Abstract
Streptococcus equi ssp. zooepidemicus (SEZ) is responsible for a wide variety of infections in many species, including pigs, horses and humans. Biofilm formation is essential for pathogenesis, and the ability to resist antibiotic treatment results in difficult-to-treat and persistent infections. However, the ability of SEZ to form biofilms is unclear. Furthermore, the mechanisms underlying SEZ biofilm formation and their attributes are poorly understood. In this study, scanning electron microscopy (SEM) demonstrated that SEZ strain ATCC35246 formed biofilms comprising a thick, heterogeneous layer with clumps on the coverslips when incubated for 24 h. In addition, we used a two-dimensional gel electrophoresis (2-DE) based approach to characterize differentially expressed protein in SEZ biofilms compared with their planktonic counterparts. The results revealed the existence of 24 protein spots of varying intensities, 13 of which were upregulated and 11 were downregulated in the SEZ biofilm compared with the planktonic controls. Most of proteins expressed during biofilm formation were associated with metabolism, adhesion, and stress conditions. These observations contribute to our understanding of the SEZ biofilm lifestyle, which may lead to more effective measures to control persistent SEZ infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus equi ssp. zooepidemicus (SEZ) is responsible for a wide variety of infections in many species, including pigs, horses and humans [10, 16]. In China, SEZ is the predominant disease causing pathogen in swine. The 1975 pandemic in Sichuan led to the death of 300,000 pigs, resulting in large economic losses [12]. The disease is generally sporadic and outbreaks are usually of short duration; however, in large populations the bacteria may be present for longer periods [5, 24, 27, 30]. Why this bacterium causes persistent infection and disease under certain conditions remains largely unknown.
A biofilm is an aggregate of microorganisms in which cells adhere to each other on a surface to form tower- and mushroom-shaped microcolonies [7]. Formation of biofilms allows microbial pathogens to create a protected environment in which sessile cells are more resistant to antibiotics compared to their planktonic counterparts. Many animal-infecting bacteria, such as Streptococcus suis (SS) [17] and Pseudomonas aeruginosa [1], have the ability to form biofilms, which plays a key role in causing persistent infections. Recent developments in proteomic technologies have paved the way for exploration of the molecular mechanisms of natural phenomena such as biofilm formation.
The ability of SEZ to form biofilms and the relationship between biofilm formation and pathogenesis or persistent SEZ infections is unclear. In this study, the ability of SEZ to form biofilms was investigated, and the differences in the protein expression profiles of SEZ grown either as planktonic cells or biofilms were identified using comparative proteomic analysis, with the aim of gaining a better understanding of SEZ biofilm formation. This study is the first examination of biofilm formation in SEZ.
Materials and Methods
Bacteria and Culture Condition
Bacterial strain SEZ ATCC35246 was originally isolated from an infected pig in Sichuan and was purchased from American Type Culture Center. It was grown at 37 °C in Todd–Hewitt broth (THB).
Microtiter Plate Biofilm Assay
Quantification of biofilm production was carried out by a microtiter plate assay [34] with some modifications. The wells of sterile 96-well flat-bottomed polystyrene microplates (Greiner, Germany) were filled with 100 μl of THB medium containing 1 % fibrinogen and, then, 10 μl of overnight inoculum was added to each well and incubated at 37 °C for 24 h without shaking. Medium alone served as a negative control. The sample was visualized by staining with 1 % crystal violet for 10 min after washing with PBS. After adding 200 μl of 95 % ethanol, A 595 nm was measured with a Tecan GENios Plus microplate reader (Tecan, Austria). All assays were performed in triplicate.
Scanning Electron Microscopy
Overnight cultures of strains were diluted to a final density of 1.0 × 106 CFU ml−1 with fresh THB medium containing 1 % fibrinogen. Scanning electron microscopy (SEM) was performed on biofilms formed on glass coverslips by dispensing 2 ml of cell suspensions into the wells of six-well microtiter plates. Plates were statically incubated at 37 °C for 24 h. The coverslips were then washed with PBS. The cells were fixed with 25 % SEM grade glutaraldehyde in PBS. Fixed samples were then dehydrated through a graded series of ethanol concentrations, mounted, and sputter coated with gold–palladium. Dried samples were adhered to metal holders with double-sided tape and finally coated in an evaporator with gold and palladium. Observations were performed at 5 kV with a scanning electron microscope (model S800; Hitachi).
Preparation of Whole-Cell Proteins
For biofilm cultures, SEZ was grown in THB medium supplemented with 1 % fibrinogen in 100 mm polystyrene petri dishes at 37 °C for 24 h. The supernatant was then removed and the plates were washed twice with 50 mM Tris/HC1 (pH 7.5). Biofilms were detached by scraping, then resuspended in 50 mM Tris–HC1. The cells were pelleted by centrifugation at approximately 8,000×g for 5 min and washed with 50 mM Tris/HC1. SEZ planktonic cell was grown in 500 Erlenmeyer flasks containing 100 ml of the above culture medium at 37 °C for 24 h. Planktonic cells were pelleted and washed as described for the biofilm cultures above. Protein precipitations were performed as described previously by Wang et al. [35]. Protein samples were treated with a 2-D Clean-up Kit (GE Healthcare) to remove contaminants that interfere with electrophoresis. The protein content was determined using the PlusOne 2-D Quant Kit (GE Healthcare).
Two-Dimensional Gel Electrophoresis
Two-dimensional gel electrophoresis (2-DE) was performed using the immobiline/polyacrylamide system. Protein sample (200 μg) was absorbed onto a 13 cm immobilized pH gradient strips and isoelectric focusing (IEF) was performed in a Protein IEF Cell (GE Healthcare) using a stepwise increase of the voltage to 8,000 V. Before electrophoreses in the second dimension, strips were equilibrated for 2 × 15 min in equilibration buffer (6 M urea, 2 % SDS, 30 %glycerol, 0.05 M Tris–HCl, pH 8.8) containing 1 % DTT and 4 % iodoacetamide. SDS-PAGE was carried out vertically in an Ettan DALT II system (GE Healthcare) using 12 % polyacrylamide gels. Resolved proteins were routinely stained with Coomassie Brilliant Blue G-250 for protein identification purposes. Samples were run in triplicate. Gel comparison analysis was conducted using Image Master Platinum 5.0 software (GE Healthcare). An analysis set was created to find the spots with a difference (minimum 2-fold increase or decrease) in the biofilm samples compared with the planktonic samples.
Mass Spectrometry Analysis of Protein Spots and Database Searches
Spots identified as being differentially expressed were excised from the triplicate 2-D gels and sent to Nanjing Ji’ao BioTechnologies Co., Ltd. for tryptic in-gel digestion and matrix-assisted laser desorption ionization time-of-flight time-of-flight-mass spectrometry (MALDI-TOF/TOF-MS) or MALDI-TOF-MS analysis. The peptide mass fingerprinting (PMF) data were analyzed using the MASCOT server (http://www.matrixscience.com/). Protein identification was assigned when the following criteria were met: presence of at least four matching peptides and sequence coverage was >15 %. The protein scores as well as the original PMF data, including extent of sequence coverage, number of peptides matched, and score of probability were used for acceptance of protein identifications.
Verification of Comparative Proteomics Results by Quantitative Real-Time PCR
Total RNA was isolated from SEZ grown as biofilms and planktonic cells for 24 h with using the E.Z.N.A bacterial RNA Kit (Omega, USA) according to the manufacturer’s protocol. cDNAs were generated by reverse transcription using a PrimeScript RT-PCR Kit (TaKaRa).The mRNA levels were measured using a two-step relative qRT-PCR. A specific primer set was used to analyze glmS, citE, gapdh, fabF, and 16s rRNA mRNA content (Table 1). 16s rRNA was used as internal control in qRT-PCR analysis. The relative change in gene expression was recorded as comparative CT (2−ΔΔCT) [23].
Results
Appearance of Biofilm In Vitro
The ability to form biofilms was investigated for SEZ strain ATCC35246 using a crystal violet microtiter plate assay. The results revealed that strain ATCC35246 had the ability to form biofilms in microtiter plates (Fig. 1). The strain showed a strong biofilm with a mean ± SD optical density at 595 nm (OD595) of 1.19 ± 0.05.
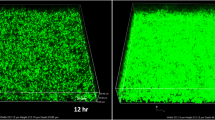
The structure of SEZ biofilms on glass coverslips was examined by SEM. SEM observations revealed that strain ATCC35246 formed a thick, heterogeneous layer with clumps on the coverslips after incubation for 24 h. The aggregates and microcolonies of SEZ were patchy with confluent areas of growth contrasting with cell-free areas (Fig. 2).
Comparative Proteomics
For further characterization of differences in protein expression between the biofilm and the planktonic cells, representative 2-DE of whole-cell protein extracts was performed. The reproducibility of separation of the total proteins was the same in all triplicate gels (data not shown). The majority of proteins were distributed in the pH range of 4–7 (IPG linear gradient) (Fig. 3a, b).
By matching and comparing the 2-DE proteomes, 24 protein spots were identified with a minimum 2-fold change in expression level. These 24 spots corresponded to 22 individual proteins, 13 of which were upregulated and 11 were downregulated in SEZ biofilms. The spots were identified by MALDI-TOF/TOF-MS. These data were compared to those in the NCBI sequence database. The probability score for the match, molecular weight (MW), isoelectric point (pI), number of peptide matches, and the percentage of the total translated ORF sequence covered by the peptides were used as confidence factors in protein identification (Tables 2, 3).
Quantitative Real-Time PCR Detection
For the analysis of gene expression in biofilm and planktonic cells, we followed the same procedure described for bacterial strain and culture condition. Quantitative RT-PCR analysis was performed on four selected genes to confirm the results of comparative proteomics analysis. The qRT-PCR results indicated that SEZ grown as biofilms expressed 2.1-fold higher glmS mRNA and gapdh mRNA (P < 0.01), 0.5-fold lower citE mRNA levels and 0.4-fold lower fabF mRNA levels (P < 0.01) than SEZ grown as planktonic cells (Fig. 4). The qRT-PCR results confirmed the results of comparative proteomic analysis.
Discussion
Biofilm production is considered as a marker of clinically relevant infection. SEM observations revealed very similar architectures of biofilms formed by SEZ. Such biofilm coverage has been already described for biofilms of S. aureus [20] and other organisms grown [8] in vitro. In this study, differences in the whole-cell protein expression of SEZ grown under either biofilm or planktonic conditions were also analyzed. Using the selective capture of transcribed sequences (SCOTS) technique, we also revealed differential gene expression in biofilms compared with planktonic cells (data not shown). Similar studies have shown differential gene expression and protein profiles in biofilm compared to planktonic cells [2, 14, 28, 35], suggesting that biofilm formation is a complex and highly regulated process. It can be speculated that marked differences in protein profiles detected under conditions of biofilm growth are responsible for the physiological changes of cells in SEZ biofilm.
The four upregulated proteins we found encode enzymes of the glycolysis or fermentation pathway. 6-phosphofructokinase (PFK) is a key enzyme in glycolysis [15]. Glutamine: fructose-6-phosphate aminotransferase (GlmS), also known as glucosamine synthase, is the rate-limiting enzyme of the hexosamine biosynthetic pathway [13]. dTDP-glucose-4,6-dehydratase RmlB (rfbB) is responsible for the conversion of dTDP-d-glucose into dTDP-4-keto-6-deoxy-d-glucose (TKDG), which is a key intermediate in most deoxysugar biosynthetic pathway [36]. Glucose-6-phosphate isomerase catalyzes the reversible aldose-ketose isomerization of d-glucose-6-phosphate in glycolysis and gluconeogenesis, and in the recycling of hexose-6-phosphate in the pentose phosphate pathway, which is important in defense against oxidative stress [38]. Previous studies of protein expression in S. mutans [22] and Mycoplasma mycoides subsp. Mycoides [21] biofilms have shown that glycolytic enzymes analogous to the ones identified in this study are highly expressed during early biofilm formation. These results strongly indicate that glycolysis and fermentation pathways in a SEZ biofilm are upregulated to produce the energy required for this process. CTP synthase (pyrG) may be important to metabolic enzyme by catalyzing the conversion of UTP to CTP [25]. Adenylosuccinate synthase (purA) is an important enzyme in purine biosynthesis [26]. The identification of proteins involved in metabolic processes, such as amino acid metabolism, carbon metabolism and cofactor biosynthesis, indicates that metabolism is important for the sessile mode of growth.
The protein most similar to B4 was glyceraldehyde-3-phosphate dehydrogenase GAPDH, which is a streptococcal surface protein that mediates cell adhesion and plays an important role in bacterial infection and invasion [6, 33]. GADPH has also been shown to be upregulated in biofilms of SS2 [35], Pseudomonas aeruginosa [18] and Staphylococus xylosus [19]. The results found in our study support the overall observation that biofilm cells express more factors for binding and sessile growth, which may play a role in early biofilm development [29].
In addition to oxidative stress and oxygen limitation, the bacteria have to cope with nutrient limitation, especially in the deeper layers of the biofilm. Regulation of transcription is needed for the cell to quickly adapt to the ever-changing outer environment and stress tolerance [32]. The stress-related proteins were upregulated expressed only in the biofilm condition, suggesting that the biofilm cells specifically express proteins in different adverse environments. It is of interest that we found the upregulation of Elongation factor-Tu (EF-Tu) protein in biofilm. EF-Tu is a translation elongation factor, responsible for the binding and transport of specific tRNA on the ribosomes. It can also intervene as a molecular chaperone in E. coli by binding to denatured proteins [3]. This factor has also been linked to stress response in M. pulmonis [11]. EF-Tu also exerts other functions. In E. coli, it regulates both transcription and translation of proteins during nutritional deprivation [9]. It has also been reported extracellular localization of EF-Tu can also increase adhesion to human plasminogen [31]. A similar result has been found EF-Tu was upregulated during biofilm formation by S. mutans [4]. In conclusion, most of upregulated proteins in the biofilm growth were associated with metabolism, adhesion, and stress conditions.
Bacteria growing in biofilms are different from those growing in planktonic cells. In this study, Y1–Y11 were identified and significantly downregulated in biofilm cells (Table 3). Most of the downregulated proteins are likely to be involved in protein synthesis or encode membrane proteins/transporters. The results support the hypothesis that general metabolic functions are downregulated in response to a reduction in growth rate during biofilm formation. Similar conclusions were found in S. mutans [37] and SS2 [35].
References
Barbosa MS, Bao SN, Andreotti PF, de Faria FP, Felipe MS, dos Santos Feitosa L, Mendes-Giannini MJ, Soares CM (2006) Glyceraldehyde-3-phosphate dehydrogenase of Paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect Immun 74(1):382–389. doi:10.1128/IAI.74.1.382-389.2006
Becker P, Hufnagle W, Peters G, Herrmann M (2001) Detection of differential gene expression in biofilm-forming versus planktonic populations of Staphylococcus aureus using micro-representational-difference analysis. Appl Environ Microbiol 67(7):2958–2965. doi:10.1128/AEM.67.7.2958-2965.2001
Caldas TD, El Yaagoubi A, Richarme G (1998) Chaperone properties of bacterial elongation factor EF-Tu. J Biol Chem 273(19):11478–11482
Carty DM, Siwy J, Brennand JE, Zurbig P, Mullen W, Franke J, McCulloch JW, Roberts CT, North RA, Chappell LC, Mischak H, Poston L, Dominiczak AF, Delles C (2011) Urinary proteomics for prediction of preeclampsia. Hypertension 57(3):561–569. doi:10.1161/HYPERTENSIONAHA.110.164285
Chalker VJ, Brooks HW, Brownlie J (2003) The association of Streptococcus equi subsp. zooepidemicus with canine infectious respiratory disease. Vet Microbiol 95(1–2):149–156
Chun YH, Johnson EM, Gabel BE, Cadogan AS (1983) Effect of vinblastine sulfate on the growth and histologic development of reaggregated hydra. Teratology 27(1):89–94. doi:10.1002/tera.1420270113
Costerton JW (1999) Introduction to biofilm. Int J Antimicrob Agents 11(3–4):217–221 discussion 237–239
Czaplicki G, Houtain JY, Mullender C, Porter SR, Humblet MF, Manteca C, Saegerman C (2012) Apparent prevalence of antibodies to Coxiella burnetii (Q fever) in bulk tank milk from dairy herds in southern Belgium. Vet J 192(3):529–531. doi:10.1016/j.tvjl.2011.08.033
Erhan D, L’Heureux PJ, Yue SY, Bengio Y (2006) Collaborative filtering on a family of biological targets. J Chem Inf Model 46(2):626–635. doi:10.1021/ci050367t
Eyre DW, Kenkre JS, Bowler IC, McBride SJ (2010) Streptococcus equi subspecies zooepidemicus meningitis—a case report and review of the literature. Eur J Clin Microbiol Infect Dis 29(12):1459–1463. doi:10.1007/s10096-010-1037-5
Fehri LF, Sirand-Pugnet P, Gourgues G, Jan G, Wroblewski H, Blanchard A (2005) Resistance to antimicrobial peptides and stress response in Mycoplasma pulmonis. Antimicrob Agents Chemother 49(10):4154–4165. doi:10.1128/AAC.49.10.4154-4165.2005
Feng ZG, Hu JS (1977) Outbreak of swine streptococcosis in sichuan province and identification of pathogen. Anim Husbandry Vet Med Lett 2:7–12
Fernandez N, Diaz EE, Amils R, Sanz JL (2008) Analysis of microbial community during biofilm development in an anaerobic wastewater treatment reactor. Microb Ecol 56(1):121–132. doi:10.1007/s00248-007-9330-2
He X, Ahn J (2011) Differential gene expression in planktonic and biofilm cells of multiple antibiotic-resistant Salmonella Typhimurium and Staphylococcus aureus. FEMS Microbiol Lett 325(2):180–188. doi:10.1111/j.1574-6968.2011.02429.x
He H, Wang Q, Sheng L, Liu Q, Zhang Y (2011) Functional characterization of Vibrio alginolyticus twin-arginine translocation system: its roles in biofilm formation, extracellular protease activity, and virulence towards fish. Curr Microbiol 62(4):1193–1199. doi:10.1007/s00284-010-9844-6
Holden MT, Heather Z, Paillot R, Steward KF, Webb K, Ainslie F, Jourdan T, Bason NC, Holroyd NE, Mungall K, Quail MA, Sanders M, Simmonds M, Willey D, Brooks K, Aanensen DM, Spratt BG, Jolley KA, Maiden MC, Kehoe M, Chanter N, Bentley SD, Robinson C, Maskell DJ, Parkhill J, Waller AS (2009) Genomic evidence for the evolution of Streptococcus equi: host restriction, increased virulence, and genetic exchange with human pathogens. PLoS Pathog 5(3):e1000346. doi:10.1371/journal.ppat.1000346
Hong K (2007) Characterization of group a streptococcal M23 protein and comparison of the M3 and M23 protein’s ligand-binding domains. Curr Microbiol 55(5):427–434. doi:10.1007/s00284-007-9012-9
Huo YY, Cheng H, Han XF, Jiang XW, Sun C, Zhang XQ, Zhu XF, Liu YF, Li PF, Ni PX, Wu M (2012) Complete genome sequence of Pelagibacterium halotolerans B2(T). J Bacteriol 194(1):197–198. doi:10.1128/JB.06343-11
Jiang X, Wang S, Cheng H, Huo Y, Zhang X, Zhu X, Han X, Ni P, Wu M (2011) Genome sequence of Halobiforma lacisalsi AJ5, an extremely halophilic archaeon which harbors a bop gene. J Bacteriol 193(24):7023–7024. doi:10.1128/JB.06282-11
Kao YC, Wang TS, Lu CW, Cheng TH, Liu YP (2012) The psychometric properties of the Peters et al. delusions inventory (PDI) in Taiwan: reliability, validity, and utility. Soc Psychiatry Psychiatr Epidemiol 47(8):1221–1234. doi:10.1007/s00127-011-0428-y
Liu LT, Liu JG, Zhao NJ, Lu CP, Chen D, Shi H, Wang CL, Zhang YJ, Liu WQ (2012) Study on the automatic recognition method of elemental spectra in laser induced breakdown spectroscopy. Guang Pu Xue Yu Guang Pu Fen Xi 32(1):20–24
Liu Z, Lu CL, Cui LP, Hu YL, Yu Q, Jiang Y, Ma T, Jiao DK, Wang D, Jia CY (2012) MicroRNA-146a modulates TGF-beta1-induced phenotypic differentiation in human dermal fibroblasts by targeting SMAD4. Arch Dermatol Res 304(3):195–202. doi:10.1007/s00403-011-1178-0
Ma Z, Fan HJ, Lu CP (2010) Molecular cloning and analysis of the UDP-glucose pyrophosphorylase in Streptococcus equi subsp. zooepidemicus. Mol Biol Rep 38(4):2751–2760. doi:10.1007/s11033-010-0420-8
Mellata M, Dho-Moulin M, Dozois CM, Curtiss R III, Brown PK, Arne P, Bree A, Desautels C, Fairbrother JM (2003) Role of virulence factors in resistance of avian pathogenic Escherichia coli to serum and in pathogenicity. Infect Immun 71(1):536–540
Moroe K, Coelho A, Chun YH, Gosselin AJ (1991) Observations on the initiation of sustained ventricular tachycardia by programmed stimulation. Pacing Clin Electrophysiol 14(3):452–459
Perkel J (2009) Comparative proteomics study suggests germ-cell-derived stem cells can stand in for ESCs. J Proteome Res 8(12):5407. doi:10.1021/pr900988y
Pesavento PA, Hurley KF, Bannasch MJ, Artiushin S, Timoney JF (2008) A clonal outbreak of acute fatal hemorrhagic pneumonia in intensively housed (shelter) dogs caused by Streptococcus equi subsp. zooepidemicus. Vet Pathol 45(1):51–53. doi:10.1354/vp.45-1-51
Ren D, Bedzyk LA, Thomas SM, Ye RW, Wood TK (2004) Gene expression in Escherichia coli biofilms. Appl Microbiol Biotechnol 64(4):515–524. doi:10.1007/s00253-003-1517-y
Resch A, Leicht S, Saric M, Pasztor L, Jakob A, Gotz F, Nordheim A (2006) Comparative proteome analysis of Staphylococcus aureus biofilm and planktonic cells and correlation with transcriptome profiling. Proteomics 6(6):1867–1877. doi:10.1002/pmic.200500531
Salasia SI, Wibawan IW, Pasaribu FH, Abdulmawjood A, Lammler C (2004) Persistent occurrence of a single Streptococcus equi subsp. zooepidemicus clone in the pig and monkey population in Indonesia. J Vet Sci 5(3):263–265
Schneider CP, Heigener D, Schott-von-Romer K, Gutz S, Laack E, Digel W, Guschall WR, Franke A, Bodenstein H, Schmidtgen C, Reck M (2008) Epidermal growth factor receptor-related tumor markers and clinical outcomes with erlotinib in non-small cell lung cancer: an analysis of patients from german centers in the TRUST study. J Thorac Oncol 3(12):1446–1453. doi:10.1097/JTO.0b013e31818ddcaa
Seaton K, Ahn SJ, Sagstetter AM, Burne RA (2011) A transcriptional regulator and ABC transporters link stress tolerance, (p)ppGpp, and genetic competence in Streptococcus mutans. J Bacteriol 193(4):862–874. doi:10.1128/JB.01257-10
Umezawa S, Obayashi T, Chun YH, Adachi H, Kanayama M, Korenaga M, Inada M, Taniguchi K (1989) Clinical significance of the technetium-99m/thallium-201 overlap in acute myocardial infarction. J Cardiol 19(4):981–990
Wang Y, Zhang W, Wu Z, Lu C (2011) Reduced virulence is an important characteristic of biofilm infection of Streptococcus suis. FEMS Microbiol Lett 316(1):36–43. doi:10.1111/j.1574-6968.2010.02189.x
Wang Y, Yi L, Wu Z, Shao J, Liu G, Fan H, Zhang W, Lu C (2012) Comparative proteomic analysis of Streptococcus suis biofilms and planktonic cells that identified biofilm infection-related immunogenic proteins. PLoS ONE 7(4):e33371. doi:10.1371/journal.pone.0033371
Williams RA, Sadler SA (1971) Electrophoresis of glucose-6-phosphate dehydrogenase, cell wall composition and the taxonomy of heterofermentative lactobacilli. J Gen Microbiol 65(3):351–358
Xiang J, Sun Z, Song F, Han LZ, Huan JN (2010) Formation of bacterial biofilm on deep vein catheters in burn patients and its significance. Zhonghua Shao Shang Za Zhi 26(2):95–99
Zhang Z, Chen D, Chen Y, Davies TM, Vaillancourt JP, Liu W (2012) Risk signals of an influenza pandemic caused by highly pathogenic avian influenza subtype H5N1: spatio-temporal perspectives. Vet J 192(3):417–421. doi:10.1016/j.tvjl.2011.08.012
Acknowledgments
This study was supported by Grants from the National Basic Research Program (973) of China (2012CB518804), the National Natural Science Foundation of China (31172319,31272581), the Jiangsu Agricultural Science and Technology Innovation Fund (CX(12)3078), the key technology program of Jiangsu province (BE2013433), the Science and Technology development project of Henan Province (142102310297), the Science and Technology Research Foundation of Henan Province Educational Committee (14A230003, 13A230261) and the project was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yi, L., Wang, Y., Ma, Z. et al. Biofilm Formation of Streptococcus equi ssp. zooepidemicus and Comparative Proteomic Analysis of Biofilm and Planktonic Cells. Curr Microbiol 69, 227–233 (2014). https://doi.org/10.1007/s00284-014-0574-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0574-z