Abstract
Under the increasing pressure of human activities, Hangzhou Bay has become one of the most seriously polluted waters along China’s coast. Considering the excessive inorganic nitrogen detected in the bay, in this study, the impact of an effluent from a coastal industrial park on ammonia-oxidizing microorganisms (AOMs) of the receiving area was interpreted for the first time by molecular technologies. Revealed by real-time PCR, the ratio of archaeal amoA/bacterial amoA ranged from 5.68 × 10−6 to 4.79 × 10−5 in the activated sludge from two wastewater treatment plants (WWTPs) and 0.54–3.44 in the sediments from the effluent receiving coastal area. Analyzed by clone and pyrosequencing libraries, genus Nitrosomonas was the predominant ammonia-oxidizing bacteria (AOB), but no ammonia-oxidizing archaea (AOA) was abundant enough for sequencing in the activated sludge from the WWTPs; genus Nitrosomonas and Nitrosopumilus were the dominant AOB and AOA, respectively, in the coastal sediments. The different abundance of AOA but similar structure of AOB between the WWTPs and nearby coastal area probably indicated an anthropogenic impact on the microbial ecology in Hangzhou Bay.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen, a prevalent pollution in aquatic environments, can be eliminated through denitrification following nitrification, in which ammonia oxidation is the first and rate-limiting step. For a long time, ammonia-oxidizing bacteria (AOB) were considered as the only group responsible for the biological oxidation of ammonia in both natural and artificial environments. Since a marine Thaumarchaeota, isolated from the rocky substratum of a tropical marine aquarium tank, was found to possess the ability of oxidizing ammonia into nitrite (Konneke et al. 2005), the ammonia oxidizers had extended to archaea.
By detecting the α subunit gene of ammonia monooxygenase (amoA), the widespread distribution of ammonia-oxidizing archaea (AOA) has been confirmed in various natural environments, i.e., soils (Bates et al. 2011), oceans (Bouskill et al. 2012), hot springs (Hatzenpichler et al. 2008), lakes (Hu et al. 2010), and rivers (Liu et al. 2013). Different from natural aquatic environments, AOA was discovered at low frequency in wastewater treatment plants (WWTPs) (Mussmann et al. 2011; Park et al. 2006; Wu et al. 2013; Zhang et al. 2009; Zhang et al. 2011). For instance, AOA amoA gene was only detected in 14 out of 52 WWTPs by real-time PCR in Mussmann et al.’s research. In Park et al.’s research, AOA was found to occur in five out of nine WWTPs by PCR and clone library. In four pilot-scale wastewater treatment reactors (Gomez-Silvan et al. 2010), AOA amoA gene was not detected in any of the 23 tested activated sludge samples by PCR. Focusing on the relative abundance, AOA overwhelmed AOB in some natural aquatic ecosystems (Beman et al. 2010; Mincer et al. 2007; Newell et al. 2011; Santoro et al. 2010), while AOB overtook AOA in some others (Christman et al. 2011; Santoro et al. 2008). In the majority of AOA reported WWTPs (Jin et al. 2010; Limpiyakorn et al. 2011; Wells et al. 2009; Yapsakli 2010), the abundance of AOB amoA gene was 2–4 orders of magnitude higher than that of AOA. Only in several municipal WWTPs reported by Kayee (Kayee et al. 2011) and Bai (Bai et al. 2012b), AOA outnumbered AOB in the activated sludge.
Based on the published amoA gene sequences, Pester et al. (2012) provided evidence for the diversification of AOA into five major clusters, Nitrosopumilis cluster, Nitrososphaera cluster, Nitrosocaldus cluster, Nitrosotalea cluster, and Nitrososphaera sister cluster. Later, Cao et al. (2013) also summarized the global ecological pattern of AOA based on amoA phylogeny; however, the non-natural environments like WWTPs were not included (Cao et al. 2013). Previous investigation indicated that AOA were mainly Nitrosophaera in WWTPs (Gao et al. 2013; Kayee et al. 2011; Limpiyakorn et al. 2011; Sonthiphand and Limpiyakorn 2011) and Nitrosopumilis cluster in marine environments (Jin et al. 2011; Li et al. 2013; Santoro et al. 2008; Zheng et al. 2013).
The discovered AOB are classified into two groups in the taxonomy, Betaproteobacteria and Gammaproteobacteria. In marine sediment, Gammaproteobacteria are considered as the minority of ammonia-oxidizing communities (Nold et al. 2000). Nitrosospira and Nitrosomonas, two genera in Betaproteobacteria, compose the majority of AOB in natural aquatic environments. In WWTPs, the high pollution aquatic environments, Nitrosomonas was found to be the ascendant AOB in most previous studies (Cao et al. 2011; Gao et al. 2013; Kayee et al. 2011; Limpiyakorn et al. 2011; Wang et al. 2012; Zhang et al. 2011).
The ammonia-oxidizing microorganisms (AOMs) between artificial WWTPs and natural aquatic environments exhibit putative differences in the abundance and predominant genera of AOA and AOB. In a natural environment being contaminated by WWTPs’ effluent, some pollutants might homologize the AOM community structure of the environment with that of the WWTPs, because pollution could alter the environmental factors and further the ecological niche of AOM. From previous research studies, salinity (Caffrey et al. 2007), dissolved oxygen (Bouskill et al. 2012; Molina et al. 2010), and ammonia (Ando et al. 2009; Wuchter et al. 2006) have been preliminarily suspected to affect the composition of AOM in aquatic environments, though the influences are still not clarified. Ratio of AOA amoA gene copies to AOB amoA gene copies and Nitrosomonas genus were put forward by Sims et al. (2012) and Cao et al. (2012), respectively, as a potential biological indicator for the contamination of natural aquatic environments.
Hangzhou Bay, surrounded by many industrial aggregations, is located in the estuary of Qiantang River and the south adjacent sea area of the Yangtze River. Large amount of industrial effluents are discharged into the bay; consequently, it has become one of the most severely polluted waters along China’s coast. As indicated by 2011 Marine Environment Quality Bulletin of Zhejiang Province, the bay was placed in the seawater quality level of inferior to class IV, the worst level for seawater quality according to the National Seawater Quality Standard (GB3097-1997). Inorganic nitrogen (up to 4 mg L−1) was a primary pollutant in Hangzhou Bay, which might influence the AOM community but lacks evidence up to now.
In this study, the Shangyu Industrial Area (SYIA), a fine chemical industrial park located on the south bank of Hangzhou Bay, was selected as our research object. The SYIA consists of over 100 enterprises, mainly producing pharmaceuticals, dyes, and amine compound. Industrial wastewaters were treated preliminarily by enterprise-owned WWTPs and followed by a SYIA-owned WWTP. The final effluent is discharged to Hangzhou Bay.
The effect of wastewater disposal on the bacterial and archaeal community of the coastal sediment of Hangzhou Bay was not distinct, though a correlation between effluent connection and bacterial communities was found (Zhang et al. 2014). AOM, the key role in nitrogen cycle, may be a more evident bioindicator for the contamination of inorganic nitrogen from wastewater disposal.
For the purpose of exploring the impact of wastewater disposal on the AOM community of the effluent receiving area, we investigated the occurrence, abundance, and compositions of bacterial and archaeal amoA genes in the samples of WWTPs’ activated sludge and coastal sediments by several molecular technologies. The results of our study would improve the knowledge of the AOM communities in industrial WWTPs and nearby coastal environment.
Materials and methods
Activated sludge and sediment sampling
About 50 g of activated sludge were sampled from the aerobic biological units of the WWTP (named as GB) in an antibiotic production factory and the WWTP (named as SY) for the whole SYIA. About 500-g surface sediment was collected by a surface sediment sampler (Van Veen, HYDRO-BIOS, Germany) at the effluent discharged site (named as HZW) of 30° 12.890′ N, 120° 51.550′ E in Hangzhou Bay.
The location of the SYIA and the two WWTPs, the characteristics of the wastewaters, and the seawater qualities were described in our previous article (Zhang et al. 2014). The samplings were conducted in winter (February 24) and summer (August 24) in 2012. Each sample was labeled as “sampling site-sampling month,” e.g., SY-August. The activated sludge and sediment samples were transported from field to our lab under 4 °C within 24 h, and stored at −70 °C until use.
DNA extraction and clone library
Total DNA was extracted from 0.5 g each sample using a PowerSoil DNA isolation kit (Mo Bio, Carlsbad, CA) according to the manual and stored at −20 °C for further application.
AOA and AOB amoA genes of the February samples were amplified to construct clone libraries. The amplifications were operated with primer pairs and thermal programs as listed in Table 1. The clone libraries were constructed as described previously (Bai et al. 2012b). Briefly, the target DNA fragments in the PCR products was firstly separated by agarose (0.8 %) gel electrophoresis and subsequently purified with a QIAquick® gel extraction kit (Qiagen, Hilden, Germany). The purified gene fragments were cloned into pGEM-T Easy vectors (Promega, Madison, WI) and then transformed into competent Escherichia coli JM109 (Promega, Madison, WI). By blue-white screening, positive clones were randomly selected and sequenced by an ABI 3730xl DNA Analyzer (Applied Biosystems, Carlsbad, CA). The rarefaction and diversity statistics were calculated by Mothur (Schloss et al. 2009). The sequences were clustered into different operational taxonomic units (OTUs) with 95 and 97 % of sequence identity threshold, respectively, for archeal (Beman et al. 2008; Mosier and Francis 2008) and bacterial (Gao et al. 2013; Wang et al. 2012) amoA genes. The representative OTU sequences were blasted against the National Center for Biotechnology Information (NCBI) database to obtain the closest published sequences. The sequences were assembled to construct phylogenetic trees applying the neighbor-joining method with the MEGA 5.2 software (Kumar et al. 2008). The representative sequences constructed on the tree were deposited in the GenBank under accession numbers of KF704372 to KF704381, KF704384 to KF704386, and KF704388 to KF704390.
Pyrosequencing
The amoA genes of August samples were amplified using barcoded primers to construct libraries through high-throughput pyrosequencing. The primers and thermal programs are listed in Table 1. Pyrosequencing was performed as described previously (Bai et al. 2012a). Briefly, PCR products were purified with an AxyPrep DNA Gel Extraction Kit (Axygen, USA). The amplicon libraries were generated by emulsion PCR according to the recommendation of 454 Life Sciences. The sequencing was performed on a Roche Genome Sequencer GS-FLX using the 454/Roche B sequencing primer kit according to the protocol. After the pyrosequencing flowgrams were converted to sequence reads without assembling by Mothur software, the sequence reads were initially trimmed to remove the barcodes, then filtered and denoised to eliminate ambiguous and low-quality reads, i.e., the reads with average sequence quality <25, ambiguous base >0, or sequence lengths <440 bp. The putative chimeras were detected and excluded with UCHIME. The remained sequences were clustered into OTUs using Mothur software with 95 and 97 % of sequence identity threshold for archeal (Beman et al. 2008; Mosier and Francis 2008) and bacterial (Cao et al. 2013; Wang et al. 2012) amoA genes, respectively. Rarefaction and diversity statistics were calculated for each sample after OTUs were clustered. To avoid the bias of libraries’ alpha diversity comparison caused by the great disparity in sequence numbers between clone libraries and pyrosequencing libraries, a hundred sequences in each pyrosequencing library were subsampled randomly by Mothur and calculated alpha diversity indexes. All the trimmed 454 sequences from this study are archived at the NCBI Sequence Read Archive (SRA) under accession SRP030141.
Real-time PCR
Real-time PCR was performed on a quantitative thermocycler (IQ5, Bio-Rad, CA) based on SYBR Green I method. The amoA genes and 16S ribosomal RNA (rRNA) genes were amplified using primers and thermal programs as listed in Table 1. The 20-μL reaction mixture consisted of 0.4 μL of primers, 10 μL of SYBRs Premix Ex Taq™ (Takara, Dalian, China), and 1.0 μL of template DNA. Standard curves were constructed as described previously (Bai et al. 2012b). The amplified efficiencies of real-time PCR ranged from 99.4 to 102.2 %, and the correlation coefficients (R 2) were all >0.99. After real-time PCR detection, gene copy in the 1 μL of template DNA were determined, while the total gene copy extracted from the sample were obtained by multiplying the gene copy in template DNA and the total DNA volume acquired by extraction. The gene abundance was calculated by dividing total gene copies extracted from the sample by the weight of the sample used for DNA extraction.
Results
Gene abundance
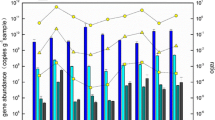
The abundance of archaeal and bacterial amoA genes as well as 16S rRNA genes measured by qPCR are shown in Fig. 1. Based on ANOVA analysis by SPSS 17.0, significant changes were found between the two sampling seasons for the abundance of AOA and AOB amoA gene in SY, the abundance of archaeal 16S rRNA and AOB amoA gene in GB, and the abundance of archaeal 16S rRNA and AOB amoA gene in HZW. The abundance of archaeal and bacterial 16S rRNA genes in sludge samples were much higher than that in sediments.
The WWTPs, SY and GB, possessed abundant AOB amoA ranged in 2.19 × 108–2.96 × 109 gene copies per gram of activated sludge but much less AOA amoA ranged in 9.15 × 103–1.68 × 104 gene copies per gram of activated sludge. The ratio of AOA amoA/AOB amoA ranged from 5.68 × 10−6 to 4.79 × 10−5, revealing that AOB was the predominant AOM in the activated sludge. The ratio of AOB amoA/bacterial 16S rRNA was 2–6 orders of magnitude higher than the ratio of AOA amoA/archaeal 16S rRNA, indicating larger proportion of bacteria, not archaea, in the sludge might participate in ammonia oxidation. In the sediment of HZW, the abundance of AOB amoA was 8.73 × 105 gene copies per gram in winter and 4.92 × 106 gene copies per gram in summer, and the abundance of AOA amoA gene shifted from 4.71 × 105 gene copies per gram in February to 1.69 × 107 gene copies per gram in August. The ratio of AOB amoA/bacterial 16S rRNA of the sediment samples (2.6 × 10−4 in HZW-February and 1.8 × 10−3 in HZW-August) were close to that in the sludge samples (6.3 × 10−4–2.7 × 10−2), while the ratio of AOA amoA/archaeal 16S rRNA of the sediment samples (6.9 × 10−3 in HZW-February and 1.3 × 10−1 in HZW-August) were much higher than that in the sludge samples (2.9 × 10−8–1.7 × 10−5). The ratio of AOA amoA/AOB amoA at HZW was 0.54 in winter and 3.44 in summer, revealing the close abundance of AOA and AOB in the sediments in the effluent receiving area in Hangzhou Bay.
Diversity of AOA
Owning to the extremely low abundance of AOA amoA gene, the amplifications of the gene from all sludge samples failed. Two libraries of AOA amoA gene from sediment samples were constructed. The coverage, diversity, and richness indexes of AOA amoA gene libraries are listed in Table 2. The Good’s coverage indicates that both libraries could well reflect the diversity of archaeal amoA gene. Six operational taxonomic units (OTUs) were detected in the winter sediment by clone library analysis, and 23 OTUs were determined in the summer sediment by 454 high-throughput pyrosequencing. The Chao1 and Shannon indexes revealed a lower richness and diversity of AOA in HZW-August than in HZW-February. The constitutions of OTUs in the two AOA libraries are shown in Fig. 2. In each library, there appeared one superdominant OTU, i.e., OTU-C3 accounted for 69.32 % of the total sequences in the winter sample and OTU-1 accounted for 96.83 % of the total sequences in the summer sample.
Distribution and relative abundance of AOA amoA gene OTUs: a HZW-February and b HZW-August. In the pyrosequencing library of HZW-August, the OTUs with relative abundance of smaller than 0.5 % were classified as others. OTU-* and OTU-C* were the OTUs from pyrosequencing and clone libraries, respectively
A phylogenetic tree was built based on the OTUs of AOA amoA gene, as shown in Fig. 3a. The OTUs were classified into two clusters, Nitrosopumilus with 10 OTUs and Nitrosophaera with 1 OTU. The Nitrosopumilus cluster was further divided into three subclusters. The relative abundance of the clusters in the two seasons was analyzed in Fig. 4. Nitrosopumilus cluster predominated in both winter and summer sediments, accounting for 95.45 and 99.18 % in the total OTUs, respectively, and Nitrosophaera cluster only appeared in the February sediment with a small abundance of 4.55 %.
Phylogenetic tree constructed for a partial AOA amoA OTUs’ sequences and b partial AOB amoA OTU sequences. The numbers (only those >50 % are shown) on the branch nodes indicate the percentages of bootstrap support for the clades based on 1000 bootstrap resamplings. Numbers in the brackets are the GenBank accession numbers of the strains in the NCBI. OTU-* and OTU-C* were the OTUs from pyrosequencing and clone libraries, respectively
Diversity of AOB
A total of six libraries of AOB amoA gene were constructed from all the six samples, including three clone libraries and three pyrosequencing libraries. The coverage, diversity, and richness indexes of all AOB amoA gene libraries are listed in Table 3. All libraries covered the majority of bacterial amoA gene diversity, as Good’s coverage ranged from 98.1 to 100 %. In winter samples, 1, 6, and 6 OTUs were detected at SY, GB, and HZW, respectively. When applying 454 high-throughput pyrosequencing to the summer samples, 26, 27, and 13 OTUs were detected at SY, GB, and HZW, respectively. Through comparison of the diversity index of clone libraries and the100 subsamples from pyrosequencing libraries, SY-August was considered to possess higher diversity than SY-February; the diversity of GB-August was slightly lower than that of GB-February; and HZW-August exhibited similar diversity with HZW-February.
A total of 10 OTUs were drawn from the three AOB clone libraries (Fig. 5a), and 35 OTUs were obtained from the three pyrosequencing libraries. The main OTUs from pyrosequencing libraries are shown in Fig. 5b. In the clone libraries, the only OTU in the sample SY-February, OTU-C1, distributed in all three winter samples and accounted for 52.83 and 75.45 % of total sequences in GB-February and HZW-February, respectively. In the pyrosequencing libraries, 12 OTUs were shared by all three libraries, the all-shared OTUs accounted for 46.15, 44.44, and 92.31 % of total OTUs, and 97.20, 98.69, and 99.96 % of total sequences in SY-August, GB-August, and HZW-August, respectively. One common OTU in particular, OTU-1, accounted for the largest proportion of total sequences in all samples, as 87.34 % in SY-August, 76.49 % in GB-August, and 74.64 % in HZW-August.
A phylogenetic tree was built based on the OTUs of AOB amoA gene (Fig. 3b). As a majority of the OTUs in the pyrosequencing libraries accounted for very small proportion in the total sequences, only the OTUs with relative abundance of more than 0.5 % were analyzed. All OTUs were classified into four clusters: Nitrosomonas europaea, Nitrosomonas halophilla, Nitrosomonas nitrosa, and Nitrosomonas-like, possessing 8, 1, 7, and 5 OTUs, respectively. The distribution of phylogenetic AOB clusters was further analyzed, as shown in Fig. 6. N. nitrosa cluster appeared in all six samples and was the most predominant AOB group in all samples. N. europaea cluster occurred in five samples and was the second abundant group in the samples of GB-February, GB-August, and HZW-August. Nitrosomonas-like cluster was only detected in the three August samples. Regarding the different sampling sites, the phylogenetic distribution of AOB was similar between winter and summer at GB, e.g., N. nitrosa and N. europaea accounted for 52.83 and 47.17 % in winter, while 82.31 and 15.20 % in summer. At SY, more AOB clusters were detected in summer. At HZW, the dominant N. nitrosa cluster maintained a large proportion of 89.10 % in winter and 79.45 % in summer, N. halophilla only occurred in winter at considerable abundance (7.27 %), and N. europaea cluster presented 3.64 % in winter and 19.85 % in summer. Focusing on the three libraries of summer samples, the all-shared 12 OTUs were identified into three clusters (shown in Table 4), in which N. nitrosa was the most abundant cluster, accounting for 91.65, 81.93, and 79.45 % of total sequences in SY-August, GB-August, and HZW-August, respectively.
Discussion
Gene abundance
The wastewater quality varied between the two WWTPs and fluctuated between two samplings in the same WWTP. The Spearman correlation between the wastewater characteristics and amoA gene abundance of AOA and AOB was analyzed using SPSS 17.0, but no significant correlation was discovered (data not shown). However, lower abundance of AOA detected in the WWTPs (Fig. 1) in this study was consistent with some previous studies with NH4 +-N of the wastewaters ranged from 14.0 to 422.3 mg/L (Limpiyakorn et al. 2011; Mussmann et al. 2011; Ozdemir et al. 2011; Park et al. 2006; Wells et al. 2009; Zhang et al. 2011). The influent NH4 +-N of both WWTPs in this study ranged from 4.7 to 158.1 mg/L (Zhang et al. 2014). Relatively high abundance of AOA in sludge samples was also reported (Kayee et al. 2011; Limpiyakorn et al. 2011; Mussmann et al. 2011), where the WWTPs’ influent NH4 +-N was in the range of 5.4–38.6 mg/L. Both pure culture-based and environmental studies indicated that at least some AOA have a high substrate affinity for ammonia and are able to grow under extremely oligotrophic conditions (Schleper and Nicol 2010). The kinetic study of the first isolated AOA strain, N. maritimus SCM1, obtained the lowest half-saturation constant (K m = 133 nM) and the minimum ammonia concentration (less than 20 nM), which was over 100-fold lower than that required by AOB (Martens-Habbena et al. 2009). Therefore, AOA may prefer to live in environments with low ammonia concentration. The high ammonia concentration may be the reason for the low abundance of AOA in the wastewater environment.
The much higher ratio of AOA amoA/archaeal 16S rRNA of the sediment samples than that in the sludge samples (Fig. 1) revealed larger proportion of archaea in the estuarine sediment than in the activated sludge could take part in ammonia oxidation. Focusing on AOA amoA/AOB amoA, the ratio in HZW (0.54 in winter and 3.44 in summer) was almost 5 orders of magnitude higher than that in WWTPs. In a subterranean estuary, Huntington Beach, log ratio of AOB to AOA was −1.0–1.5 (Santoro et al. 2008); in the low salinity area of San Francisco Bay, log ratio of AOA to AOB was 0.5–2.0 (Mosier and Francis 2008); in the Pearl River Estuary, AOA amoA gene copy numbers (9.6 × 106–5.1 × 107 copies per gram of sediment) overwhelmed AOB amoA gene copy numbers (9.5 × 104–6.2 × 105 copies per gram of sediment) (Jin et al. 2011); in Douro estuary, the sediments possessed AOB amoA/AOA amoA of 1–39, and the AOB abundance slightly exceeded the AOA’s (Magalhaes et al. 2009). In this study, the ratio of AOA/AOB in the effluent receiving area was similar with that in other estuaries, but much different from WWTPs, though the seawater had been contaminated.
Diversity of AOM
As shown in Fig. 3a, the OTUs classified in Nitrosopumilus cluster were similar to the AOA sequences from ocean (Francis et al. 2005), estuaries (Jin et al. 2011; Li et al. 2013; Santoro et al. 2008; Zheng et al. 2013), and a biofilter treating artificial seawater (Brown et al. 2013), while the only OTU classified in Nitrosophaera cluster has been detected with high abundance in WWTPs in previous studies (Gao et al. 2013; Kayee et al. 2011; Limpiyakorn et al. 2011; Sonthiphand and Limpiyakorn 2011). In a study on eight WWTPs (Gao et al. 2013), for example, 18 out of 19 AOA amoA OTUs were affiliated to Nitrososphaera cluster, only one OTU (two sequences) was in Nitrosopumilus cluster. It seems that Nitrososphaera cluster is more likely to predominate in wastewater systems, while Nitrosopumilus cluster prefers to prevail in marine and estuary environments.
In this study, the AOB groups in the WWTPs and the effluent receiving area in Hangzhou Bay were all classified as Nitrosomonas, but another AOB genus Nitrosospira was not detected. The same phenomena were confirmed in many WWTPs. Gao et al. (2013) found all AOB classified in Nitrosomonas lineage in eight full-scale WWTPs, and Nitrosospira linage was not detected. In another group of eight WWTPs treating municipal wastewater (Kayee et al. 2011), N. oligotropha cluster dominated in seven WWTPs, N. communis cluster dominated in the last WWTP, and AOB of Nitrosospira cluster was not detected. In a study on six full-scaled wastewater treatment bioreactors (Zhang et al. 2011), though a lower abundance of Nitrosospira was detected, Nitrosomonas genus was predominant in all bioreactors. The similar results were also reported from a municipal WWTP in Palo Alto (Newell et al. 2011), seven full-scale WWTPs in Thailand (Limpiyakorn et al. 2011), and a pilot-scale WWTP in China (Wang et al. 2012). It seems that Nitrosomonas genus is more likely to flourish in the aerobic artificial environment for wastewater treatment.
However, the AOB composition in the sediments in Hangzhou Bay was different from that in other sea areas. The AOB amoA sequences in the intertidal sediments of the Yangtze River estuary (Zheng et al. 2013) as well as in the sediments of the Pearl River estuary (Jin et al. 2011) were grouped into two genera, Nitrosospira and Nitrosomonas. Both genera distributed in the San Francisco Bay estuary (Mosier and Francis 2008) and the offshore Rovinj, Croatia (Bayer et al. 2008). The amoA gene sequence lineages of Nitrosospira clade contained the sequences mainly from estuarine, coastal, and deep sea, while Nitrosomonas genus could be a potential bioindicator for pollution or land-sourced effluents (freshwater or wastewater) flowing into coastal environments (Cao et al. 2012). As for Hangzhou Bay, Nitrosospira cluster was determined as the dominant AOB group in the Qiantang River (Liu et al. 2013); thus, the low salinity of the industrial effluent receiving area might be a minor reason for the dominance of Nitrosomonas. Moreover, we deduced that the pollution, mainly caused by industrial effluent discharge, should be the key factor that determined the dominance of Nitrosomonas and absence of Nitrosospira in the coastal sediments of Hangzhou Bay.
In conclusion, although the ratio of AOA to AOB in the coastal sediment was 5–6 orders of magnitude different from that in the activated sludge, the homologous AOB between the effluent receiving coastal area and the WWTPs probably indicated an impact on the microbial ecology in Hangzhou Bay by the effluent disposal.
References
Ando Y, Nakagawa T, Takahashi R, Yoshihara K, Tokuyama T (2009) Seasonal changes in abundance of ammonia-oxidizing archaea and ammonia-oxidizing bacteria and their nitrification in sand of an eelgrass zone. Microbes Environ 24(1):21–27
Bai Y, Shi Q, Wen D, Li Z, Jefferson WA, Feng C, Tang X (2012a) Bacterial communities in the sediments of Dianchi Lake, a partitioned eutrophic waterbody in China. PLoS ONE 7(5):e37796
Bai Y, Sun Q, Wen D, Tang X (2012b) Abundance of ammonia-oxidizing bacteria and archaea in industrial and domestic wastewater treatment systems. FEMS Microbiol Ecol 80(2):323–330
Bates ST, Berg-Lyons D, Caporaso JG, Walters WA, Knight R, Fierer N (2011) Examining the global distribution of dominant archaeal populations in soil. ISME J 5(5):908–917
Bayer K, Schmitt S, Hentschel U (2008) Physiology, phylogeny and in situ evidence for bacterial and archaeal nitrifiers in the marine sponge Aplysina aerophoba. Environ Microbiol 10(11):2942–2955
Beman JM, Popp BN, Francis CA (2008) Molecular and biogeochemical evidence for ammonia oxidation by marine Crenarchaeota in the Gulf of California. ISME J 2(4):429–441
Beman JM, Sachdeva R, Fuhrman JA (2010) Population ecology of nitrifying archaea and bacteria in the Southern California Bight. Environ Microbiol 12(5):1282–1292
Bouskill NJ, Eveillard D, Chien D, Jayakumar A, Ward BB (2012) Environmental factors determining ammonia-oxidizing organism distribution and diversity in marine environments. Environ Microbiol 14(3):714–729
Brown MN, Briones A, Diana J, Raskin L (2013) Ammonia-oxidizing archaea and nitrite-oxidizing nitrospiras in the biofilter of a shrimp recirculating aquaculture system. FEMS Microbiol Ecol 83(1):17–25
Caffrey JM, Bano N, Kalanetra K, Hollibaugh JT (2007) Ammonia oxidation and ammonia-oxidizing bacteria and archaea from estuaries with differing histories of hypoxia. ISME J 1(7):660–662
Cao H, Auguet J-C, Gu J-D (2013) Global ecological pattern of ammonia-oxidizing archaea. PLoS ONE 8(2):e52853
Cao H, Hong Y, Li M, Gu J-D (2012) Community shift of ammonia-oxidizing bacteria along an anthropogenic pollution gradient from the pearl river delta to the South China Sea. Appl Microbiol Biotechnol 94(1):247–259
Cao H, Li M, Hong Y, Gu J-D (2011) Diversity and abundance of ammonia-oxidizing archaea and bacteria in polluted mangrove sediment. Syst Appl Microbiol 34(7):513–523
Chen X-P, Zhu Y-G, Xia Y, Shen J-P, He J-Z (2008) Ammonia-oxidizing archaea: important players in paddy rhizosphere soil? Environ Microbiol 10(8):1978–1987
Christman GD, Cottrell MT, Popp BN, Gier E, Kirchman DL (2011) Abundance, diversity, and activity of ammonia-oxidizing prokaryotes in the coastal Arctic Ocean in summer and winter. Appl Environ Microbiol 77(6):2026–2034
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci U S A 102(41):14683–14688
Gao J-F, Luo X, Wu G-X, Li T, Peng Y-Z (2013) Quantitative analyses of the composition and abundance of ammonia-oxidizing archaea and ammonia-oxidizing bacteria in eight full-scale biological wastewater treatment plants. Bioresour Technol 138:285–296
Gomez-Silvan C, Molina-Munoz M, Poyatos JM, Ramos A, Hontoria E, Rodelas B, Gonzalez-Lopez J (2010) Structure of archaeal communities in membrane-bioreactor and submerged-biofilter wastewater treatment plants. Bioresour Technol 101(7):2096–2105
Hatzenpichler R, Lebedeva EV, Spieck E, Stoecker K, Richter A, Daims H, Wagner M (2008) A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc Natl Acad Sci U S A 105(6):2134–2139
He S, Gall DL, McMahon KD (2007) “Candidatus Accumulibacter” population structure in enhanced biological phosphorus removal Sludges as revealed by polyphosphate kinase genes. Appl Environ Microbiol 73(18):5865–5874
Hu A, Yao T, Jiao N, Liu Y, Yang Z, Liu X (2010) Community structures of ammonia-oxidising archaea and bacteria in high-altitude lakes on the Tibetan Plateau. Freshw Biol 55(11):2375–2390
Jin T, Zhang T, Yan Q (2010) Characterization and quantification of ammonia-oxidizing archaea (AOA) and bacteria (AOB) in a nitrogen-removing reactor using T-RFLP and qPCR. Appl Microbiol Biotechnol 87(3):1167–1176
Jin T, Zhang T, Ye L, Lee OO, Wong YH, Qian PY (2011) Diversity and quantity of ammonia-oxidizing archaea and bacteria in sediment of the Pearl River Estuary, China. Appl Microbiol Biotechnol 90(3):1137–1145
Kayee P, Sonthiphand P, Rongsayamanont C, Limpiyakorn T (2011) Archaeal amoA Genes outnumber bacterial amoA genes in municipal wastewater treatment plants in Bangkok. Microb Ecol 62(4):776–788
Konneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437(7058):543–546
Kumar S, Nei M, Dudley J, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9(4):299–306
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442(7104):806–809
Li Z, Jin W, Liang Z, Yue Y, Lv J (2013) Abundance and diversity of ammonia-oxidizing archaea in response to various habitats in Pearl River Delta of China, a subtropical maritime zone. J Environ Sci (China) 25(6):1195–1205
Limpiyakorn T, Sonthiphand P, Rongsayamanont C, Polprasert C (2011) Abundance of amoA genes of ammonia-oxidizing archaea and bacteria in activated sludge of full-scale wastewater treatment plants. Bioresour Technol 102(4):3694–3701
Liu S, Shen L, Lou L, Tian G, Zheng P, Hu B (2013) Spatial distribution and factors shaping the niche segregation of ammonia-oxidizing microorganisms in the Qiantang River, China. Appl Environ Microbiol 79(13):4065–4071
Magalhaes CM, Machado A, Bordalo AA (2009) Temporal variability in the abundance of ammonia-oxidizing bacteria vs. archaea in sandy sediments of the Douro River estuary, Portugal. Aquat Microb Ecol 56(1):13–23
Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA (2009) Ammonia oxidation kinetics determine niche separation of nitrifying archaea and bacteria. Nature 461(7266):976–981
Mincer TJ, Church MJ, Taylor LT, Preston C, Kar DM, DeLong EF (2007) Quantitative distribution of presumptive archaeal and bacterial nitrifiers in Monterey Bay and the North Pacific Subtropical Gyre. Environ Microbiol 9(5):1162–1175
Molina V, Belmar L, Ulloa O (2010) High diversity of ammonia-oxidizing archaea in permanent and seasonal oxygen-deficient waters of the eastern South Pacific. Environ Microbiol 12(9):2450–2465
Mosier AC, Francis CA (2008) Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ Microbiol 10(11):3002–3016
Mussmann M, Brito I, Pitcher A, Damste JSS, Hatzenpichler R, Richter A, Nielsen JL, Nielsen PH, Mueller A, Daims H, Wagner M, Head IM (2011) Thaumarchaeotes abundant in refinery nitrifying sludges express amoA but are not obligate autotrophic ammonia oxidizers. Proc Natl Acad Sci U S A 108(40):16771–16776
Newell SE, Babbin AR, Jayakumar A, Ward BB (2011) Ammonia oxidation rates and nitrification in the Arabian Sea. Glob Biogeochem Cycles 25(4):Gb4016. doi:10.1029/2010gb003940
Nold SC, Zhou JZ, Devol AH, Tiedje JM (2000) Pacific northwest marine sediments contain ammonia-oxidizing bacteria in the β subdivision of the Proteobacteria. Appl Environ Microbiol 66(10):4532–4535
Ozdemir B, Mertoglu B, Yapsakli K, Aliyazicioglu C, Saatci A, Yenigun O (2011) Investigation of nitrogen converters in membrane bioreactor. J Environ Sci Health A 46(5):500–508
Park H-D, Wells GF, Bae H, Criddle CS, Francis CA (2006) Occurrence of ammonia-oxidizing archaea in wastewater treatment plant bioreactors. Appl Environ Microbiol 72(8):5643–5647
Pester M, Rattei T, Flechl S, Grongroft A, Richter A, Overmann J, Reinhold-Hurek B, Loy A, Wagner M (2012) amoA-based consensus phylogeny of ammonia-oxidizing archaea and deep sequencing of amoA genes from soils of four different geographic regions. Environ Microbiol 14(2):525–539
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63(12):4704–4712
Santoro AE, Casciotti KL, Francis CA (2010) Activity, abundance and diversity of nitrifying archaea and bacteria in the central California current. Environ Microbiol 12(7):1989–2006
Santoro AE, Francis CA, de Sieyes NR, Boehm AB (2008) Shifts in the relative abundance of ammonia-oxidizing bacteria and archaea across physicochemical gradients in a subterranean estuary. Environ Microbiol 10(4):1068–1079
Schleper C, Nicol GW (2010) Ammonia-oxidising archaea—physiology, ecology and evolution. In: Poole RK (ed). Adv Microb Physiol 57:1–41
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541
Sims A, Horton J, Gajaraj S, McIntosh S, Miles RJ, Mueller R, Reed R, Hu Z (2012) Temporal and spatial distributions of ammonia-oxidizing archaea and bacteria and their ratio as an indicator of oligotrophic conditions in natural wetlands. Water Res 46(13):4121–4129
Sonthiphand P, Limpiyakorn T (2011) Change in ammonia-oxidizing microorganisms in enriched nitrifying activated sludge. Appl Environ Microbiol 89(3):843–853
Wang X, Wen X, Xia Y, Hu M, Zhao F, Ding K (2012) Ammonia oxidizing bacteria community dynamics in a pilot-scale wastewater treatment plant. PLoS ONE 7(4) doi:e36272
Wells GF, Park H-D, Yeung C-H, Eggleston B, Francis CA, Criddle CS (2009) Ammonia-oxidizing communities in a highly aerated full-scale activated sludge bioreactor: betaproteobacterial dynamics and low relative abundance of Crenarchaea. Environ Microbiol 11(9):2310–2328
Wu Y-J, Whang L-M, Fukushima T, Chang S-H (2013) Responses of ammonia-oxidizing archaeal and betaproteobacterial populations to wastewater salinity in a full-scale municipal wastewater treatment plant. J Biosci Bioeng 115(4):424–432
Wuchter C, Abbas B, Coolen MJL, Herfort L, van Bleijswijk J, Timmers P, Strous M, Teira E, Herndl GJ, Middelburg JJ, Schouten S, Damste JSS (2006) Archaeal nitrification in the ocean. Proc Natl Acad Sci U S A 103(33):12317–12322
Xia W, Zhang C, Zeng X, Feng Y, Weng J, Lin X, Zhu J, Xiong Z, Xu J, Cai Z, Jia Z (2011) Autotrophic growth of nitrifying community in an agricultural soil. ISME J 5(7):1226–1236
Yapsakli K (2010) Co-occurrence of nitrogen-converting organisms in full-scale treatment plants. J Environ Sci Health A 45(9):1060–1070
Zhang T, Jin T, Yan Q, Shao M, Wells G, Criddle C, Fang HHP (2009) Occurrence of ammonia-oxidizing archaea in activated sludges of a laboratory scale reactor and two wastewater treatment plants. J Appl Microbiol 107(3):970–977
Zhang T, Ye L, Tong AHY, Shao M-F, Lok S (2011) Ammonia-oxidizing archaea and ammonia-oxidizing bacteria in six full-scale wastewater treatment bioreactors. Appl Microbiol Biotechnol 91(4):1215–1225
Zhang Y, Chen L, Sun R, Dai T, Tian J, Liu R, Wen D (2014) Effect of wastewater disposal on the bacterial and archaeal community of sea sediment in an industrial area in China. FEMS Microbiol Ecol 88(2):320–332
Zheng Y, Hou L, Liu M, Lu M, Zhao H, Yin G, Zhou J (2013) Diversity, abundance, and activity of ammonia-oxidizing bacteria and archaea in Chongming eastern intertidal sediments. Appl Microbiol Biotechnol 97(18):8351–8363
Acknowledgments
This study was supported by a General Project (No. 51178002) granted by the Natural Science Foundation of China. We are sincerely grateful to the Zhoushan Environmental Protection Bureau and the Zhoushan Marine Ecological Envionmental Monitoring Station, Zhejiang Province, for providing the background data of Hangzhou Bay. We also thank Zhongyuan Zheng, Jing Zhang, Zhichao Li, Chengfeng Zhang from Peking University, Cong Liu from Tsinghua University, and Yin Zhang from Shanghai Normal University for helping in the sample collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Chen, L., Sun, R. et al. Ammonia-oxidizing bacteria and archaea in wastewater treatment plant sludge and nearby coastal sediment in an industrial area in China. Appl Microbiol Biotechnol 99, 4495–4507 (2015). https://doi.org/10.1007/s00253-014-6352-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6352-9