Abstract
Antimicrobial peptides are the promising candidates for withstanding multidrug-resistant bacteria (MDRB) which were caused by the misuse and extensive use of antibiotics. In this research, in vitro activities of one antimicrobial cationic peptide, brevinin-2CE alone and in combination with five kinds of antibiotics were assessed against clinical isolates of extended-spectrum β-lactamase-producing Escherichia coli and methicillin-resistant Staphylococcus aureus. The results showed that most of the combination groups had synergistic effects. Also, it was obvious that brevinin-2CE had more rapid and severe action on the tested MDRBs which demonstrated that brevinin-2CE and the antibiotics had different antimicrobial mechanisms. Thus, it was presumed that the antimicrobial peptides destroyed the bacterial cells via pore formation mechanisms which lead to the increasing of membrane permeability; and then the other compounds like antibiotics might enter into the cells and accomplish the antimicrobial activities more rapidly and efficiently.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The extensive use of antibiotics in medicine, food industry, and agriculture has resulted in the frequent emergence of multidrug-resistant bacteria (MDRB) which might cause severe health problems. Methicillin-resistant Staphylococcus aureus (MRSA), as the representative of Gram-positive MDRB, was first found in 1961, only 2 years after the antibiotic methicillin was created to battle the bacterial infections. Till now, MRSA is one of the most important pathogenic bacteria which are spread all over the world [25]. Moreover, several MRSA strains were found to be resistant to multiple antibiotics; which are not only the beta-lactam antibiotics, but also erythromycin, cipro, and gentamicin, even the antibiotic of last resort—vancomycin [4]. Similar to MRSA, extended-spectrum β-lactamase (ESBL)-producing Escherichia coli, as the representative of Gram-negative MDRB, spread at an alarming rate. Usually, the plasmids responsible for production of ESBL also carried genes encoding resistance to the other drugs. Therefore, antibiotic options in the treatment of ESBL-producing microorganisms were extremely restricted [15, 22].
Although the cases of antimicrobial drug resistance were expanding, limited numbers of new antibiotics had been successfully developed in the last few decades. Thus, new antimicrobial agents against MDRB infections were urgently required [23]. In the process of searching for alternative chemotherapeutic compounds, antimicrobial peptides (AMPs) had gradually received increasing attentions. As a kind of new antimicrobial agents, AMPs had been isolated from diverse organisms including animals, plants, and bacteria, especially from amphibians. They are small, no more than 100 amino acid residues long, cationic peptides acting in a variety of ways, among which the most common mechanism is permeabilization and disruption of the target cell membrane [21]. It is now widely recognized that AMPs could play a promising role in fighting MDRB.
However, it is costly to isolate the natural AMPs from the hosts, and the mass production methods to manufacture AMPs are not yet well developed, so using a large scale of AMP drugs to fight against MDRB is unrealistic. Some researches tried to test the effect of the combination of AMPs with the conventional antibiotics against MDRB, and the results showed that the combinations usually achieved synergistic or enhanced activities [2, 8, 9, 14, 16]. Such treatment not only provided new possibilities for curing MDRB, but also reduced the risks of creating new MDRB strains with the decrease of the antibiotics dosage.
In our previous study, brevinin-2CE, a Rana chensinensis AMP, had been identified. And it was demonstrated to be a potential therapeutic agent with high antimicrobial activity and strong cytotoxic effect on cancer cells, but comparatively low hemolytic activity [30]. Now, the independent and combined activities of brevinin-2CE with five conventional antibiotics, rifampicin, clindamycin, levofloxacin, amoxicillin, and chloramphenicol, were examined against two MDRB strains. Our research tried to assess whether the combinations of AMP with the conventional antibiotics were effective in the treatment of MDRB infections and whether these combinations were more active than each component individually.
Materials and Methods
Antimicrobial Peptide and Antibiotics
The antimicrobial peptide brevinin-2CE was synthesized by Sinoasis Pharmaceuticals, INC (Guangzhou, China). The final purity of the synthetic peptide was higher than 95 % after purified by reverse-phase high-performance liquid chromatography (RP-HPLC). Furthermore, the identity was confirmed by ion trap mass spectrometer (ITMS) [30]. The AMP stock solution from dry powder was prepared at a concentration of 538 μmol/L and stored at −80 °C.
Rifampicin (Shanghai Xinyi Wanxiang pharmaceutical company, China), clindamycin (Xi’an Lijun Pharmaceutical Co., LTD, China), levofloxacin (Liaoning Aoda Pharmaceutical Co., LTD, China), amoxicillin (Shanxi Tongda Pharmaceutical Co., LTD, China), and chloramphenicol (Xi’an Lijun Pharmaceutical Co., LTD, China) were purchased in a local pharmacy. These antibiotics were diluted in accordance with the manufacturers’ recommendations and then stored at −80 °C.
Bacterial Strains and Growth Conditions
Escherichia coli 44102 (E. coli), Staphylococcus aureus 22401 (S. aureus), the ESBL-producing E. coli I1 (E. coli I1), and the methicillin-resistant S. aureus B2 (S. aureus B2) were purchased from the Institute of Microbiology (Shaanxi, China). The bacteria were cultured in Mueller-Hinton broth at 37 °C.
Determination of Minimal Inhibitory Concentrations
The minimal inhibitory concentrations (MICs) of brevinin-2CE and the antibiotics were determined using microbroth dilution technique, as described by the Clinical and Laboratory Standards Institute (CLSI) [27]. Briefly, bacteria cells grown overnight were diluted in Mueller-Hinton broth to 107 CFU/mL. In addition, the peptide and antibiotics were also diluted in the broth medium to the working concentration. Then, 100 μL of test bacteria and an equal volume of the peptide or antibiotic were added into the 96-well culture plate. All the tests were performed in triplicate. After 16 h of incubation at 37 °C with shaking, microbial sedimentation was measured by the absorbance at 600 nm of each sample using a microtiter plate reader (BioTek ELx800). The experiments were repeated for three times. MIC was defined as the lowest concentration of the antimicrobial agent that produced the complete inhibition of visible growth. The fractional inhibitory concentration (FIC) index for the combination of two antimicrobial agents was calculated according to the following equation: FIC index = (MIC drug A in combination)/(MIC drug A alone) + (MIC drug B in combination)/(MIC drug B alone). FIC indices were interpreted as follows: ≤0.5, synergy; 0.5–1, additivity, 1–4, indifference; and >4, antagonism [26].
Killing Test of the Synergistic Groups
The inoculums of log-phase E. coli I1 or S. aureus B2 at the concentration of 107 CFU/mL were suspended in the Mueller-Hinton broth in the 96-well plate. Then brevinin-2CE and the antibiotics were added into the broth at the MICs, alone or combined. All the tests were performed in triplicate and repeated three times. Then the mixtures were incubated at 37 °C for 30 min. Every 10 min, serial tenfold dilutions of each mixture were prepared and plated onto plate count agar for counting the colony number. The bactericidal activities of the antimicrobial agents were evaluated by the bacteriostasis rate (η) which was calculated as follows: \( \eta = (N_{0} - N_{\text{t}} )/N_{0} \times 100\,\% \), N 0 and N t representing the colony number of the negative control and test group, respectively [29].
Morphological Changes Observed by Scanning Electron Microscopy
The clinical isolates E. coli I1 and S. aureus B2 were treated with brevinin-2CE or/and the antibiotics for 30 min at MIC concentrations. The samples were washed twice with PBS (0.01 mol/L, pH 7.2) to remove the dead cells and then fixed in 2.5 % glutaraldehyde in PBS for 6 h at 4 °C. The cells were then dehydrated with a graded ethyl alcohol series from 30 to 100 %. The morphological changes of the cells were observed using an S-3400N (II) Scanning Electron Microscopy (Hitachi, Japan).
Results
Brevinin-2CE was More Active than Most of the Tested Antibiotics
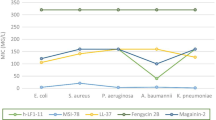
The MICs of brevinin-2CE and the antibiotics against wild-type and multidrug-resistant bacteria are summarized in Table 1. Among all the antibiotics, levofloxacin showed the highest antimicrobial activity against both Gram-positive and Gram-negative MDRB strains. Rifampicin and clindamycin had more activities against E. coli I1, while amoxicillin and chloramphenicol had more activities against S. aureus B2. Compared to the antibiotics, the antimicrobial peptide brevinin-2CE demonstrated the higher and broader spectrum antimicrobial effect with respect to both of the MDRB strains. In a word, brevinin-2CE and the antibiotics exhibited diverse activities against clinical isolates. Brevinin-2CE was more active than most of the tested antibiotics. On the other hand, Gram-negative strain E. coli I1 was more sensitive than Gram-positive strain S. aureus B2 to brevinin-2CE, which was similar to the effect of brevinin-2CE against the standard E. coli and S. aureus strains [30].
In addition to MIC data, the concentration variation range of each antimicrobial agent was detected along with their bacteriostasis rates ranging from 0 to 100 % (Fig. 1). Obviously, brevinin-2CE had the narrowest range from 2.9 to 11.6 μg/mL for E. coli I1 and 5.8 to 23.2 μg/mL for S. aureus B2. For the antibiotics, chloramphenicol had the narrowest active concentration range from 2 to 32 μg/mL for S. aureus B2. While, all the other antibiotics had wider ranges, no matter for E. coli I1 or S. aureus B2.
Most of the Brevinin-2CE–Antibiotic Combinations Had Synergy Effect
The MICs of brevinin-2CE and the antibiotics in the combination groups were decreased dramatically (Table 2). For E. coil I1, the MIC of brevinin-2CE in the brevinin-2CE–rifampicin combination was reduced to 25 % compared to the MIC of brevinin-2CE. The rifampicin in the combination was also reduced to 25 %. In the combination of brevinin-2CE–chloramphenicol, both the AMP and the antibiotic MICs were also reduced to 25 %. While in the brevinin-2CE–clindamycin and brevinin-2CE–levofloxacin group, the MICs of each agent were reduced to 12.5 %, respectively. For S. aureus B2, the MICs of brevinin-2CE, levofloxacin, amoxicillin, and chloramphenicol in the combination groups were reduced to 25 %, respectively. While in the brevinin-2CE–rifampicin and brevinin-2CE–clindamycin groups, brevinin-2CE’s MIC was reduced to 33 %, rifampicin’s and clindamycin’s MICs were reduced to 50 %, respectively.
The FIC indices indicated that most of the tested groups had synergistic effect (FIC ≤ 0.5). Especially, the combination of brevinin-2CE–clindamycin and brevinin-2CE–levofloxacin showed the strongest synergistic activity against E. coli I1 with the FICs reaching to 0.25. On the other hand, the combination of rifampicin or clindamycin with brevinin-2CE against S. aureus B2 showed only enhancement effect (0.5 < FIC < 1).
Brevinin-2CE Helped to Increase the Bacteriostasis Rates
No matter whether it is E. coil I1 (Fig. 2) or S. aureus B2 (Fig. 3), the bacteriostasis rates (η) of each group treated with the antibiotics alone increased gradually. While the groups treated with brevinin-2CE had the dramatic increasing bacteriostasis rates, i.e., more than 95 % bacteria cells were killed at 30 min; for all the combination groups, brevinin-2CE helped to accelerate the bacteriostasis rates at every time point. The number of living bacteria cells decreased at every time point.
Brevinin-2CE–Antibiotic Combinations Caused More Severe Cell Damage
The SEM micrographs of E. coli I1 (Fig. 4) and S. aureus B2 (Fig. 5) treated with brevinin-2CE and different antibiotics, alone or combined, are shown below. It was obvious that both brevinin-2CE and the antibiotics caused damage to E. coli I1 and S. aureus B2. When the cells were treated with brevinin-2CE in combination with the antibiotics at the combined MIC concentration, more severe cell damages were observed than when treated with the antibiotics alone, which indicated that the antibacterial activities of the combinations were much more effective than the antibiotics alone.
Micrographs of E. coil I1 treated by different antimicrobial agents. E. coil I1 without any treatment was used as the control (a); the other groups were E. coil I1 treated by rifampicin (b), clindamycin (c), levofloxacin (d), chloramphenicol (e), brevinin-2CE (f), rifampicin and brevinin-2CE (g), clindamycin and brevinin-2CE (h), levofloxacin and brevinin-2CE (i), and chloramphenicol and brevinin-2CE (j). The meaning of the labels 1 cell fracture, 2 cell elongation, 3 cell surface roughening, and 4 cell clubbing
Micrographs of S. aureus B2 treated by different antimicrobial agents. S. aureus B2 without any treatment was used as the control (a); the other groups were S. aureus B2 treated by rifampicin (b), clindamycin (c), levofloxacin (d), amoxicillin (e), chloramphenicol (f), brevinin-2CE (g), rifampicin and brevinin-2CE (h), clindamycin and brevinin-2CE (i), levofloxacin and brevinin-2CE (j), amoxicillin and brevinin-2CE (k), and chloramphenicol and brevinin-2CE (l). The meaning of the labels 1 cell fracture, 2 cell leakage, 3 cell surface roughening, 4 cell shrinkage, and 5 cell clubbing
Discussion
The emergence of multidrug-resistant bacteria had been an invariable accompaniment of the therapeutic use of antimicrobial agents. To combat this problem, researchers developed different kinds of extended-spectrum antibiotics and more potent selective antimicrobial agents. Many AMPs were found to be efficient in the treatment of MDRB infections; for example, truncated AMPs from marine organisms were active against MRSA [17]; a dicarba derivative of the antimicrobial peptide bevinin-1BYa displayed potent bactericidal activity against MRSA and multidrug-resistant Acinetobacter baumannii [11]; brevinin-2 related peptide (B2RP) could potently inhibit the growth of nosocomial isolates of multidrug-resistant A. baumannii [1, 7]; and brevinin-2TSa showed growth inhibitory activity against MRSA [6].
In previous studies, brevinin-2CE was identified to inhibit the growth of the standard Gram-positive and Gram-negative bacteria to different extents [20, 30]. In this study, the findings showed that brevinin-2CE was active against the tested MRSA and ESBL-producing E. coli. Also, the MICs of brevinin-2CE against wild-type and multidrug-resistant bacteria were almost the same; suggesting that the bactericidal mechanisms of brevinin-2CE against the bacteria strains were the same, with no relationship to their resistance spectrum. As expected, the five kinds of antibiotics had slight effect on the tested MRSA and ESBL-producing E. coli, while brevinin-2CE exhibited more effective bactericidal effect compared with the antibiotics. This suggested that brevinin-2CE, compared with antibiotics, had different bactericidal mechanisms to deal with the MDRBs.
The killing tests indicated that brevinin-2CE had more rapid killing rate. This was in accordance with the researches conducted by Andrea Giacometti in which the killing effects to S. aureus caused by the nisin and ranalexin were complete after a 20 and 30-min exposure time, respectively. However, lots of antibiotics need more time to inhibit the bacteria [9]. When treated with brevinin-2CE and the antibiotics for the same time, more serious distortions of the bacteria cells were exposed by SEM in the brevinin-2CE group. Similar to our results, the SEM studies on the AMPs extracted from the plateau frog revealed that the cells displayed various alterations of the cellular shape and morphology changes of the bacteria surface under the AMPs treatment [18]. Thus, the SEM results confirmed the rapid action of brevinin-2CE on the tested MDRBs.
It was known that the antibiotics could inhibit the bacteria through interfering with nucleic acid synthesis, blocking protein synthesis, suppressing cell wall synthesis, and so on. In the combination studies, although the tested antibiotics had different antibacterial mechanisms, most of the test groups displayed synergistic effect, which suggested that the action mode of brevinin-2CE was not the same as any mode of the investigated antibiotics. Moreover, many synergistic or enhanced activities were reported in other combination studies of antibiotics and antimicrobial peptides. It was found that the proline-rich antimicrobial peptide dimer, A3-APO, was able to recover the lost activity of chloramphenicol, β-lactams, sulfonamides, or trimethoprim against MDRB with partial or full synergy [5]. Also, the researches indicated that the recombinant mouse β-defensin 3 not only had synergy effect with ampicillin against S. aureus, but also showed synergistic activities with itraconazole, amphotericin, or 5-fluorocytosine against Candida albicans [14]. As the antibacterial mechanisms of all these antibiotics were different, we could presume that the antimicrobial mechanisms of AMPs must be different from the modes of the most conventional antibiotics.
However, the mechanisms of the synergy or enhancement effects of the AMP–antibiotics combinations were still unclear. Studies used model membrane systems, such as supported lipid bilayers, vesicles, and lamellaes, which showed that many AMPs were able to lyse biomembranes and form membrane pores via mechanisms such as barrel stave and toroidal pore [10, 12, 13, 19, 28]. So one interpretation was that the AMPs, by triggering the activity of bacterial murein hydrolases, might cause degradation of the peptidoglycan and had a direct membrane permeabilizing activity; and then these damages probably allowed the entry of hydrophobic compounds such as antibiotics [9]. Another hypothesis may be involved in the peptide–hydrophobic antibiotic interaction [3, 9]. From our data, the possible mechanism of brevinin-2CE and the tested antibiotics combinations might be the first explanation. As the SEM results indicated that, in the combination groups, the cells appeared as roughening surfaces, crimple, and bend; which were serious than the groups only treated with antibiotics but not severe than the groups treated with brevinin-2CE at MIC concentration alone. Thus, we may infer that the morphology changes of the bacteria were caused not only by brevinin-2CE, but also by the antibiotics which just need more time to act. It was suggested that the antibiotics might pass the cell barriers easier with the help of brevinin-2CE. The killing test data agree with the SEM results. Brevinin-2CE inhibited the MDRBs growth in advance of the antibiotics, while the peptide could also accelerate the bactericidal velocity of the antibiotics when they were combined together. In conclusion, we presumed that the AMPs destroyed the bacterial cells via pore formation mechanisms which led to the increasing of membrane permeability, and then the other compounds like antibiotics might enter into the cells and accomplish the activities more rapidly and efficiently. Based on the hypothesis, brevinin-2CE degraded the thin peptidoglycan layer of E. coli I1 much easier than the thick layer of S. aureus B2; which explained why brevinin-2CE showed stronger synergistic effect on E. coli I1 than S. aureus B2.
Although the speculated mode of the antimicrobial peptide antibiotics action still needs more direct proofs, the anti-MDRB activities and the synergy effects demonstrated by several combination groups made the brevinin-2CE–antibiotics potentially useful for antimicrobial chemotherapy. However, only very few in vivo studies about the cationic peptide actions had been published [8, 24]. Therefore, future researches concerning in vivo efficacy and unknown toxicities of the brevinin-2CE combinations are needed.
References
Al-Ghaferi N, Kolodziejek J, Nowotny N, Coquet L, Jouenne T, Leprince J, Vaudry H, King JD, Conlon JM (2010) Antimicrobial peptides from the skin secretions of the South-East Asian frog Hylarana erythraea (Ranidae). Peptides 31(4):548–554. doi:10.1016/j.peptides.2009.12.013
Baranska-Rybak W, Cirioni O, Dawgul M, Sokolowska-Wojdylo M, Naumiuk L, Szczerkowska-Dobosz A, Nowicki R, Roszkiewicz J, Kamysz W (2011) Activity of antimicrobial peptides and conventional antibiotics against superantigen positive Staphylococcus aureus isolated from the patients with neoplastic and inflammatory erythrodermia. Chemother Res Pract 2011:270932. doi:10.1155/2011/270932
Bevins CL, Zasloff M (1990) Peptides from frog skin. Annu Rev Biochem 59:395–414. doi:10.1146/annurev.bi.59.070190.002143
Borde JP, Kern WV (2012) Treatment of MRSA infections. Dtsch Med Wochenschr 137(49):2553–2557. doi:10.1055/s-0032-1327283
Cassone M, Vogiatzi P, La Montagna R, De Olivier Inacio V, Cudic P, Wade JD, Otvos L Jr (2008) Scope and limitations of the designer proline-rich antibacterial peptide dimer, A3-APO, alone or in synergy with conventional antibiotics. Peptides 29(11):1878–1886. doi:10.1016/j.peptides.2008.07.016
Conlon JM, Al-Ghaferi N, Abraham B, Sonnevend A, Coquet L, Leprince J, Jouenne T, Vaudry H, Iwamuro S (2006) Antimicrobial peptides from the skin of the Tsushima brown frog Rana tsushimensis. Comp Biochem Physiol Toxicol Pharmacol 143(1):42–49. doi:10.1016/j.cbpc.2005.11.022
Conlon JM, Ahmed E, Condamine E (2009) Antimicrobial properties of brevinin-2-related peptide and its analogs: efficacy against multidrug-resistant Acinetobacter baumannii. Chem Biol Drug Des 74(5):488–493. doi:10.1111/j.1747-0285.2009.00882.x
Desbois AP, Gemmell CG, Coote PJ (2010) In vivo efficacy of the antimicrobial peptide ranalexin in combination with the endopeptidase lysostaphin against wound and systemic methicillin-resistant Staphylococcus aureus (MRSA) infections. Int J Antimicrob Agents 35(6):559–565. doi:10.1016/j.ijantimicag.2010.01.016
Giacometti A, Cirioni O, Barchiesi F, Scalise G (2000) In-vitro activity and killing effect of polycationic peptides on methicillin-resistant Staphylococcus aureus and interactions with clinically used antibiotics. Diagn Microbiol Infect Dis 38(2):115–118
He K, Ludtke SJ, Huang HW, Worcester DL (1995) Antimicrobial peptide pores in membranes detected by neutron in-plane scattering. Biochemistry 34(48):15614–15618
Hossain MA, Guilhaudis L, Sonnevend A, Attoub S, van Lierop BJ, Robinson AJ, Wade JD, Conlon JM (2011) Synthesis, conformational analysis and biological properties of a dicarba derivative of the antimicrobial peptide, brevinin-1BYa. Eur Biophys J 40(4):555–564. doi:10.1007/s00249-011-0679-2
Huang HW (1999) Peptide–lipid interactions and mechanisms of antimicrobial peptides. Novartis Found Symp 225:188–200 discussion 200-186
Huang HW, Wu Y (1991) Lipid–alamethicin interactions influence alamethicin orientation. Biophys J 60(5):1079–1087
Jiang Y, Yi X, Li M, Wang T, Qi T, She X (2012) Antimicrobial activities of recombinant mouse beta-defensin 3 and its synergy with antibiotics. J Mater Sci Mater Med 23(7):1723–1728. doi:10.1007/s10856-012-4645-z
Lee BS, Hwang JH, Lee SH, Jang SE, Jang ES, Jo HJ, Shin CM, Park YS, Kim JW, Jung SH, Kim N, Lee DH, Lee JK, Ahn S (2012) Risk factors of organ failure in patients with bacteremic cholangitis. Dig Dis Sci. doi:10.1007/s10620-012-2478-8
Li Q, Huang J, Guo H, Guo X, Zhu Y, Dong K (2012) Bactericidal activity against meticillin-resistant Staphylococcus aureus of a novel eukaryotic therapeutic recombinant antimicrobial peptide. Int J Antimicrob Agents 39(6):496–499. doi:10.1016/j.ijantimicag.2012.03.003
Lin MC, Hui CF, Chen JY, Wu JL (2013) Truncated antimicrobial peptides from marine organisms retain anticancer activity and antibacterial activity against multidrug-resistant Staphylococcus aureus. Peptides 44:139–148. doi:10.1016/j.peptides.2013.04.004
Lu Z, Zhai L, Wang H, Che Q, Wang D, Feng F, Zhao Z, Yu H (2010) Novel families of antimicrobial peptides with multiple functions from skin of Xizang plateau frog, Nanorana parkeri. Biochimie 92(5):475–481. doi:10.1016/j.biochi.2010.01.025
Ludtke SJ, He K, Wu Y, Huang HW (1994) Cooperative membrane insertion of magainin correlated with its cytolytic activity. Biochim Biophys Acta 1190(1):181–184
Morikawa N, Hagiwara K, Nakajima T (1992) Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem Biophys Res Commun 189(1):184–190
Nicolas P, Vanhoye D, Amiche M (2003) Molecular strategies in biological evolution of antimicrobial peptides. Peptides 24(11):1669–1680. doi:10.1016/j.peptides.2003.08.017
Nuotio L, Schneitz C, Nilsson O (2013) Effect of competitive exclusion in reducing the occurrence of Escherichia coli producing extended-spectrum beta-lactamases in the ceca of broiler chicks. Poult Sci 92(1):250–254. doi:10.3382/ps.2012-02575
Rennie RP (2012) Current and future challenges in the development of antimicrobial agents. Handb Exp Pharmacol 211:45–65. doi:10.1007/978-3-642-28951-4_4
Rishi P, Preet S, Bharrhan S, Verma I (2011) In vitro and in vivo synergistic effects of cryptdin 2 and ampicillin against Salmonella. Antimicrob Agents Chemother 55(9):4176–4182. doi:10.1128/AAC.00273-11
Schroppel K, Riessen R (2013) Multiresistant gram-negative bacteria: a bacterial challenge of the twenty-first century. Medizinische Klinik Intensivmedizin und Notfallmedizin. doi:10.1007/s00063-012-0160-8
Sueke H, Kaye SB, Neal T, Hall A, Tuft S, Parry CM (2010) An in vitro investigation of synergy or antagonism between antimicrobial combinations against isolates from bacterial keratitis. Invest Ophthalmol Vis Sci 51(8):4151–4155. doi:10.1167/iovs.09-4839
Wayne (2012) Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 9th edn, Approved standard M07-A9
Wu Y, He K, Ludtke SJ, Huang HW (1995) X-ray diffraction study of lipid bilayer membranes interacting with amphiphilic helical peptides: diphytanoyl phosphatidylcholine with alamethicin at low concentrations. Biophys J 68(6):2361–2369. doi:10.1016/S0006-3495(95)80418-2
Yang Y, Liu H, Liu G, Ran X (2010) Antibacterial effect of autologous platelet-rich gel derived from health volunteers in vitro. Zhongguo xiu fu chong jian wai ke za zhi 24(5):571–576
Zhao J, Sun Y, Li Z, Su Q (2011) Molecular cloning of novel antimicrobial peptide genes from the skin of the Chinese brown frog, Rana chensinensis. Zoolog Sci 28(2):112–117. doi:10.2108/zsj.28.112
Acknowledgments
The work described in this paper was supported by the Fundamental Research Funds for the Central Universities (2014 grants from SNNU) and the Innovative Experiment Projects of Educational Ministry of China for Undergraduate (201210781028, cx13075).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Y., Liu, Y., Sun, Y. et al. In Vitro Synergistic Activities of Antimicrobial Peptide Brevinin-2CE with Five Kinds of Antibiotics Against Multidrug-Resistant Clinical Isolates. Curr Microbiol 68, 685–692 (2014). https://doi.org/10.1007/s00284-014-0529-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0529-4