Abstract
The adherence ability and biofilm production are the characteristic of enhanced virulence among isolates of vancomycin-intermediate Staphylococcus aureus (VISA) strains. Although biofilm-forming properties have been well demonstrated in S. aureus, they still remain unclear among the recently emerged types of VISA strains. The aim of this study was to investigate correlations between the distribution of genes encoding staphylococcal microbial surface components which recognise adhesive matrix molecules (MSCRAMMs), the surface protein A (Spa) types, MLST types and the ability of VISA strains to biofilm formation. Microtiter plate assay (Mtp) results showed that all eleven biofilm producer isolates were adherent at various levels. PCR experiments showed that nine MSCRAMM genes, clfA, clfB, fnbA and fib were detected in all of the strains, indicating a high prevalence. The prevalences of other MSCRAMMs and icaABCD genes were found to be variable and not equally distributed among the VISA strains. There was no direct correlation between the distribution of adhesion-related genes and biofilm formation, which indicates that the presence or absence of these genes cannot be employed as an indicator of the ability to biofilm formation. Isolates which belong to the same Spa and ST types showed similar adherence capacities in the Mtp assay, but significant differences were observed between different Spa types. The findings of this study, using quantitative methods, have shown that genotypically different strains of VISA have different capabilities to produce biofilms. This may be caused by a difference in the spa types of VISA isolates or due to their differences in the expression of MSCRAMM and icaABCD genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus is one of the most important pathogens responsible for both nosocomial and community-acquired infections [11]. These infections, particularly those caused by methicillin-resistant Staphylococcus aureus (MRSA) strains, have been treated primarily with glycopeptide antibiotics, such as vancomycin. Unfortunately, the increased incidence of nosocomial MRSA infections and extensive use of vancomycin have led to the expansion of S. aureus strains with varying grades of resistance to vancomycin [13]. The precise mechanisms which cause reduced susceptibility to vancomycin in S. aureus isolates remain unclear. Several phenotypic, genetic and biochemical alterations can be found in vancomycin-intermediate S. aureus (VISA) strains [15]. The ability to attach to surfaces and biofilm formation is one of the most important virulence factors in S. aureus infection [6]. The development of biofilms by S. aureus is a two-step process involving an initial attachment and a subsequent maturation step. These steps are affected by several physiological factors and require step-specific factors and environmental condition [24]. In the human body, attachment to matrix proteins on the cell surface is a critical step toward S. aureus attachment and biofilm formation [20]. Although biofilm-forming properties have been well demonstrated in S. aureus, they still remain unclear among the recently emergent VISA strains. S. aureus expresses several microbial surface components recognising adhesive matrix molecules (MSCRAMMs) that interact with host extracellular ligands, such as elastin-binding protein (ebpS), laminin-binding protein (eno), collagen-binding protein (cna), fibronectin-binding proteins A and B (finbA, finbB), fibrinogen-binding protein (fib), clumping factors A and B (clfA, clfB) and bone sialoprotein-binding protein (bbp) [2]. Another significant factor required for the formation and maturation of biofilm is the polysaccharide intercellular adhesin (PIA), which is also called poly-N-acetylglucosamine (PNAG) because of its chemical composition [22]. PIA biosynthesis is catalysed by the products of the ica gene locus, which contains an N-acetylglucosamine transferase (icaA and icaD), a PIA deacetylase (icaB), a putative PIA exporter (icaC) and a regulatory gene (icaR) [10, 17, 18]. The role of biofilms in clinical infections has received increasing interest because of the detection and characterisation of genes involved in biofilm formation [8, 9]. In this study, we investigated the prevalence of MSCRAMMs and biofilm-related genes and biofilm formation abilities in VISA strains.
Materials and Methods
Bacterial Isolates
Eleven VISA isolates were chosen from a collection of 415 clinical isolates of S. aureus during 3 years (Aug 2010 to March 2013), from four university hospitals of Tehran. Isolates were confirmed as VISA by vancomycin agar screening test and agar dilution method. The minimum inhibitory concentrations (MICs) to vancomycin for all VISA isolates were between 4 and 8 μg/ml. All isolates were also reevaluated for the presence of the vanA gene by PCR. vanA gene was not found in any of the VISA strains. VISA isolates were stored at −70 °C in tripticase soy broth supplemented by 10 % glycerol before tested.
Biofilm Formation Assay
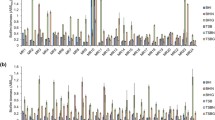
A modified microtiter plate method was followed as previously described [26]. Briefly, the wells of microtiter plate are filled with 180 µl of trypticase soy broth (TSB) containing 1 % glucose. Then, a 20 µl quantity of previously prepared bacterial suspension with turbidity equal to 0.5 Macfarland standards is added to each well. The negative control wells contain 200 µl of TSB supplemented with 1 % glucose. Incubation was carried out at 37 °C for 24 h before removal of the cultures. Then, the cells were decanted, and each well is washed three times with sterile phosphate buffered saline, fixed by methanol for 20 min, dried at room temperature and finally strained with 0.1 % saferanin. The safranin dye bound to the adherent cells was dissolved with 1 mL of 95 % ethanol per well, and the plates were read at 490 nm (A 490) using ELISA reader. Optical density cut-off (ODc) was determined. It is defined as average OD of negative control + 3 × standard deviation (SD) of negative control. Formation of biofilm by isolates was analysed and categorised relying on the absorbance of the safranin-stained attached cells. The data calculation has been shown in Table 1. Biofilm-producing S. aureus ATCC 35556 strains were used for strongly biofilm-producing control, while S. epidermidis ATCC 12228 strains were used to negative control.
Polymerase Chain Reaction (PCR) for icaABCD Genes
VISA isolates were selected to molecular screening for icaABCD genes using polymerase chain reaction which was carried out as previously described [10] [22]. PCR amplification was performed with an Eppendorf thermal cycler (Mastercycler® gradient). The primers, PCR condition and sizes of the expected amplification product for PCR amplification are listed in Table 2.
Multiplex PCR for MSCRAMMs Genes
Nine MSCRAMM genes in different VISA isolates were examined by multiplex PCR method. Five microlitres of template DNA was added to a 50 μL reaction mixture (Amplicon. Denmark) containing 25 mM Tris/HCl, 50 mM KCl, 1.5 mM MgCl2, 0.2 mM each dNTP, all primers at various concentrations and 2.5 U Taq polymerase. Two primer groups were prepared for multiplex PCR: PCR1 to amplify bbp, cna, ebpS and eno and PCR2 to amplify fnbA, fnbB, fib, clfA and clfB [27]. The PCR products were analysed by electrophoresis in a 1.4 % agarose gel and stained with gel red. The primers, PCR condition and sizes of the expected amplification product are listed in Table 2.
Spa Typing
Determination of spa type was performed by PCR with primers forward (5′-AGACGATCCTTCGGTGAGC-3′) and reverse (5′-GCTTTTGCAATGTCATTTACTG-3′), and sequencing of polymorphic X region of spa gene was carried out as previously described [25]. The amplified spa gene fragments were purified and sequenced.
MLST
For all VISA isolates, the ST was determined by MLST. Partial gene sequences of seven housekeeping genes (arcC,aroE, glpF, gmk, pta, tpi and yqiL) were determined by PCR and direct sequencing, and their allelic profile (allele numbers) and ST were obtained using the S. aureus MLST database (http://www.mlst.net) hosted by Imperial College in London, UK [12].
Results and Discussion
Attachment to cell surfaces and inanimate surfaces, such as catheters and prosthetic devices, as well as biofilm formation are the most important processes of pathogenesis in infections caused by staphylococcal species, such as S. aureus and S. epidermidis [21]. Recently, a rapid increase in the populations of S. aureus with reduced susceptibility and resistance to vancomycin has been reported from different parts of the world [3–5, 14]. There are several reports concerning the prevalence of MSCRAMMs and biofilm-related genes from different sources of S. aureus in the literature. The study of the ability of VISA strains to adhere to surfaces and the genetic characteristics concerning MSCRAMMs and biofilm-related genes in VISA strains will improve our understanding of the correlation between reduced susceptibility to vancomycin in S. aureus and the ability of these strains to biofilm formation [2]. In the present study, all of the VISA clinical isolates examined were found to be adherent, although at different levels (Table 3). In this study, microtiter tissue culture plates were chosen to determine the ability of VISA strains to biofilm formation because using this method is easy to differentiate between weakly, moderately and strongly adherent isolates. Howden et al. analysed the biofilm-forming ability of clinical VISA strains isolated from bacteraemic patients and demonstrated reduced biofilm formation compared with vancomycin-susceptible S. aureus (VSSA) strains [16]. In another study, Sakoulas et al. analysed the biofilm-forming ability of laboratory-induced VISA strains and demonstrated enhanced biofilm formation compared with VSSA strains [23]. Our results showed that the ability of VISA strains to biofilm formation is variable because among our VISA strains, 54.5 % of isolates showed strong, 27.3 % showed moderate and 18.2 % showed weak adhesion. In contrast to previous reports, we demonstrated that the ability of biofilm formation by VISA strains is not related to the source of isolation such as laboratory-induced or clinically derived strains. These results may be caused by of the low number of VISA strains obtained in previous studies. Additionally, the significant cell wall and physiological changes occurring in VISA strains could limit or enhance biofilm formation by affecting the initial attachment or subsequent intercellular adhesion.
Several reports have been published about the correlation between phenotypic methods, such as the use of microtiter tissue culture plates and the presence of ica locus and MSCRAMMs genes in S. aureus isolates from different parts of the world [2, 19]. In most of these studies, no correlation was seen between the genetic characterisation and the phenotypic biofilm assay. In a study conducted by Atshan et al. that was similar to our study, there was no direct correlation between morphology and the distribution of MSCRAMM genes [2]. Thus, the disagreement between phenotypic and genotypic characterisation may be due to variation and heterogeneity in the genetic backgrounds. In addition, biofilm formation is affected by a variety of factors such as environmental conditions [1]. The nine MSCRAMMs and four biofilm-related genes were examined in VISA and other strains using a PCR method. The clfaA, clfB, fnbA, icaD and fib genes were detected in all of the mentioned isolates. However, the bbp gene was not detected in any of the VISA strains. Other MSCRAMMs had varying prevalence among the VISA strains: the prevalence of eno and cna was 90.1 %, whereas the prevalences of ebpS, bbp and fnbB in VISA strains were 18.2, 0 and 54.5 %, respectively. The prevalences of icaA, icaB, icaC and icaD in VISA strains were 63.7, 63.7, 90.1 and 100 %, respectively (Table 4). No relationship was observed between the source of bacteria, such as wards and type of specimens and the potential for biofilm formation among VISA strains. No direct correlation between the distribution of adhesin genes and biofilm formation was seen. However, VISA strains with the same frequency of MSCRAMMs and biofilm-related genes give different results in phenotypic tests. All of the examined VISA strains harboured at least four MSCRAMM genes including clfA, clfA, fnbA and fib. According to Howden et al., the variation in the prevalence of MSCRAMM genes depends on the several factors, such as species and source of isolation [15]. There was no direct relationship between the distribution of MSCRAMMs and biofilm-related genes and the ability to biofilm formation, which indicates that a single gene or a subset of biofilm-related genes cannot be employed as an indicator of the ability to biofilm formation.
Spa typing of the 11 isolates revealed six different spa types. Repeats among the spa types varied from 2 (t2467) to 12 (t12925). A dendrogram was constructed for all eleven isolates in order to visualise the relationships among the spa types (Fig. 1). Among the spa types, t030 was the most prevalent and was detected in 4 (36.4 %) strains. In addition, among the six spa types, one new spa type was identified and deposited in the spa database. Isolates that belong to same Spa and ST types showed similar adherence capacity in the Mtp assay, but significant differences were observed between different Spa types. For example, all isolates belonging to spa type t030 were found to form a strongly adherent biofilm, whereas all isolates belonging to spa type t037 were observed to have weak biofilm formation using the microtiter plate method. This is in agreement with the findings of Croes et al., who reported differences in biofilm formation among S. aureus isolates related to the genetic background of protein A (Spa) [7].
In summary, the findings of this study, which used both phenotypic and genotypic methods, demonstrated that genotypically different VISA isolates have different abilities to produce biofilms. This diversity in biofilm characteristics is because of the differences in genetic background, such as Spa type. It has been shown that VISA strains have different capacities to adhere to surfaces and biofilm formation. This may be caused by several factors, such as physiological and structural conditions, and not because of their differences in the presence of MSCRAMMs or biofilm-related genes.
References
Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW (2012) Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 33(26):5967–5982
Atshan SS, Nor Shamsudin M, Sekawi Z, Lung LT, Hamat RA, Karunanidhi A, Mateg Ali A, Ghaznavi-Rad E, Ghasemzadeh-Moghaddam H, Chong Seng JS, Nathan JJ, Pei CP (2012) Prevalence of adhesion and regulation of biofilm-related genes in different clones of Staphylococcus aureus. J Biomed Biotechnol 2012:976972
Azimian A, Asghar Havaei S, Fazeli H, Naderi M, Ghazvini K, Mirab Samiee S, Soleimani M, Najar Peerayeh S (2012) Genetic analysis of a vancomycin-resistant Staphylococcus aureus strain isolated in Iran. J Clin Microbiol 50(11):3581–3585
Azimian A, Havaei SA, Fazeli H, Naderi M, Ghazvini K, Samiee SM, Soleimani M, Peerayeh SN (2012) Genetic characterization of a vancomycin-resistant Staphylococcus aureus isolate from the respiratory tract of a patient in a university hospital in northeastern Iran. J Clin Microbiol 50(11):3581–3585
Butt T, Ahmad RN, Usman M, Mahmood A (2003) Vancomycin-resistance Staphylococcus aureus. J Coll Phys Surg Pak 13(7):428–429
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284(5418):1318–1322
Croes S, Deurenberg RH, Boumans M-LL, Beisser PS, Neef C, Stobberingh EE (2009) Staphylococcus aureus biofilm formation at the physiologic glucose concentration depends on the S. aureus lineage. BMC Microbiol 9(1):229
Cucarella C, Tormo MA, Knecht E, Amorena B, Lasa I, Foster TJ, Penades JR (2002) Expression of the biofilm-associated protein interferes with host protein receptors of Staphylococcus aureus and alters the infective process. Infect Immun 70(6):3180–3186
Cucarella C, Tormo MA, Ubeda C, Trotonda MP, Monzon M, Peris C, Amorena B, Lasa I, Penades JR (2004) Role of biofilm-associated protein bap in the pathogenesis of bovine Staphylococcus aureus. Infect Immun 72(4):2177–2185
Cue D, Lei MG, Luong TT, Kuechenmeister L, Dunman PM, O’Donnell S, Rowe S, O’Gara JP, Lee CY (2009) Rbf promotes biofilm formation by Staphylococcus aureus via repression of icaR, a negative regulator of icaADBC. J Bacteriol 191(20):6363–6373
David MZ, Daum RS (2010) Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 23(3):616–687
Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG (2000) Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol 38(3):1008–1015
Havaei SA, Azimian A, Fazeli H, Naderi M, Ghazvini K, Samiee SM, Masoumi Z, Akbari M (2012) Genetic characterization of methicillin resistant and sensitive, vancomycin intermediate Staphylococcus aureus strains isolated from different Iranian Hospitals. ISRN Microbiol 2012:6
Hiramatsu K (2001) Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect Dis 1(3):147–155
Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML (2010) Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev 23(1):99–139
Howden BP, Johnson PD, Ward PB, Stinear TP, Davies JK (2006) Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 50(9):3039–3047
Jefferson KK, Pier DB, Goldmann DA, Pier GB (2004) The teicoplanin-associated locus regulator (TcaR) and the intercellular adhesin locus regulator (IcaR) are transcriptional inhibitors of the ica locus in Staphylococcus aureus. J Bacteriol 186(8):2449–2456
Kiem S, Oh WS, Peck KR, Lee NY, Lee JY, Song JH, Hwang ES, Kim EC, Cha CY, Choe KW (2004) Phase variation of biofilm formation in Staphylococcus aureus by IS 256 insertion and its impact on the capacity adhering to polyurethane surface. J Korean Med Sci 19(6):779–782
Novick RP (2003) Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48(6):1429–1449
Periasamy S, Joo HS, Duong AC, Bach TH, Tan VY, Chatterjee SS, Cheung GY, Otto M (2012) How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci USA 109(4):1281–1286
Rani SA, Pitts B, Beyenal H, Veluchamy RA, Lewandowski Z, Davison WM, Buckingham-Meyer K, Stewart PS (2007) Spatial patterns of DNA replication, protein synthesis, and oxygen concentration within bacterial biofilms reveal diverse physiological states. J Bacteriol 189(11):4223–4233
Rohde H, Knobloch JK, Horstkotte MA, Mack D (2001) Correlation of Staphylococcus aureus icaADBC genotype and biofilm expression phenotype. J Clin Microbiol 39(12):4595–4596
Sakoulas G, Eliopoulos GM, Moellering RC Jr, Wennersten C, Venkataraman L, Novick RP, Gold HS (2002) Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother 46(5):1492–1502
Seo YS, Lee DY, Rayamahji N, Kang ML, Yoo HS (2008) Biofilm-forming associated genotypic and phenotypic characteristics of Staphylococcus spp. isolated from animals and air. Res Vet Sci 85(3):433–438
Shopsin B, Gomez M, Montgomery S, Smith D, Waddington M, Dodge D, Bost D, Riehman M, Naidich S, Kreiswirth B (1999) Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol 37(11):3556–3563
Stepanovic S, Vukovic D, Hola V, Di Bonaventura G, Djukic S, Cirkovic I, Ruzicka F (2007) Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115(8):891–899
Tristan A, Ying L, Bes M, Etienne J, Vandenesch F, Lina G (2003) Use of multiplex PCR to identify Staphylococcus aureus adhesins involved in human hematogenous infections. J Clin Microbiol 41(9):4465–4467
Acknowledgments
This study was supported by the Tarbiat Modares University and conducted in the Department of Bacteriology, Faculty of Medical Sciences as part of Ph.D thesis.The authors would like to thank Dr. Ehsanollah Ghaznavi-Rad (Arak University of Medical Sciences) for providing control strains.
Disclosure
All authors report no conflicts of interest relevant to this article.
Funding
This work was supported by Grants from Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirzaee, M., Najar-Peerayeh, S., Behmanesh, M. et al. Relationship Between Adhesin Genes and Biofilm Formation in Vancomycin-Intermediate Staphylococcus aureus Clinical Isolates. Curr Microbiol 70, 665–670 (2015). https://doi.org/10.1007/s00284-014-0771-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0771-9