Abstract
The impact of an early food introduction on the microbiota composition and microbial metabolism in colon was investigated using a new-born piglet model. At day 4 after birth, 10 litters of piglets were randomly allocated to a sow-rearing group (SR group) and a milk-replacer supplementing group (MRS group) (n = 5). A commercial milk replacer was given to the suckling piglets in the MRS group from the 4th day to the 28th day. Pyrosequencing of the V3–V4 region of the 16S rRNA genes showed that the milk replacer supplementation significantly decreased the relative abundance of Lactobacillus, Clostridium XI, Blautia, Clostridium sensustricto and Escherichia (p = 0.08) in the colon of the piglets, but significantly increased the relative abundance of Paraprevotella on the 28th day. In addition, the abundance of Rumminococcus, Clostridium XlVa, Succiniclasticum, Clostridium IV tended to increase in the MRS group. The concentrations of acetate, propionate, butyrate, valerate and branch-chain fatty acids (BCFAs) in the colonic digesta increased with the milk replacer supplementary in the MRS group. In addition, the milk replacer supplementary increased the expression level of Toll-like receptor 4 (TLR4), but decreased the expression level of interleukin-6 (IL-6) in the colonic mucosa of the piglets. In conclusion, an early food introduction can influence the gut bacterial composition and metabolism, and may further affect the intestinal health by modifying the gene transcription related to the colonic function. These findings may provide some guidelines for the early nutrition supplementation for infants during the lactation period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast-feeding has been endorsed as an optimum way of feeding infants [1, 2]. The WHO recommends an exclusive breast-feeding for 6 months, and a supplemental breast-feeding up to 2 years and beyond [3]. However, the prevalence of exclusive breast-feeding infants for 6 months is at low percentage in both developing and developed countries [4,5,6,7]. Hence, many infants are fed with formula milk or other supplemental foods in addition to breast milk [8,9,10]. Previous studies have pointed out that an early food introduction would increase the later risk of some chronic diseases, such as obesity and food allergies [5, 11]. On the other hand, De Lange et al. [12] reported that an early food introduction would stimulate the development of gastrointestinal tract to adapt to a complicated dietary environment [12, 13]. Moreover, such practice can prevent growth retardation by providing additional complementary nutrition, especially for the children at age of 6–12 months [14, 15]. Therefore, whether the early food introduction is beneficial or harmful to the infant health needs to be further studied.
The maintenance of colonic homeostasis and normal physiological function is essential to infant health. The colon harbors the highest density of microbes, and the colonic microbiota changes rapidly in response to dietary treatments [16,17,18]. Recent studies have revealed that colonic microbiota plays a crucial role in the host’s metabolic, physiological and immune processes [7]. Duncan et al. indicated that the colonic microbiota affects energy harvest, storage, and expenditure including short-chain fatty acids (SCFAs) as the main energy source of colonocytes [19]. Moreover, the changes of microbial composition induced by antibiotics affect the immune status of the host and influence disease susceptibility [20]. Therefore, we hypothesize that an early food introduction affects the host health by modulating the microbiota composition, microbial metabolism in the colon and the expressions of genes related to colonic function. To date, few data are available regarding how the colonic microbiota responds to the early food introduction. To verify this hypothesis, we developed an early food introduction model by feeding new-born piglets with a milk replacer in addition to sow milk to investigate the changes in microbiota composition, microbial metabolites in the colon and the expressions of genes related to the colonic function during the lactation period.
Materials and Methods
Animal Trial
The animal experiment was approved by the Animal Experiment Committee of Nanjing Agricultural University, in accordance with the Regulations for the Administrations of Affairs Concerning the Experimental Animals (The State Science and Technology Commission of China, 1988). All experiments were performed in accordance with the approved guidelines and regulations.
10 Landrace × Yorkshire crossbred sows were artificially inseminated by one Duroc boar to minimize the genetic variation among their off-springs. The sows were allowed to farrow in concrete-floored farrowing pens at the experimental farm of Nanjing Agricultural University, Jiangsu, China. The piglets within each litter were adjusted for an average body weight of 1.87 ± 0.05 kg and balanced gender with a standardized litter size of 8 piglets (half male and half female).
Piglets were completely reared by the sows to acquire passive immunity from colostrum till the 4th day after farrowing. Afterwards, 5 litters of piglets were randomly allocated to a sow-rearing group (SR group), while the other 5 litters of piglets were assigned to a milk-replacer supplementing group (MRS group). A commercial milk replacer (Table S1) was given ad libitum to the suckling piglets in the MRS group from the 4th to the 28th day. The milk replacer was mixed with warm water and fed ad libitum to piglets. To avoid non-experimental effects, the piglets had no access to creep feed during the experiment. Water was fed ad libitum throughout the experiment. The piglets were weaned on the 28th day and fed with a commercial-weaning diet. The growth performance and diarrhea frequency were monitored for 7 days after weaning to investigate the effect of early dietary introduction on the weaning transition of piglets. On the 28th day, five piglets from each group were sacrificed for sampling. The abdomen of piglets was opened and the whole gastrointestinal tract was removed to collect colonic digesta and mucosa for subsequent analysis. In addition, blood was collected before the piglets were killed.
DNA Extraction, PCR Amplification and Illumina MiSeq Sequencing
Total bacterial DNA was extracted from the colonic digesta using the bead-beating method as described by Zoetendal et al. [21]. The concentration of DNA was determined using a Nano-Drop 1000 spectrophotometer (Thermo Scientific Inc., Wimington, DE, USA). The V3–V4 regions of bacterial 16S ribosomal RNA gene were amplified by polymerase chain reaction (PCR) using the bacterial universal primers 319F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT). The PCR amplification was performed using the following program: 95 °C for 2 min, followed by 25 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s and final extension 72 °C for 7 min. The amplicons extracted from 2% agarose gel were purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences; Union City, CA, USA) according to the recommended instructions, and were quantified using QuantiFluor™-ST (Promega, USA). The purified amplicons were pooled in equimolar and paired-end on the Illumina platform according to the standard protocols [22].
Bioinformatics Analysis
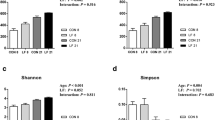
The raw sequencing data were uploaded to GeneBank in NCBI with the accession number SRP105910. Then the Raw FASTQ files were de-multiplexed and quality-filtered using QIIME (version 1.70) with a reported standard criteria [23]. Operational taxonomic units (OTUs) were clustered with a 97% similarity cut-off using UPARSE (version 7.1 http://drive5.com/uparse/), and the chimeric sequences were identified and removed using UCHIME. The rarefaction curves (Fig. S1) generated by the number of reads through the number of OTUs tend to approach the saturation plateau. The bacterial diversity was assessed using the observed species, Simpson, Chao1 and Shannon indices.
Determination of Microbial Metabolites
The concentrations of SCFAs in the colonic digesta were determined by gas chromatography (Shimadzu, GC-14A with an FID detector, Japan) as previously described by Mao et al. [24]. A Nukol™ Capillary GC Column (Sigma-Aldrich, US) was used. The temperature of injector, column and detector were 110, 150, and 180 °C, respectively. The lactic acid was detected using a commercial kit (Nanjing Jiancheng Bioengineering Institute, China) according to the specification. The colorimetric method was used to measure the concentration of ammonia-N in the colonic digesta as described by Nyachoti et al. [25].
RNA Extraction, cDNA Synthesis and Real-Time PCR
Total RNA of the colonic mucosa was isolated using TRIzol (Invitrogen, China), and 1 µg RNA was reverse transcribed to cDNA with a Prime Script RT reagent kit (TaKaRa Biotechnology (Dalian) Co., China) according to the manufacturer’s protocols. Real-time PCR was performed using the StepOne-Plus (Applied Biosystems, California, USA) with StepOne Software (version 2.2.2, Applied Biosystems). For the amplification, a 20 µL reaction mixture contains 10 µL SYBR Premix Ex Taq (TliRNaseH Plus), 0.4 µL of each primer, 0.4 µL of ROX Reference Dye (50×), 6.8 µL of ddH2O and 2 µL of cDNA. The primers used are listed in Table S2 as described by Zhang et al. [26]. The expression of genes (TLR4, Toll-like receptor 4; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; TGF-β, transforming growth factor-β; IFN-γ, interferon-γ; ZO-1, zonula occludin 1; occludin) was calculated relative to the expression of β-actin using the 2−ΔΔCt method as previous described by Herfel et al. [27]. The relative mRNA expression of the target gene was normalized to the control group.
Immunoglobulin Analysis
The concentrations of total immunoglobulin A (IgA), immunoglobulin M (IgM), immunoglobulin G (IgG) in serum and secretory immunoglobulin A (sIgA) in the colonic mucosa were measured using the porcine IgA, IgM, IgG and sIgA ELISA kits (Nanjing AOGENE Technology Science Co., Ltd., China), respectively. The concentration of sIgA in the mucosa was expressed as the relative amount of sIgA to total protein. The total protein content was quantified using the commercial kit (Nanjing AOGENE Technology Science Co., Ltd., China).
Statistical Analysis
Data were analyzed with SPSS 20.0 and are shown as means ± SEM. The Student’s t test and Mann–Whitney U test were used to assess the differences between the MRS group and the SR group. The normality of the distribution of variables was tested by the Shapiro–Wilk test. The variables which had a non-normal distribution were analyzed using the nonparametric methods. The t test and the Mann–Whitney U test were used to analyze the data that had a normal or non-normal distribution. Significant differences were declared when p < 0.05.
Results
Bacterial Diversity and Bacterial Composition Determined by MiSeq Sequencing
As shown in Table S3, the diversity indices (Shannon and Simpson) and richness estimators (observed species and Chao1) of colonic microbiota are similar in the MRS group and the SR group. The bacterial composition was assessed at different taxonomic levels. At the phylum level (Fig. S2), there is no statistical difference in bacterial composition between the two groups (p > 0.05). At the family level, Lactobacillaceae, Peptostreptococcaceae and Clostridiaceae 1 (Fig. S3) have lower abundance in the colonic digesta of piglets in the MRS group than those in the SR group (p < 0.05).
At the genus level (Fig. 1a), the abundance of Lactobacillus, Clostridium XI, Blautia, and Clostridium sensu stricto (p < 0.05) and Escherichia (p = 0.08) tend to decrease in the MRS group compared with the SR group. On the other hand, the abundance of Rumminococcus (p = 0.06), Clostridium XlVa (p = 0.05), Succiniclasticum (p = 0.05), Clostridium IV (p = 0.06) and Paraprevotella (p < 0.05) tends to increase in the MRS group. At the species level (Fig. 1b), the abundance of Faecalibacterium prausnitzii (p = 0.10) and Ruminococcus bromii (p = 0.09) tends to increase in the MRS group, the abundance of Lactobacillus salivarius (p = 0.05), Lactobacillus reuteri (p = 0.10) and Escherichia coli (p = 0.08) tends to decrease.
Comparisons of relative abundance of altered bacteria at the genus level (a) and at the species level (b) in each group. Dotted bar SR, a sow-rearing group; checked bar MRS, a milk-replacer supplementing group. Different letters among group at the same day indicated a significant difference, p < 0.05. n = 5, per group per day
Microbial Metabolites in the Colonic Digesta
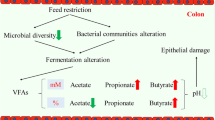
To reveal the microbial metabolism in the colonic digesta, the concentrations of lactic acid, SCFAs and ammonia-N were examined (Table 1). The concentrations of total SCFA, acetate, propionate, butyrate, valerate and BCFA (isobutyric acid and isovaleric acid) in the colonic digesta of piglets in the MRS group are significantly increased compared with those in the SR group. In addition, there is no statistical difference in the concentrations of lactic acid and ammonia-N in the colonic digesta of piglets in the two groups (p > 0.05).
Serum Immunoglobulin Concentration and Colonic Immune Function
There was no difference in the serum concentration of IgA, IgM and IgG and colonic mucosa sIgA of piglets in the SR and MRS groups (Table S4). However, the piglets in the MRS group have lower expression level of IL-6 (p < 0.05) but higher expression level of TLR4 (p < 0.05) in colonic mucosa than the piglets in the SR group (Fig. 2). In addition, the piglets in the MRS group have an increased expression level of IL-8 (p = 0.06) but a decreased expression level of IFN-γ (p = 0.09) and TNF-α (p = 0.08) in colonic mucosa compared with the piglets in the SR group. There is no difference in the expression levels of IL-1β, IL-10, TGF-β, ZO-1 and occludin in colonic mucosa of piglets from both groups (p > 0.05).
The mRNA relative expression in colonic mucosa. Dotted bar SR, a sow-rearing group; checked bar MRS, a milk-replacer supplementing group; different letters among groups at the same day indicated a significant difference, p < 0.05. n = 5, per group per day; IL-8 (p = 0.06); IFN-γ (p = 0.09); TNF-α (p = 0.08)
Discussion
Previous studies indicated that an early food introduction would damage the gut histological structure and might increase the risk of some chronic diseases, such as obesity and food allergies [28,29,30]. However, Caulfield reported that an early food introduction can prevent infants’ growth retardation by providing additional complementary nutrition [8, 14]. In this study, an early food introduction model of new-born piglets using a milk replacer was used to investigate the changes in microbiota composition, microbial metabolism and colonic function during the lactation period. Our results showed no significant differences in ADG (MRS: 0.21 kg ± 0.01 kg; SR: 0.20 kg ± 0.01 kg, p = 0.50) of piglets in the two groups from the 4th to 28th day. There were no significant differences in ADG (MRS: 0.17 kg ± 0.03 kg; SR: 0.18 kg ± 0.02 kg, p = 0.71) and ADFI (MRS: 428.52 g ± 11.96 g; SR: 419.11 g ± 7.52 g, p = 0.52) of piglets from the 28th to 35th day. In addition, no significant differences in colonic mucosal morphology and the concentration of sIgA in the colonic mucosa were observed on the 28th day in the MRS group and the SR group. These results indicated that an early food introduction did not have a clearly negative influence on the hosts’ health and colonic morphology in the study. Moreover, it was found that the early food introduction would modulate the colonic bacterial composition and bacterial metabolism, and could further affect the host health by modulating the expression of genes related to the colonic function. Similar results were also reported by others regarding the altered bacterial composition, the increased concentrations of SCFAs [5, 11, 30] in response to the early food introduction during the lactation period.
SCFA formation is one of the most important processes mediated by colonic microorganisms, and has multiple effects on the intestinal health [31,32,33]. In our study, the early food introduction increased the concentrations of SCFAs in the colon on the 28th day, which was consistent with previous studies [5]. First, the increased concentration of total SCFAs might be caused by the increased indigested substrate such as carbohydrate and protein in the colon as the milk replacer was supplemented to piglets from the 4th day to the 28th day [34, 35]. Second, the altered bacterial composition could lead to the increased concentrations of SCFAs. Our results showed that the early food introduction did not significantly change the bacterial composition at phylum level, but altered some genera and species in the colonic digesta on the 28th day. The enriched abundances of Ruminococcus, Clostridium XlVa, Clostridium IV and Paraprevotella in the MRS group also contributed to the increased amount of total SCFAs as they were all shown to be able to improve SCFA production [23, 36,37,38,39,40]. The increased abundance of Succiniclasticum contributed to the increased concentration of propionate as it could ferment succinate and converted succinate quantitatively to propionate [41]. In addition, for the altered genera and species, our results showed that the abundance of Escherichia and Escherichia coli tended to decrease in the colonic digesta after the early food introduction, which contributed to the decreased diarrhea frequency of piglets after weaning in this study [42]. The diarrhea frequency of piglets in the MRS group after weaning had a decreasing trend compared with the piglets in the SR group (MRS: 5.57 ± 0.60; SR: 7.29 ± 0.58, p = 0.08). These results indicated that the early food introduction could decrease the diarrhea rate of piglets after weaning. Furthermore, the early food introduction decreased the abundance of Lactobacillus, which agreed with previous studies [11, 43]. The decreased abundance of Lactobacillus may be caused by the decreased amount of ingested oligosaccharide which is associated with the reduced amount of breast milk after the early food introduction [43,44,45]. In addition, our results showed that the abundance of F. prausnitzii in the MRS group and the SR group were 0.65% and 0.10% (p = 0.10), respectively. F. prausnitzii is a butyrate producer and has anti-inflammatory effects, making it a key member of microbiota that contributes to the intestinal health [36, 46, 47]. Overall, these results indicated that an early food introduction would increase the concentrations of SCFA and might affect the host health by the modified microbiota composition and metabolism.
The homeostasis of gut is maintained by the combined actions of microbiota, microbial metabolites and host cells [48]. The altered microbiota composition and metabolism in the colon would then modulate local gene transcription in colonic mucosa [49, 50]. In our study, although the early food introduction decreased the expression of TNF-α and IL-6, the expression levels of other inflammatory genes did not change significantly. The decreased expression of TNF-α and IL-6 might be caused by the increased butyrate in the colonic digesta. When pathogen invades, butyrate could stimulate the macrophage to produce the expression of anti-inflammation cytokine IL-10, and suppressed the expression of pro-inflammatory cytokine TNF-α and IL-6 [51] at the same time. In addition, the F. prauznitzii also contributed to the decreased IL-6 as Sokol et al. reported that it was correlated inversely with IL-6 after a surgery [52]. In addition, the up-regulated expression of TLR4 in the MRS group was also associated with these modified expressions of inflammatory cytokines in colonic mucosa [53,54,55]. For the barrier function in the colon, the expression of tight junction proteins was also positively associated with butyrate [38]. Evidences showed that an early food introduction would increase intestinal permeability and reduced the ZO-1 mRNA expression [34]. However, the early food introduction did not affect the expression of ZO-1 and occludin, or the colonic morphology in our study. Therefore, the increased concentrations of SCFAs and altered microbial composition caused by the early food introduction might affect the intestinal health by modulating the gene transcription related to the barrier function and innate immune function.
In summary, the increased SCFAs might be caused by the increased ingestion of protein and carbohydrate from milk replacer, resulting in more substrates for the microbial fermentation. Moreover, the increased SCFAs might contribute to the decreased expression of pro-inflammatory cytokines and help maintain an acidic environment to inhibit the proliferation of pathogens. Therefore, early food introduction by milk replacer would increase microbial fermentation, inhibit pathogen proliferation and decrease the risk of inflammation in colon. In conclusion, an early food introduction can influence the gut bacterial composition and metabolism, and may affect the intestinal health by modifying the gene transcription related to colonic function. These findings may provide some guidelines for the early nutrition supplementation for infants during the lactation period.
References
Salone LR, Vann WF Jr, Dee DL (2013) Breastfeeding: an overview of oral and general health benefits. J Am Dent Assoc 144:143–151
Walker A (2010) Breast milk as the gold standard for protective nutrients. J Pediatr 156:s3-7. https://doi.org/10.1016/j.jpeds.2009.11.021
Kramer MS, Kakuma R (2004) The optimal duration of exclusive breastfeeding: a systematic review. Adv Exp Med Biol 554:63–77
Colen CG, Ramey DM (2014) Is breast truly best? Estimating the effects of breastfeeding on long-term child health and wellbeing in the United States using sibling comparisons. Soc Sci Med 109:55–65. https://doi.org/10.1016/j.socscimed. 2014.01.027
Le Huerou-Luron I, Blat S, Boudry G (2010) Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev 23:23–36. https://doi.org/10.1017/S0954422410000065
Pang WW, Aris IM, Fok D, Soh SE, Chua MC, Lim SB, Saw SM, Kwek K, Gluckman PD, Godfrey KM, van Dam RM, Kramer MS, Chong YS (2016) Determinants of breastfeeding practices and success in a multi-ethnic Asian population. Birth 43:68–77. https://doi.org/10.1111/birt.12206
Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, Murch S, Sankar MJ, Walker N, Rollins NC (2016) Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387:475–490. https://doi.org/10.1016/s0140-6736(15)01024-7
Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M, Gil A, Vieites JM, Norin E, Young D, Scott JA, Dore J, Edwards CA (2011) Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 157:1385–1392. https://doi.org/10.1099/mic.0.042143-0
Issaka AI, Agho KE, Page AN, Burns P, Stevens GJ, Dibley MJ (2014) Determinants of early introduction of solid, semi-solid or soft foods among infants aged 3–5 months in four Anglophone West African countries. Nutrients 6:2602–2618. https://doi.org/10.3390/nu6072602
Penny ME, Creed-Kanashiro HM, Robert RC, Narro MR, Caulfield LE, Black RE (2005) Effectiveness of an educational intervention delivered through the health services to improve nutrition in young children: a cluster-randomised controlled trial. Lancet 365:1863–1872. https://doi.org/10.1016/s0140-6736(05)66426-4
Poroyko V, White JR, Wang M, Donovan S, Alverdy J, Liu DC, Morowitz MJ (2010) Gut microbial gene expression in mother-fed and formula-fed piglets. PLoS ONE 5:e12459. https://doi.org/10.1371/journal.pone.0012459
De Lange CFM, Pluske J, Gong J, Nyachoti CM (2010) Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest Sci 134(1–3):124–134. https://doi.org/10.1016/j.livsci.2010.06.117
Kamitsuka MD, Horton MK, Williams MA (2000) The incidence of necrotizing enterocolitis after introducing standardized feeding schedules for infants between 1250 and 2500 grams and less than 35 weeks of gestation. Pediatrics 105:379–383
Caulfield LE, Huffman SL, Piwoz EG (1999) Interventions to improve intake of complementary foods by infants 6 to 12 months of age in developing countries: impact on growth and on the prevalence of malnutrition and potential contribution to child survival. Food Nutr Bull 20:183–200
Schroeder DG, Martorell R, Floras R (1999) Infant and child growth and fatness and fat distribution in guatemalan adults. Am J Epidemiol 149:177–185
Kim HB, Isaacson RE (2015) The pig gut microbial diversity: understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet Microbiol 177:242–251. https://doi.org/10.1016/j.vetmic.2015.03.014
Sekirov I, Russell SL, Antunes LC, Finlay BB (2010) Gut microbiota in health and disease. Physiol Rev 90:859–904. https://doi.org/10.1152/physrev.00045.2009.-Gut
Tan H, O’Toole PW (2015) Impact of diet on the human intestinal microbiota. Curr Opin Food Sci 2:71–77. doi.https://doi.org/10.1016/j.cofs.2015.01.005
Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, Flint HJ (2008) Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 32:1720–1724. https://doi.org/10.1038/ijo.2008.155
Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, Gill N, Blanchet MR, Mohn WW, McNagny KM, Finlay BB (2012) Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep 13:440–447. https://doi.org/10.1038/embor.2012.32
Zoetendal EG, Akkermans ADL, Vos WMD (1998) Temperature Gradient Gel Electrophoresis Analysis of 16S rRNA from Human Fecal Samples Reveals Stable and Host-Specific Communities of Active Bacteria. Appl Environ Microbiol 64:3854–3859
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R (2012) Ultra-high-throughput microbial community analysis on the illumina hiSeq and miSeq platforms. ISME J 6:1621–1624. https://doi.org/10.1038/ismej.2012.8
Sun Y, Zhou L, Fang L, Su Y, Zhu W (2015) Responses in colonic microbial community and gene expression of pigs to a long-term high resistant starch diet. Front Microbiol 6:877. https://doi.org/10.3389/fmicb.2015.00877
Mao S, Zhang R, Wang D, Zhu W (2012) The diversity of the fecal bacterial community and its relationship with the concentration of volatile fatty acids in the feces during subacute rumen acidosis in dairy cows. BMC Vet Res 8:237. https://doi.org/10.1186/1746-6148-8-237
Nyachoti CM, Omogbenigun FO, Rademacher M, Blank G (2006) Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J Anim Sci 84:125–134
Zhang CJ, Yu M, Yang YX, Mu CL, Su Y, Zhu WY (2016) Differential effect of early antibiotic intervention on bacterial fermentation patterns and mucosal gene expression in the colon of pigs under diets with different protein levels. Appl Microbiol Biotechnol 101:1–13
Herfel TM, Jacobi SK, Lin X, Fellner V, Walker DC, Jouni ZE, Odle J (2011) Polydextrose enrichment of infant formula demonstrates prebiotic characteristics by altering intestinal microbiota, organic acid concentrations, and cytokine expression in suckling piglets. J Nutr 141:2139–2145. https://doi.org/10.3945/jn.111.143727
Huh SY, Rifas-Shiman SL, Taveras EM, Oken E, Gillman MW (2011) Timing of solid food introduction and risk of obesity in preschool-aged children. Pediatrics 127:e544-551. https://doi.org/10.1542/peds.2010-0740
Kuo AA, Inkelas M, Slusser WM, Maidenberg M, Halfon N (2011) Introduction of solid food to young infants. Matern Child Health J 15:1185–1194. https://doi.org/10.1007/s10995-010-0669-5
Lin HY, Chang JH, Chung MY, Lin HC (2014) Prevention of necrotizing enterocolitis in preterm very low birth weight infants: is it feasible? J Formos Med Assoc 113:490–497. https://doi.org/10.1016/j.jfma.2013.03.010
Blaut M (2015) Gut microbiota and energy balance: role in obesity. Proc Nutr Soc 74:227–234. https://doi.org/10.1017/S0029665114001700
De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G (2014) Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156:84–96. https://doi.org/10.1016/j.cell.2013.12.016
Lin HV, Frassetto A, Kowalik EJ, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ (2012) Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 7:e35240. https://doi.org/10.1371/journal.pone.0035240
Chen H, Mao X, He J, Yu B, Huang Z, Yu J, Zheng P, Chen D (2013) Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br J Nutr 110:1837–1848. https://doi.org/10.1017/S0007114513001293
Howard MD, Gordon DT, Pace LW, Garleb KA, Kerley MS (1995) Effects of dietary supplementation with fructooligosaccharides on colonic microbiota populations and epithelial cell proliferation in neonatal pigs. J Pediatr Gastroenterol Nutr 21:297–303
Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A (2013) Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog 5:23. https://doi.org/10.1186/1757-4749-5-23
Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J (2010) Resistant Starches Types 2 and 4 Have Differential Effects on the Composition of the Fecal Microbiota in Human Subjects. PLoS ONE 5:e15046. https://doi.org/10.1371/journal.pone.0015046
Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ (2002) The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett 217:133–139
Singhal A, Farooqi IS, O’ Rahilly S, Cole TJ, Fewtrell M, Lucas A (2002) Early nutrition and leptin concentrations in later life. Am J Clin Nutr 75:993–999
Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A, Louis P, McIntosh F, Johnstone AM, Lobley GE, Parkhill J, Flint HJ (2011) Dominant and diet-responsive groups of bacteria within the human colonic microbtioa. ISME J 5:220–230. https://doi.org/10.1038/ismej.2010.118
Kanengoni AT, Chimonyo M, Tasara T, Cormican P, Chapwanya A, Ndimba BK, Dzama K (2015) A comparison of faecal microbial populations of South African Windsnyer-type indigenous pigs (SAWIP) and Large White x Landrace (LW × LR) crosses fed diets containing ensiled maize cobs. FEMS Microbiol Lett 362:fnv100. https://doi.org/10.1093/femsle/fnv100
Shi C, Wang J, Zhu Y, Niu Q, Wang J (2017) Effects of early supplementary feeding milk replacer on post weaning piglets’ diarrhea frequency, bacterial community and metabolites. Anim Husb Vet Med 49:51–57
Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE (2006) Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118:511–521
Drissi F, Raoult D, Merhej V (2016) Metabolic role of lactobacilli in weight modification in humans and animals. Microb Pathog. https://doi.org/10.1016/j.micpath.2016.03.006
Grummer-Strawn LM, Scanlon KS, Fein SB (2008) Infant feeding and feeding transitions during the first year of life. Pediatrics 122:S36-42. https://doi.org/10.1542/peds.2008-1315d
Macfarlane S, Cleary S, Bahrami B, Reynolds N, Macfarlane GT (2013) Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double-blind, placebo-controlled crossover study. Aliment Pharmacol Ther 38:804–816. https://doi.org/10.1111/apt.12453
Miquel S, Martín R, Rossi O, Bermúdez-Humarán LG, Chatel JM, Soko H, Thomas M, Wells JM, Langella P (2013) Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol 16:255–261. https://doi.org/10.1016/j.mib.2013.06.003
Cassir N, Simeoni U, La Scola B (2016) Gut microbiota and the pathogenesis of necrotizing enterocolitis in preterm neonates. Future Microbiol 11:273–292. https://doi.org/10.2217/fmb.15.136
Aufreiter S, Kim JH, O’Connor DL (2011) Dietary oligosaccharides increase colonic weight and the amount but not concentration of bacterially synthesized folate in the colon of piglets. J Nutr 141:366–372. https://doi.org/10.3945/jn.110.135343
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA (2005) Diversity of the human intestinal microbial flora. Science 308:1635–1638. https://doi.org/10.1126/science.1110591
Jacobi SK, Odle J (2012) Nutritional factors influencing intestinal health of the neonate. Adv Nutr 3:687–696. https://doi.org/10.3945/an.112.002683
Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P (2008) Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA 105:16731–16736. https://doi.org/10.1073/pnas.0804812105
Dheer R, Santaolalla R, Davies JM, Lang JK, Phillips MC, Pastorini C, Vazquez- Pertejo MT, Abreu MT (2016) Intestinal epithelial toll-like receptor 4 signaling affects epithelial function and colonic microbiota and promotes a risk for transmissible colitis. Infect Immun 84:798–810. https://doi.org/10.1128/IAI.01374-15
Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y, Soliman A, Arditi M, Abreu MT (2005) Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol 288:G1055-1065. https://doi.org/10.1152/ajpgi.00328.2004
Hooper LV, Littman DR, Macpherson AJ (2012) Interactions between the microbiota and the immune system. Science 336:1268–1273. https://doi.org/10.1126/science.1223490
Acknowledgements
The authors thank National Center for International Research on Animal Gut Nutrition for financial support. This study was supported by National Key R&D Program of China 2017YFD0500505 and the Fundamental Research Funds for the Central Universities, China (KYZ201722).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, C., Zhu, Y., Niu, Q. et al. The Changes of Colonic Bacterial Composition and Bacterial Metabolism Induced by an Early Food Introduction in a Neonatal Porcine Model. Curr Microbiol 75, 745–751 (2018). https://doi.org/10.1007/s00284-018-1442-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-018-1442-z