municipal waste incineration by Aspergillus niger Environ Sci Technol 30 3066 3070
Brandl H, Bosshard R, Wegmann M 2001 Computer-munching microbes: metal leaching from electronic scrap by bacteria and fungi Hydrometallurgy 59 319 326
Bullen HA, Oehrle SA, Bennett AF, Taylor NM, Barton HA 2008 Use of attenuated total reflectance Fourier transform infrared spectroscopy to identify microbial metabolic products on carbonate mineral surfaces Appl Environ Microbiol 74 14 4553 5455
W Burgstaller F Schinner 1993 Leaching of metals with fungi J Biotechnol 27 91 116
Chinner F, Burgstaller W 1989 Extraction of zinc from industrial waste by Penicillium sp Appl Environ Microbial 55 1153 1156
Das N 2010 Recovery of precious metals through biosorption, a review Hydrometallurgy 103 180 189
Faraji F, Navid A, Golmohammadzadeh R, Fereshteh R 2018 Fungal bioleaching of WPCB using Aspergillus niger: Observation optimization and kinetics J Environ Manage 217 775 787
Felsenstein J 1985 Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39(4): 783–791.
Fischer G, Braun S, Thissen R, Dott W 2006 FTIR spectroscopy as a tool for rapid identification and intraspecies characterization of airborne filamentous fungi J Microbiol Methods 64 63 77
Golmohammadzadeh R, Rashchi F, Vahidi E 2017 Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: process optimization and kinetic aspects Waste Manag 64 244 254
Gosh S, Paul AK 2016 Bioleaching of nickel by Aspergillus humicola SKP102 isolated from Indian lateritic overburden. J Sustain Min 1–7.
Grube M, Muter O, Strikauska S, Gavare Limane M 2008 Application of FT-IR spectroscopy for control of the medium composition during the biodegradation of nitro aromatic compounds J Ind Microbiol Biotechnol 39 1545 2154
Hennebel T, Boon N, Maes S, Lenz M 2015 Biotechnologies for critical raw material recovery from primary and secondary sources: R&D priorities and future perspectives. N Biotechnol 32.
Hester R E, Harrison RM 2009 In: Electronic waste management. Royal society of chemistry 258–263
Horeh NM, Mousavi SM, Shojaosadati S 2016 Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. 320: 257–266
Jadhav U, Su C, Hocheng H 2016 Leaching of metals from printed circuit board powder by an Aspergillus niger culture supernatant and hydrogen peroxide RSCAdv 6 43442 43452
Jorge E MA, Rosalba Argumedo-Delira Alejandro Alarcón, Ma. Remedios Mendoza- López, Oscar García-Barradas, Jesús Samuel Cruz-Sánchez, Ronald Ferrera-Cerrato, and Maribel Jiménez-Fernández 2015 Braz J Microbiol 46(3): 707–713.
Kimura M 1980 A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences J Mol Evol 16 2 111 120
Kratochvil D, Volesky B 1998 Advances in the biosorption of heavy metals Trends Biotechnol 16 7 291 300
Kuyucak N, Volesky B 1988 Bio sorbents for recovery of metals from industrial solutions J Biotechnol Lett 10 2 137 142
Li J, Liang C, Ma C 2015 Bioleaching of gold from waste printed circuit boards by Chromobacterium violaceum J Mater Cycles and Waste Manag 17 529 539
Liang G, Mo Y, Zhou Q 2010 Novel strategies of bioleaching metals from printed circuit boards (PCBs) in mixed cultivation of two acidophiles Enzyme MicrobTechnol 47 322 326
Mack C, Wilhelmi B, Duncan JR, Burgess JE 2007 Biosorption of precious metals Biotechnol 25 264 271
Nwagu NT, Okolo BN 2011 Growth profile and Amylase hydrolytic activity of a thermophilic fungi Aspergillus fumigatus isolated from soil Asian J Biotechnol 3 1 46 57 https://doi.org/10.3923/ajbkr.2011.46.57
Ongondo FO, Williams ID, Whitlock G 2015 Distinct Urban Mines: Exploiting secondary resources in unique anthropogenic spaces Waste Manag 45 4 9
Silva RA, Park J, Lee E, Park J, Choi SQ, Kim H 2015 Influence of bacterial adhesion on copper extraction from printed circuit boards Sep Purif Technol 143 169 176
Smidt E, Schwanninger M 2007 Characterization of waste materials using FTIR spectroscopy process monitoring and quality assessment Spectrosc Lett 38 3 247 270
Sundar Rao PSS, Richard J 2012 Analysis of variance. In: Introduction to Biostatistics and Research Methods 108–113.
Vanessa L, Brisson W-Q, Alvarez-cohen L 2019 Metabolomic analysis reveals contributions of citric and citramalic acids to rare earth bioleaching by a Paecilomyces fungus Front Microbiol 10 3008
Vanessa Forti, Cornelis PeterBalde, Reudieger Kuehr and Graam Bel 2020 The Global E waste Monitor 1–120
Walaszczyk E, Podgórski W, Janczar-Smuga M 2018 Effect of medium pH on chemical selectivity of oxalic acid biosynthesis by Aspergillus niger W78C in submerged batch cultures with sucrose as a carbon source Chem Pap 72 1089 1093
Wellmer FW, Hagelüken 2015 The feedback control cycle of mineral supply increase of raw material efficiency and sustainable development Minerals 5 527
Willner J 2012 Leaching of selected heavy metals from electronic waste in the presence of the A. ferrooxidans bacteria J Achiev Mater Manuf Eng 55 860 863
Xu TJ, Ramanathan T, Ting YP 2014 Bioleaching of incineration fly ash by Aspergillus niger precipitation of metallic salt crystals and morphological alteration of the fungus Biotechnol Rep 3 8 14
Yoo JM, Jeong J, Yoo K, Lee J, Chun KW 2009 Enrichment of the metallic components from waste printed circuit boards by a mechanical separation process using a stamp mill Waste Manag 29 1132 1137
Young RJ, Veasey TJ 2000 Application of the ring loaded strength (RLS) disc test to monitors the effects of thermal pre-treatments on ore grind ability Miner Eng 13 783 787
Acknowledgements
The authors are thankful to Gujarat Council on Science and Technology (GUJCOST), Gujarat, India, for the project Grant. We are also thankful to Kadi Sarva Vishwavidyalaya for providing the laboratory facilities and support to carry out this work and also to SICART, Gujarat, for helping us in carrying out a different analysis like ICP-OES, FTIR, XRD, TEM, SEM, and particle size analysis. Different mine effluent samples were collected by Richa Solanki and a special thanks to her also.
Funding
This study is supported by the Gujarat Council On Science And Technology (GUJCOST), Gujarat, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Aspergillus fumigatus ability to leach metals from PCB of mobile phone has not been reported. The strain isolated showed genotypic differences from the already reported strains of Aspergillus fumigatus, so the isolates was named as Aspergillus fumigatus A2DS.
• Fifty-seven percent of copper and thirty-two percent of nickel were leached by the organism after one-step leaching at a temperature of 30 °C (shaking condition for 7 days). Effects of various factors like pH, temperature, inoculum %, and pulp density conditions were also analyzed. Maximum percentage of copper and nickel were obtained at a pH of 6 (58.7% Cu and 32% Ni), temperature of 40 °C (61.8% Cu and 27.7% Ni), a pulp density of 0.5% (62% Cu and 42.37% Ni), and inoculum of 1% (58% Cu and 32.29% Ni).
• ICP-OES of the leachate obtained using spent media (containing organic acids like citric acid, succinic acid, and fumaric acid, confirmed by HPLC and FTIR) indicate acidolysis of the metal. Sixty-one percent of the copper and 35% of nickel were leached out after seven days of incubation at shaking condition and 57% of copper and 32.8% of nickel at static condition confirming acidolysis property of the strain.
• The main highlight of the study is that the prolonged incubation of the strain until 30 days did not show much leaching ability. The variation in pH and biomass were noted throughout the 20 days. pH decreased up to the sixth day and there after the pH remained constant, confirming the correlation between acid metabolites and leaching.
• TEM and SEM–EDX of the residue obtained after leaching confirmed the ability to absorb and adsorb metals on the mycelium. Ten percent copper was adsorbed by the mycelium. Metals like copper and nickel were also absorbed and adsorbed by the fungal mycelium, which will provide an economical alternative for removing toxic heavy metals from industrial wastewater and aid in environmental remediation.
Rights and permissions
About this article
Cite this article
Patel, F., Lakshmi, B. Bioleaching of copper and nickel from mobile phone printed circuit board using Aspergillus fumigatus A2DS. Braz J Microbiol 52, 1475–1487 (2021). https://doi.org/10.1007/s42770-021-00526-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00526-y