Abstract
Ammonia-oxidizing bacteria (AOB) play an important role in nitrification in estuaries. The aim of this study was to examine the spatial abundance, diversity, and activity of AOB in coastal sediments of the Liaohe Estuary using quantitative PCR, high-throughput sequencing of the amoA gene coding the ammonia monooxygenase enzyme active subunit, and sediment slurry incubation experiments. AOB abundance ranged from 8.54 × 104 to 5.85 × 106 copies g−1 of wet sediment weight and exhibited an increasing trend from the Liaohe Estuary to the open coastal zone. Potential nitrification rates (PNRs) ranged from 0.1 to 336.8 nmol N g−1 day−1 along the estuary to the coastal zone. Log AOB abundance and PNRs were significantly positively correlated. AOB richness decreased from the estuary to the coastal zone. High-throughput sequencing analysis indicated that the majority of amoA gene sequences fell within the Nitrosomonas and Nitrosomonas-like clade, and only a few sequences were clustered within the Nitrosospira clade. This finding indicates that the Nitrosomonas-related lineage may be more adaptable to the specific conditions in this estuary than the Nitrosospira lineage. Sites with high nitrification rates were located in the southern open region and were dominated by the Nitrosomonas-like lineage, whereas the Nitrosospira lineage was found primarily in the northern estuary mouth sites with low nitrification rates. Thus, nitrification potentials in Liaohe estuarine sediments in the southern open region were greater than those in the northern estuary mouth, and the Nitrosomonas-related lineage might play a more important role than the Nitrosospira lineage in nitrification in this estuary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen pollution in coastal zones is a widespread and severe issue, particularly in ecosystems with urban or agricultural watersheds [9]. Human activities, discharge of wastewater treatment plant effluent, application of nitrogen-based fertilizers, and other activities result in increasing amounts of biologically available nitrogen. Much of the anthropogenic nitrogen ultimately enters rivers and nearshore coastal areas [2], which poses a serious threat to the balance of estuarine and coastal ecosystems. Nitrification, which consists of the sequential oxidation of ammonium (NH4 +) to nitrite (NO2 −) and then nitrate (NO3 −), is a key part of nitrogen transformation in estuarine and coastal ecosystems. Excess nitrogen can be greatly diminished by tight coupling between nitrification and denitrification or anaerobic ammonium oxidation [42], thereby mitigating eutrophication of estuary and coastal environments. The removal of nitrogen, however, often depends directly on the supply of products from nitrification [21]. Thus, nitrification plays a critical role in facilitating the removal of nitrogen, particularly in estuarine and coastal ecosystems. Due to the great importance of nitrification in coastal ecosystems and the pronounced impact of human activities on nitrogen supply, key microbial communities involved in nitrification have been the focus of remarkably active research.
Ammonia oxidation, which is the first and rate-limiting step of nitrification, plays a central role in the global nitrogen cycle [24]. Ammonia-oxidizing bacteria (AOB) are generally thought to perform this crucial process, and known AOB are categorized into two distinct monophyletic groups (β-proteobacteria and γ-proteobacteria) [18, 31]. All AOB carry the amoA gene that encodes the α subunit of the key ammonia monooxygenase enzyme, which is responsible for catalyzing the oxidation of ammonia to hydroxylamine. The ubiquity of the amoA gene among AOB has facilitated the development of amoA as a molecular marker [20], allowing the detection and quantification of AOB in a variety of environments, including lakes [27, 40], estuaries [14, 23, 30], wastewater treatment plant sludge [39], and constructed wetlands [10, 34], without requiring laboratory culture. It also has led to accurate assessment of abundance and diversity features of AOB.
To date, most studies have focused on the distribution and diversity of AOB and on exploration of complex microbial communities to determine which strains are present and which environmental factors drive their distribution [36] and abundance. However, whether these organisms are actively involved in nitrification is poorly understood. Wankel et al. [37] reported that sites with high and low nitrification rates were dominated by marine/estuarine Nitrosospira-like bacterial amoA sequences and estuarine Nitrosomonas-like amoA sequences, respectively, which suggested that Nitrosospira-like AOB may be more active and play a more critical role in nitrification. However, little is known about the linkage between functional dynamics and ammonia oxidation microbial community composition in estuarine systems.
The Liaohe Delta, with an area of approximately 4000 km2, is located in northeast China [22]. As an important industrial base, the Liaohe River watershed has been subjected to heavy anthropogenic influences due to rapid development of the social economy [29]. Therefore, nitrogen transformations are of major concern in the Liaohe Estuary. Past investigations have focused mostly on variations of AOB abundance [1, 7] and nutrient concentrations [38] seasonally and spatially in the Liaohe Estuary. Thus, studies of the relationships between the nitrification rates and AOB are needed. The objectives of the present study were to (a) investigate the abundance, diversity, and activity of AOB along an estuarine environmental gradient in the surface oxic sediments where nitrification can occur and (b) explore the relationships between AOB abundance, community composition, and potential nitrification rates (PNRs).
Materials and Methods
Sample Collection and Physicochemical Parameters
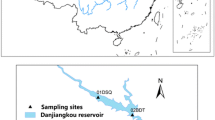
During a spring research cruise (May 2014), samples were collected from three sites (station 5, 40°50′N 121°46′E; station 12, 40°44′N 121°41′E; and station 24, 40°38′N 121°34′E) along the Liaohe Estuary to the coastal zone of Liaodong Bay, Bohai Sea, China (Fig. 1). The surface sediment samples from each site were sectioned into the upper layer (0–2 cm) and the lower layer (2–4 cm), and the sections were placed in sterile plastic bags. Sediment samples were labeled with the layer and location collected (e.g., U5 and L5) and transported to the laboratory on ice. At each site, triplicate subcores were taken and mixed together for homogenization. Upon return to the laboratory, each sediment sample was further split into two parts: one part was stored at 4 °C for sediment physiochemical analysis and the other part was preserved at −80 °C for DNA extraction and subsequent analysis.
Environmental parameters, including salinity and dissolved oxygen (DO) concentration, in the water column were measured in situ using an YSI 6600 Sonde. The concentrations of nutrients, including NH4 +, NO2 −, and NO3 −, were determined according to the “The Specification for Marine Monitoring. Part 4: Seawater Analysis” [16]. The concentrations of total phosphate (TP) and total nitrogen (TN) in sediments were measured according to the “The Specification for Marine Monitoring. Part 5: Sediment Analysis” [17]. Replicate chemical tests were performed for each sample.
PNRs
PNR assays were conducted in triplicate for each site using the method developed by Bernhard et al. [5]. Approximately 10 g (wet weight) of sediment were added to 100 mL of sterile artificial seawater (adjusted to site-specific salinity) amended with 300 μM NH4Cl and 60 μM KH2PO4. Sediment slurries from each site were kept in the dark at room temperature for 24 h with continuous shaking (120 rpm). Subsamples were obtained at several intervals over the incubation period. Subsequently, the samples were centrifuged, filtered, and stored at −20 °C until analysis. The resulting filtrate was analyzed for the accumulation of NO3 − and NO2 − using an AA3 continuous flow analyzer (Bran + Luebbe GmbH, Norderstedt, Germany). Although NO2 − concentrations over the nitrification incubation period were negligible because it can be quickly oxidized to NO3 − by nitrite-oxidizing bacteria, the PNRs were calculated based on the changes in NO3 − (plus NO2 −) concentrations with time [5].
DNA Extraction and Gene Amplification
Total genomic DNA was extracted from sediment samples using the Rapid Soil DNA Isolation Kit (Sangon Biotech, Shanghai, China) according to the manufacturer’s directions. The extracted DNA was verified on a 1% agarose gel. DNA extracts were stored at −20 °C before using them as a template for PCR. The bacterial amoA gene fragments were amplified using the primer set composed of amoA-1F and amoA-2R [32]. PCR was performed in a total volume of 25 μL containing 12.5 μL 2 × Taq Master Mix (TaKaRa, Dalian, China), 0.5 µM of each primer, and 1 μL template DNA. PCRs for AOB were carried out as follows: 3 min at 94 °C followed by 35 cycles of 1 min at 94 °C, 30 s at 55 °C, and 1 min at 72 °C, with a final extension of 10 min at 72 °C. Appropriately sized fragments were separated by electrophoresis in 1.5% agarose gels.
Real-time Quantitative PCR (qPCR)
Copy numbers of the amoA gene of AOB were determined in triplicate with an ABI 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using the SYBR green qPCR method. Plasmids carrying bacterial amoA gene fragment constructed in this study were extracted from Escherichia coli hosts using a TaKaRa MiniBEST Plasmid Purification Kit (TaKaRa). Concentrations of plasmid DNA were measured using a NanoDrop 2000 C spectrophotometer (Thermo, Winmington, DE, USA). Standard curves were created using a dilution series of standard ranging from 106 to 101 gene copies μL−1 and no-template controls that substituted sterile water for DNA. The primer set amoA-1F and amoA-2R was used for the amplification of the AOB amoA gene. The qPCR assay was performed in a total volume of 25 μL containing 12.5 μL of SYBR® Premix Ex Taq™, 2 μL template DNA, and 0.5 μM of each primer. The PCR protocols were performed as follows: 2 min at 50 °C and 10 min at 95 °C followed by 40 cycles of 30 s at 95 °C, 40 s at 58 °C, and 40 s at 72 °C. Standard curve correlation coefficients (R2) were >0.99, and PCR amplification efficiencies were 83.3%. Specificity was determined using melt curves to exclude primer–dimer artifacts and nonspecific PCR amplification products. Occasionally, obvious outliers were excluded, and the midpoint of duplicate values was used.
High-Throughput Sequencing and Data Analysis
The AOB amoA gene was amplified using primers amoA-1F and amoA-2R [32], yielding a fragment of 491 bp, to construct libraries, and amoA gene PCR amplicons were sequenced using the Illumina MiSeq technology at BGI Co., Ltd. (Shenzhen, China). Paired-end reads were generated by the Illumina MiSeq platform, the raw data were pre-processed to obtain clean data using an in-house procedure [13], and the paired reads were joined with FLASH (v1.2.11) [28]. The joined pairs were then quality filtered and chimera checked using scripts of the software UCHIME (v4.2.40) [12]. The resulting sequences were clustered into operational taxonomic units (OTUs) at a 97% similarity threshold using UPARSE [11]. The most abundant sequence of each OTU was selected as the representative sequence. OTU representative sequences were taxonomically classified using the BLASTn tool. A neighbor-joining phylogenic tree was generated using MEGA software (v5.05) [35]. The relative confidence of the tree topologies was evaluated by performing 1000 bootstrap replicates. Alpha-diversity indices, including Chao1, Shannon, and Simpson values, were subsequently calculated by Mothur (v1.31) [33]. Principal component analysis (PCA) of OTUs was performed based on the relative abundance of each OTU in each sample with R.
Nucleotide Sequence Accession Number
The nucleotide sequences obtained in this study were deposited in the Sequence Read Archive of the National Center for Biotechnology Information under the accession number SRP071260.
Results and Discussion
Environmental Characterization
Table S1 shows the environmental characteristics of the sampling sites. In this study, obvious gradient variations of environmental variables were found in Liaohe Estuary coastal zones. Briefly, salinity and DO concentration increased along the Liaohe Estuary to the coastal zone of Liaodong Bay, Bohai Sea. Salinity increased from 24.6 to 28.4 psu, and DO concentration ranged from 7.4 to 8.3 mg L−1. However, concentrations of nutrients generally decreased as salinity increased. Concentrations of NH4 +, the reactant of ammonia oxidation, decreased from 4.11 to 2.16 μM. NO3 − concentrations decreased from 23.25 to 15.35 μM, and NO2 − concentrations decreased from 1.71 to 0.40 μM. This result was consistent with the findings of prior studies of the Yangtze Estuary and Pearl River Delta [26, 42]. The TP concentrations in sediment were 213.1–364.5 μg g−1, and the TN concentrations were 160.0–203.7 μg g−1.
In general, all nutrient concentrations were the highest at station 5, which was the nearest offshore station located at the northern tip of the sampling area. This area is subjected to several sources of contamination from anthropogenic activity. The lowest nutrient concentrations were observed at station 24, which was the station farthest offshore. This difference in nutrient concentrations suggests that riverine N inputs from upstream human activities led to high nutrient concentrations in the estuary mouth, and these inputs may be the most pronounced nitrogen source for estuaries and coasts. Due to the dilution of estuarine waters, nitrogen nutrient contents were lower in the coastal areas.
PNRs and AOB amoA Gene Abundance
In this study, PNRs were used as a proxy of activity of AOB. The upper layer of the sediment had higher PNRs than the lower at each site, with upper rates ranging from 18.5 to 336.8 nmol N g−1 day−1 and lower rates ranging from 0.1 to 92.1 nmol N g−1 day−1 (Fig. 2). In addition, PNRs showed an increasing trend from the Liaohe Estuary mouth to the open coastal zone. A previous study of Chongming eastern intertidal sediments [41] revealed that PNRs in summer increased gradually from the high tidal flat to the low tidal flat. In our study, rates at station 5 were the lowest, but the greatest nutrient concentrations were found here. In contrast, rates at station 24 were the highest and nutrient concentrations were the lowest.
The abundance of AOB in surface sediments was determined using a real-time PCR assay targeting the amoA gene. Similar to the observed PNR variations, bacterial amoA gene abundance increased from the Liaohe Estuary mouth to the open coastal zone. AOB abundance in the upper layer was 4.87 × 105–5.85 × 106 copies g−1 wet sediment weight, whereas that in the lower layer ranged between 8.54 × 104 and 2.69 × 106 copies g−1 wet sediment weight (Fig. 2). Numbers of AOB amoA gene copies in this study were similar to those previously reported in other riverine or estuarine ecosystems [9, 42]. PNRs are considered to roughly reflect the abundance of AOB [19], so we expected these two parameters to correlate significantly. The correlation between AOB abundance and PNRs was positively correlated and statistically significant (r = 0.953, P < 0.01, n = 18; Fig. 2) when the logarithmic transformation was applied to the data, which suggests that AOB may be of great importance in nitrification in the study area. Li et al. [25] also reported that AOB (possibly Nitrosomonas spp.) were of major significance in nitrification in a nutrified estuary. The greatest number of copies of the bacterial amoA gene was detected at site 24 and the lowest was recorded at site 5, suggesting that AOB may play a more important role in mediating the oxidation of ammonia to nitrite in the southern open region than in the northern estuary mouth.
Diversity and Distribution of AOB
Bacterial amoA sequences were obtained from all six samples (U5, U12, U24, L5, L12, and L24), and more than 24,000 amoA gene fragment tag sequences were obtained for each sample (Table S2). When analyzed using a 97% sequence similarity cutoff, the 151,009 total AOB amoA sequences formed 41 OTUs. In general, the estimated coverage values showed that more than 93% of bacterial amoA diversity was captured (Table 1). This result indicates that these libraries represented the majority, but not the full extent, of the total bacterial amoA gene sequences present in the samples and that many OTUs were shared among sites (Fig. 3).
Chao1 and Shannon indices were calculated to evaluate the richness and diversity of the AOB at each site. Richness showed a decreasing trend along the estuary to the coastal zone based on the Chao 1 index, which ranged from 26.0 to 22.3 (Table 1). Overall, the bacterial amoA gene in the upper layer exhibited greater richness than that in the lower layer. Samples U5 and L5 had the highest Chao1 indices in the upper and lower layer, respectively, and these sediments were both obtained from site 5. Thus, station 5 harbored the greatest AOB richness. It also had the greatest number of unique OTUs (3, 4 in the upper and lower layer, respectively). Research conducted in the Sacramento-San Joaquin River Delta [9] showed a similar result. The Shannon diversity index also tended to decrease from the estuary mouth to the open coastal zone, but the greatest diversity was observed in sample U12 (index value 1.71). Both the Chao1 and Shannon indices had the lowest values at site 24. Diversity generally decreased as salinity increased, thus the higher salinity sites were less diverse than the lower salinity sites. Similar results were reported for the Plum Island Sound Estuary [4]. Neither the Shannon nor Chao1 indices were significantly correlated with PNRs.
A majority of amoA OTUs were shared among the samples (Fig. 3), indicating that the amoA genes were widely disseminated. However, the relative abundances of the majority of amoA OTUs were quite low; only the proportion of OTU 1 in each sample reached more than 30%. Figure S1 shows the percentage of AOB amoA gene OTUs for all samples combined. OTUs accounting for >1% of the total included OTU1, OTU2, OTU4, OTU3, OTU7, OTU32, OTU6, and OTU5, and together they accounted for 19.51% of all OTUs; however, they accounted for 97.19% of the whole sequences recovered. Previous research in the Changjiang Estuary and its adjacent area in the East China Sea [8] reported similar results. The high percentage of these eight OTUs among all samples suggests that they may play critical roles in nitrification in the study region.
Composition of the Bacterial amoA Gene
The bacterial amoA sequences were grouped into 41 unique OTUs, but most of them were not closely related to sequences from any cultured AOB isolates (Fig. 4). Phylogenetic analysis indicated that the bacterial amoA gene sequences grouped with known sequences from the Nitrosomonas and Nitrosospira genera. The majority of amoA gene sequences (31 OTUs, 150357 sequences) fell within Nitrosomonas, and only a few sequences belonged to Nitrosospira (10 OTUs, 652 sequences). However, the opposite result was found in a study of the Changjiang Estuary and its adjacent area in the East China Sea [8]. This difference may be explained by the adaptation of microorganisms to the environment in different regions. The Nitrosomonas sequences grouped with sequences in the lineages of Nitrosomonas marina (HM345612), Nitrosomonas aestuarii (HM345616), and the Nitrosomonas-like lineage (18 OTUs), with the latter being the most dominant species. In the Nitrosospira lineage, sequences were affiliated with Nitrosospira briensis (AY123821). High-throughput sequencing analysis indicated that the majority of amoA gene sequences fell within the Nitrosomonas and Nitrosomonas-like clade, suggesting that the Nitrosomonas-related lineage may be more adaptable than the Nitrosospira lineage in this geographic region.
Neighbor-joining phylogenetic tree of AOB amoA OTUs. Sequences obtained in this study are shown with “OTU” in the names. Numbers in parentheses indicate the number of sequences per OTUs. The symbol next to each OTU indicates the composition of the OTU by sample. Reference sequences from named organisms are indicated with GenBank accession numbers. Clades referred to in the text are marked to the right of the tree. Bootstrap values are indicated on branch nodes (1000 neighbor-joining bootstrap replicates)
AOB communities in estuary sediments generally are dominated by Nitrosospira-like amoA sequences in marine-influenced regions and by a mix of Nitrosospira-like and Nitrosomonas-like sequences in oligohaline/freshwater regions [3, 6, 15]. In this study, Nitrosomonas and the Nitrosomonas-like lineage had a widespread distribution, ranging from the estuary to the open coastal region. Similar results were reported from the Yangtze Estuary [42] and Plum Island Sound Estuary [4]. However, the spatial distribution of the Nitrosospira lineage was very restricted, as they were primarily found at lower salinity sites. Nitrosospira was mainly obtained from site 5, which had low PNRs. A previous study revealed that sites with high nitrification rates primarily contained marine/estuarine Nitrosospira-like bacterial amoA sequences [37]. Our results illustrate that Nitrosospira may be inactive in the studied habitats. Compared with Nitrosospira, Nitrosomonas showed a broad geographic distribution, and sites with high nitrification rates were dominated by the Nitrosomonas-like lineage. This finding suggests that Nitrosomonas-related species might play a more important role in nitrification in this estuarine coastal zone.
To evaluate whether AOB composition in the sediments differed by region, we performed PCA based on OTU counts of AOB data from each site. The first two principal component axes explained 91.1% of the total variability in OTU compositions of AOB among stations, which suggests a strong clustering of station samples geographically in the Liaohe estuarine coastal regions (Fig. 5). The amoA genotype assemblages of samples U24 and L24 (from station 24) were grouped together; this site had the lowest nutrient concentrations and was the farthest from the shore. However, the sediment samples collected from stations 5 and 12 were not grouped together, likely because the site near the shore was subjected to larger variability (e.g., nutrient pulses, tidal changes [4, 42], and disturbance due to human activity) than the site far from the shore.
PCA of AOB communities with weighted UniFrac using the bacterial amoA sequences recovered from the Liaohe Estuary. Shown is the plot of the first two principal coordinate axes for PCA and the distribution of the AOB communities of each sample in response to these axes. Similarity is high if two samples are located close together
Conclusions
In conclusion, the spatial abundance, diversity, and activity of AOB in sediments of the Liaohe Estuary were investigated to provide insights into the microbial mechanisms driving nitrification in estuary environments. Nitrification potentials in Liaohe estuarine sediments were greater in the southern open region than in the northern estuary mouth. Additionally, PNRs were higher in the upper layer than in the lower layer. Abundances of the bacterial amoA gene showed an increasing trend from the Liaohe Estuary to the open coastal zone. Log AOB abundance was significantly correlated with log PNRs, indicating that AOB may be of great importance in nitrification in the study area. High-throughput sequencing analysis demonstrated that Liaohe Estuary study sites were primarily dominated by Nitrosomonas and the Nitrosomonas-like lineage rather than the Nitrosospira lineage, which suggests that the Nitrosomonas-related lineage may be more adaptable to the specific conditions than the Nitrosospira lineage in this geographic region. PCA revealed a strong geographical clustering of station samples in the Liaohe estuarine sediments. Sites with high nitrification rates were dominated by the Nitrosomonas-like lineage, whereas the Nitrosospira lineage was principally found at sites with low nitrification rates. Thus, the Nitrosomonas-related lineage might play a more important role in nitrification than the Nitrosospira lineage in this estuary. These results revealed that AOB (especially the Nitrosomonas-related lineage) may play a vital role in ammonia oxidation during nitrification in Liaohe Estuary sediments. This information may help elucidate the roles of AOB in the nitrogen cycling of estuary sediment.
References
Bai J, Chen CT, Zhao YG, Tian WJ, Dong X, Yin NN (2010) Studies on nitrobacteria and nitrification in Liaohe estuary wetland sediments. Environ Sci 31(12):3011–3017 (Chinese)
Bernhard A (2010) The Nitrogen cycle: processes, players, and human impact. Nat Educ Knowl 3(10):25
Bernhard AE, Bollmann A (2010) Estuarine nitrifiers: New players, patterns and processes. Estuar Coastal Shelf Sci 88(88):1–11
Bernhard AE, Donn T, Giblin AE, Stahl DA (2005) Loss of diversity of ammonia-oxidizing bacteria correlates with increasing salinity in an estuary system. Environ Microbiol 7(9):1289–1297
Bernhard AE, Tucker J, Giblin AE, Stahl DA (2007) Functionally distinct communities of ammonia-oxidizing bacteria along an estuarine salinity gradient. Environ Microbiol 9(6):1439–1447
Cao H, Hong Y, Li M, Gu JD (2012) Community shift of ammonia-oxidizing bacteria along an anthropogenic pollution gradient from the Pearl River Delta to the South China Sea. Appl Microbiol Biotechnol 94(1):247–259
Chen LG, Fan JF, Guan DM, Zhao HD, Ming HX, Wu LJ, Chen JY (2010) Analysis of temporal and spatial distribution of nitrobacteria in sediment of Liaohe Estuary. Mar Environ Sci 29(2):174–178 (Chinese)
Chen Y, Zhen Y, He H, Lu X, Mi T, Yu Z (2014) Diversity, abundance, and spatial distribution of ammonia-oxidizing β-proteobacteria in sediments from Changjiang Estuary and its adjacent area in East China Sea. Microb Ecol 67(4):788–803
Damashek J, Smith JM, Mosier AC, Francis CA (2015) Benthic ammonia oxidizers differ in community structure and biogeochemical potential across a riverine delta. Front Microbiol 5:743
Dong X, Reddy GB (2012) Ammonia-oxidizing bacterial community and nitrification rates in constructed wetlands treating swine wastewater. Ecol Eng 40(3):189–197
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10(10):996–998
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27(16):2194–2200
Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, Ravel J (2014) An improved dual-indexing approach for multiplexed 16 S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2 (1):1–7. doi:10.1186/2049-2618-2-6
Francis CA, O’Mullan GD, Ward BB (2003) Diversity of ammonia monooxygenase (amoA) genes across environmental gradients in Chesapeake Bay sediments. Geobiology 1(2):129–140
Freitag TE, Chang L, Prosser JI (2006) Changes in the community structure and activity of betaproteobacterial ammonia-oxidizing sediment bacteria along a freshwater-marine gradient. Environ Microbiol 8(4):684–696
General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (2008) The specification for marine monitoring-Part 4: seawater analysis. Standards Press of China, Beijing
General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (2008) The specification for marine monitoring-Part 5: sediment analysis. Standards Press of China, Beijing
Head IM, Hiorns WD, Embley TM, Mccarthy AJ, Saunders JR (1993) The phylogeny of autotrophic ammonia-oxidizing bacteria as determined by analysis of 16 S ribosomal RNA gene sequences. J Gen Microbiol 139(6):1147–1153
Henriksen K (1980) Measurement of in situ rates of nitrification in sediment. Microb Ecol 6(4):329–337
Horz HP, Barbrook A, Field CB, Bohannan BJ (2004) Ammonia-oxidizing bacteria respond to multifactorial global change. Proc Nat Acad Sci USA 101(42):15136–15141
Jensen K, Sloth NP, Risgaard-Petersen N, Rysgaard S, Revsbech NP (1994) Estimation of nitrification and denitrification from microprofiles of oxygen and nitrate in model sediment systems. Appl Environ Microb 60(6):2094–2100
Ji Y, Zhou G, New T (2009) Abiotic factors influencing the distribution of vegetation in coastal estuary of the Liaohe Delta, Northeast China. Estuar Coasts 32 (5):937–942
Jin T, Zhang T, Ye L, Lee OO, Yue HW, Qian PY (2011) Diversity and quantity of ammonia-oxidizing Archaea and Bacteria in sediment of the Pearl River Estuary, China. Appl Microbiol Biotechnol 90(3):1137–1145
Junier P, Molina V, Dorador C, Hadas O, Kim OS, Junier T, Witzel KP, Imhoff JF (2010) Phylogenetic and functional marker genes to study ammonia-oxidizing microorganisms (AOM) in the environment. Appl Microbiol Biotechnol 85(3):425–440
Li J, Nedwell DB, Beddow J, Dumbrell AJ, Mckew BA, Thorpe EL, Whitby C (2015) amoA Gene abundances and nitrification potential rates suggest that benthic ammonia-oxidizing bacteria and not Archaea dominate N cycling in the Colne Estuary, United Kingdom. Applied &. Environ Microbiol 81(1):159–165
Li Z, Jin W, Liang Z, Yue Y, Lv J (2013) Abundance and diversity of ammonia-oxidizing archaea in response to various habitats in Pearl River Delta of China, a subtropical maritime zone. J Environ Sci 25(6):1195–1205
Liu B, Li Y, Zhang J, Zhou X, Wu C (2014) Abundance and diversity of ammonia-oxidizing microorganisms in the sediments of Jinshan Lake. Curr Microbiol 69(5):751–757
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21):2957–2963
Men B, He MC, Li T, Lin CY, Quan XC (2009) Distributions of polycyclic aromatic hydrocarbons in the Daliao River Estuary of Liaodong Bay, Bohai Sea (China). Mar Pollut Bull 58(6):818–826
Mosier AC, Francis CA (2008) Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ Microbiol 10(11):3002–3016
Purkhold U, Wagner M, Timmermann G, Pommerening-Röser A, Koops HP (2003) 16 S rRNA and amoA-based phylogeny of 12 novel betaproteobacterial ammonia-oxidizing isolates: extension of the dataset and proposal of a new lineage within the nitrosomonads. Int J Syst Evol Microbiol 53(5):1485–1494
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541
Sims A, Gajaraj S, Hu Z (2012) Seasonal population changes of ammonia-oxidizing organisms and their relationship to water quality in a constructed wetland. Ecol Eng 40(3):100–107
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5 : molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol Int J org Evol 28(10):2731–2739
Tsiknia M, Paranychianakis NV, Varouchakis EA, Nikolaidis NP (2015) Environmental drivers of the distribution of nitrogen functional genes at a watershed scale. Fems Microbiol Ecol 91 (6):fiv052
Wankel SD, Mosier AC, Hansel CM, Paytan A, Francis CA (2011) Spatial variability in nitrification rates and ammonia-oxidizing microbial communities in the agriculturally impacted Elkhorn Slough estuary, California. Appl Environ Microbiol 77(1):269–280
Zhang JH, Yu LX, Yao QZ, Tian L (2014) Mixing behavior of nutrients in different seasons at Liaohe Estuary. Environ Sci 35(2):569–576 (Chinese)
Zhang Y, Chen L, Sun R, Dai T, Tian J, Wen D (2015) Ammonia-oxidizing bacteria and archaea in wastewater treatment plant sludge and nearby coastal sediment in an industrial area in China. Appl Microbiol Biotechnol 99(10):4495–4507
Zhao D, Zeng J, Wan W, Liang H, Huang R, Wu QL (2013) Vertical distribution of ammonia-oxidizing archaea and bacteria in sediments of a Eutrophic Lake. Curr Microbiol 67(3):327–332
Zheng Y, Hou L, Min L, Min L, Hui Z, Yin G, Zhou J (2013) Diversity, abundance, and activity of ammonia-oxidizing bacteria and archaea in Chongming eastern intertidal sediments. Appl Microbiol Biotechnol 97(18):8351–8363
Zheng Y, Hou L, Newell S, Liu M, Zhou J, Zhao H, You L, Cheng X (2014) Community dynamics and activity of ammonia-oxidizing prokaryotes in intertidal sediments of the Yangtze estuary. Appl Environ Microbiol 80(1):408–419
Acknowledgements
We thank Hongjun Li and Chunxin Zhang for collecting the sediment samples and physiochemical data during the spring cruise. This work was supported by the National Natural Science Foundation (Grant 41676115), the National Key Research Program (Grant 2016YFA0601400), and the State Oceanic Administration (Grant GASI-03-01-02-05) of China. This study is a contribution to the international IMBER project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, Y., Fan, J., Su, J. et al. Spatial Abundance, Diversity, and Activity of Ammonia-Oxidizing Bacteria in Coastal Sediments of the Liaohe Estuary. Curr Microbiol 74, 632–640 (2017). https://doi.org/10.1007/s00284-017-1226-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-017-1226-x