Abstract
Brevinin-1BYa (FLPILASLAAKFGPKLFCLVTKKC), first isolated from skin secretions of the frog Rana boylii, displays broad-spectrum antimicrobial activity and potent haemolytic activity. This study investigates the effects on conformation and biological activity of replacement of the intramolecular disulphide bridge in the peptide by a non-reducible dicarba bond. Dicarba-brevinin-1BYa was prepared by microwave irradiation of [Agl18,Agl24]-brevinin-1BYa (Agl = allylglycine) in the presence of a second generation Grubbs’ catalyst. Circular dichroism spectroscopy in 50% trifluoroethanol-water indicated that the degree of α-helicity of the dicarba derivative (22%) was less than that of brevinin-1BYa (27%) but comparable to that of the acyclic derivative [Ser18,Ser24]-brevinin-1BYa (23%). Dicarba-brevinin-1BYa showed a two-fold increase in potency against reference strains of Escherichia coli, Staphylococcus aureus, and Candida albicans compared with the native peptide and displayed potent bactericidal activity against clinical isolates of methicillin-resistant S. aureus (MRSA) and multidrug-resistant Acinetobacter baumannii (MIC in the range 1–8 μM). Compared with brevinin-1BYa and [Ser18,Ser24]-brevinin-1BYa, the dicarba derivative was associated with increased cytotoxicity against human erythrocytes (2.5-fold), MDA-MB-231 breast carcinoma cells (1.3-fold) and HepG2 hepatoma-derived cells (1.5-fold).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emergence, particularly during the past 10–15 years, of strains of pathogenic bacteria and fungi that are resistant to commonly used antibiotics poses a potentially serious threat to public health (Norrby et al. 2005; Martinez and Silley 2010). There is an urgent need for new types of antimicrobial agents with activity against these microorganisms that also possess appropriate pharmacokinetic and toxicological profiles. Peptides with antimicrobial activity play an important role in the innate immunity that constitutes the first-line defence against invading pathogens for a wide range of vertebrate and invertebrate species (Auvynet and Rosenstein 2009; Diamond et al. 2009). Compounds based upon these naturally occurring anti-infective and immunomodulatory peptides are being increasingly considered as potential therapeutic agents (Mookherjee and Hancock 2007; Zhang and Falla 2010).
Frogs belonging to the extensive family Ranidae, currently 342 species distributed worldwide (Frost 2010), have proved to be a particularly rich source of peptides with antibacterial and antifungal activities (reviewed in Conlon 2008; Conlon et al. 2009b). These peptides are synthesized in granular glands in the skin and are released into skin secretions, often in very high concentrations, in response to environmental stress. There are no conserved amino acid sequences that are associated with antimicrobial activity, but the peptides are almost invariably cationic, relatively hydrophobic, and have the propensity to form an amphipathic α-helix in a membrane-mimetic solvent or in the environment of a phospholipid vesicle (Powers and Hancock 2003). In contrast to antimicrobial peptides from species of other well-studied families such as the Hylidae (Pukala et al. 2006; Nicolas and El Amri 2009) and Pipidae (Mechkarska et al. 2010), most of the peptides from frogs of the Ranidae family contain an intramolecular disulphide bridge defining a cyclic domain at the C-terminus that generally comprises six or seven amino acid residues. Examples of such cystine-containing peptides include brevinin-1, brevinin-2, esculentin-1, esculentin-2, ranatuerin-1, ranatuerin-2, and palustrin-2 (reviewed in Conlon 2008, 2011).
Major obstacles to the development of peptide-based anti-infective drugs, particularly if they are to be administered systemically, are their toxicities and their short half-lives in the circulation. In the case of peptides containing a disulphide bond, protein-disulphide oxidoreductases can disrupt these cysteine bridges rendering the peptide inactive and more susceptible to proteolytic degradation. Microwave-assisted ring-closing metathesis provides a relatively simple means of preparing a metabolically stable and non-reducible dicarba bond to replace a cysteine bridge and has found wide application (Jacobsen et al. 2010). The method involves chemical bond formation between the side chains of two olefinic residues such as allylglycine (Agl), in the presence of a ruthenium-alkylidene catalyst (Grubbs’ catalyst). Microwave irradiation is thought to prevent peptide aggregation and potentiates the activity of the catalyst (van Lierop et al. 2010). Examples include synthesis of dicarba derivatives of oxytocin (Stymiest et al. 2003), conotoxins (Robinson et al. 2007, 2009), the A-chain of human relaxin-3 (Hossain et al. 2009), and the somatostatin analogue, octreotide (Barragán et al. 2009).
The 24 amino-acid-residue peptide brevinin-1BYa (FLPILASLAAKFGPKLFCLVTKKC) represents a candidate for drug development. The peptide was first isolated from norepinephrine-stimulated skin secretions from the North American foothill yellow-legged frog Rana boylii (Conlon et al. 2003), and preliminary data indicated potent growth-inhibitory activity against both reference strains and clinical isolates of Gram-positive and Gram-negative bacteria and against opportunistic yeast pathogens of the genus Candida (Pál et al. 2006). However, the therapeutic potential of the peptide, particularly if it is to be administered systemically, is limited by its potent haemolytic activity against human erythrocytes (LC50 = 10 μM).
The aim of the present study was to investigate the effects on the conformation, antimicrobial activity and cytolytic activity against human cells of replacing the disulphide bridge in brevinin-1BYa by a non-reducible, metabolically stable dicarba bond. The properties of this analogue are compared with those of the acyclic peptide [Ser18,Ser24]brevinin-1BYa in which the cysteines are replaced by isosteric serine residues.
Methodology
Materials
9-Fluorenylmethoxycarbonyl (Fmoc)-protected α-l-amino acids and 1-[bis(dimethylamino)methylene]-1H-benzotriazolium hexafluorophosphate 3-oxide (HBTU) were purchased from GL Biochem (Shanghai, China). Piperidine and trifluoroacetic acid (TFA) were purchased from Auspep (Melbourne, Australia). Fmoc-PAL-PEG-PS resins with substitution of ca. 0.19 mmol/g were purchased from Applied Biosystems (Melbourne, Australia). Dimethylformamide (DMF), methanol, diethyl ether and dichloromethane were purchased from Merck (Melbourne, Australia). Triisopropylsilane and diisopropylethylamine were purchased from Sigma-Aldrich (Sydney, Australia). Fmoc-l-allylglycine (Agl) was purchased from AnaSpec (Fremont, CA, USA). Tricyclohexylphosphine [1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydro-imidazol-2-ylidene] (benzylidene) ruthenium(II) dichloride (second generation Grubbs’ catalyst) was supplied by Aldrich and stored under nitrogen. Acetonitrile was purchased from BDH Laboratory Supplies (Poole, UK). All other reagents were from Sigma-Aldrich (Sydney, Australia). Solvents and chemicals were all of peptide synthesis or analytical grade.
Solid phase peptide synthesis
The linear Alg-containing brevinin-1BYa analogue was prepared by continuous flow Fmoc solid-phase synthesis using an automatic PerSeptive Biosystems Pioneer peptide synthesiser (Framingham, MA, USA) as previously described (Bathgate et al. 2006). All amino acid side chains were protected by TFA-labile protecting groups. Fmoc-l-Agl-OH was used in the place of each Fmoc-l-Cys(Trt)-OH residue in positions 18 and 24. After sequence completion, a small aliquot of the resin-bound peptide was subjected to TFA-mediated cleavage for reverse-phase HPLC and mass spectral analysis. This supported formation of the Fmoc-protected brevinin-1BYa peptide at a high purity (M r calc. 2,819.6 Da; M r obs. 2,819.0).
Microwave-assisted ring-closing metathesis
Dichloromethane and a 0.4 M solution of LiCl in DMF were degassed with high purity argon prior to use. The microwave reaction was carried out on a CEM Discover™ system fitted with the Benchmate™ option. The instrument produces a continuous focussed beam of microwave radiation at a maximum power delivery selected by the user, reaching and maintaining a selected temperature. Reactions were performed in 10 ml high pressure glass microwave vessels fitted with self-sealing Teflon septa as a pressure release device. The vessels employed magnetic stirrer beads, and the temperature of the reaction was monitored continuously with a non-contact infrared sensor located below the microwave cavity floor. Reaction times were measured from the time the microwave reached its maximum temperature until the reaction period had lapsed (cooling periods not included).
In a nitrogen-filled glove box, the microwave reactor vessel was loaded with resin-bound Fmoc-brevinin-1BYa analogue (0.05 mmol), dichloromethane (5 ml), 0.4 M LiCl in DMF (200 μl) and second generation Grubbs’ catalyst (8.5 mg, 10 μmol, 20 mol%). The system was sealed, and the reaction mixture was then irradiated with 100 W of microwave energy and stirred at 100°C for 2 h. The reaction mixture was cooled to room temperature, and the resin then transferred to a fritted syringe and soaked in a 1:1 mixture of dimethyl sulphoxide-DMF for 18 h. After filtration, the resin was washed with dichloromethane and methanol then left to dry in vacuo for 1 h. Post-metathesis, a small aliquot of resin-bound peptide was subjected to Fmoc-deprotection in the presence of 20% v/v piperidine in DMF and washed with dimethylformamide, dichloromethane and methanol. After TFA-mediated cleavage, reverse-phase HPLC and mass spectral analysis of the resultant solid supported formation of the desired cyclic dicarba (DC) peptide as two components in a 70:30 ratio. The newly installed dicarba bridges possess cis- and trans- geometry. The target peptides are described as DC-brevinin-1BYa (peak 1) and DC-brevinin-1BYa (peak 2).
The remaining resin-bound Fmoc-brevinin-1BYa was then treated with 20% v/v piperidine in DMF as described above. Final cleavage of the peptide from its solid support and simultaneous side chain deprotection were achieved by treatment with TFA (95%) in the presence of the scavengers anisole (3%) and triisopropylsilane (2%) for 2 h, and the crude peptide (48 mg) was then purified by analytical reverse phase HPLC on a 25 × 0.46 cm Vydac 218TP54 (C18) analytical column (Grace, Deerfield, IL, USA). The concentration of acetonitrile in the eluting solvent was raised from 35 to 65% at a flow rate of 1.5 ml/min. Selected fractions were combined and lyophilised to give two isomers of the desired peptide as colourless solids (DC-brevinin-1BYa peak 1:6.8 mg and DC-brevinin-1BYa peak 2:3.0 mg) at >98% purity (Fig. 1). The identity of each peptide was confirmed by ESI-MS analysis (DC-brevinin-1BYa peak 1: M r calc 2,568.3 Da, M r obs 2,567.8 and DC-brevinin-1BYa peak 2: M r calc 2,568.3 Da, M r obs 2,568.0).
The synthesis and purification of brevinin-1BYa and [Ser18,Ser24]-brevinin-1BYa have been described previously (Pál et al. 2006).
Estimation of hydrophobicity
Peptides (approximately 5 nmol) were injected onto a 25 × 0.46 cm Vydac 218TP54 (C-18) column equilibrated with acetonitrile/water/TFA (21.0/78.9/0.1) at a flow rate of 1.5 ml/min. The concentration of acetonitrile was raised to 49.0% over 40 min using a linear gradient. Absorbance was measured at 214 nm, and the retention time of each peptide was recorded.
Secondary structure prediction
Prediction of secondary structure and determination of helicity per residue for brevinin-1BYa were undertaken using the AGADIR program (Muñoz and Serrano 1994). AGADIR is a prediction algorithm based on the helix/coil transition theory, which predicts the helical behaviour of monomeric peptides. Calculations were performed at pH 7 and 298 K. A minimum percentage of 1% helicity/residue was considered to predict the presence of a helix.
Circular dichroism spectroscopy
Circular dichroism spectra were acquired on a CD6 dichrograph (Jobin-Yvon, Longjumeau, France) calibrated using D-10-camphorsulfonic acid. Peptides were analysed at a concentration of 0.5 mg/ml in (a) water and (b) trifluoroethanol (TFE)/water (50/50, v/v). Data points were collected from 260 to 185 nm at room temperature with a 0.5 mm path length quartz cell. For each spectrum, three scans were accumulated and then averaged. A baseline was obtained by recording a spectrum of the solvent, and the mean residue molar ellipticity (θ), expressed in deg cm2 dmol−1, was calculated from the observed ellipticity after baseline correction. The helical content of the peptides was estimated using the Forood formula (Forood et al. 1993).
Microorganisms
Reference strains of Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922) and Candida albicans (ATCC 90028) were purchased from the American Type Culture Collection (Rockville, MD, USA). Six strains of methicillin-resistant S. aureus (MRSA) were isolated from patients admitted to Tawam Hospital (Al-Ain, UAE). The characteristics of the strains including antibiotic resistance are shown in Table 2. Five independent strains of multidrug-resistant Acinetobacter baumannii (MDRAB) isolated at four different hospitals in Abu Dhabi Emirate were included in the study. The origin of the strains has been described previously (Conlon et al. 2009a). The bacteria were resistant to all antibiotics commonly used to treat Acinetobacter infections including cephalosporins, carbapenems, fluoroquinolones and aminoglycosides but remained sensitive to tigecycline and colistin. These strains were positive for the bla OXA-51 gene detected by PCR (Turton et al. 2006). The clonal lineage of the strains, determined by a multiplex PCR assay (Turton et al. 2007) is shown in Table 3.
Antimicrobial assays
Minimum inhibitory concentration (MIC) of the peptides was determined in duplicate in three independent experiments by standard microdilution methods using 96-well microtiter cell-culture plates (Clinical Laboratory and Standards Institute 2008a, b). Serial dilutions of the peptides in Mueller-Hinton broth (50 μl) were mixed with an inoculum [50 μl of 106 colony forming units (CFU)/ml] from a log-phase culture. Bacteria were incubated for 18 h at 37°C in a humidified atmosphere of air. C. albicans was incubated in RPMI 1640 medium for 48 h at 35°C, with an inoculum of 5 × 104 CFU/ml. After incubation, the absorbance at 630 nm of each well was determined using a microtiter plate reader. Minimum bactericidal concentration (MBC) was measured by incubating 10 μl of culture from the wells of the MIC titration plate containing 1× MIC, 2× MIC and 4× MIC in Mueller-Hinton broth for 18 h at 37°C in a humidified atmosphere of air. MBC was considered to be the lowest concentration for which no visible growth was detected. In order to monitor the validity and reproducibility of the assays, incubations with bacteria were carried out in parallel with increasing concentrations of ampicillin and incubations with C. albicans in parallel with amphotericin B.
Cytotoxicity assays
Peptides in the concentration range 0.6–20 μM were incubated with washed human erythrocytes (2 × 107 cells) from a healthy donor in Dulbecco’s phosphate-buffered saline, pH 7.4 (100 μl) for 1 h at 37°C. After centrifugation (12,000×g for 15 s), the absorbance at 450 nm of the supernatant was measured. A parallel incubation in the presence of 1% v/v Tween-20 was carried out to determine the absorbance associated with 100% haemolysis. The LD50 value was taken as the mean concentration of peptide producing 50% haemolysis in three independent experiments.
Human breast cancer cells MDA-MB-231 (kindly provided by Prof. Joan Massague, Memorial Sloan-Kettering Cancer Center, New York, USA) were maintained at 37°C in DMEM medium supplemented with antibiotics (penicillin 50 U/ml; streptomycin 50 μg/ml) (Invitrogen, Cergy Pontoise, France) and 10% fetal bovine serum (FBS, Biowest, Nouaille, France). Cells were seeded in 96-well plates at a density of 5 × 103 cells/well into 96-well plates. After 24 h, cells were treated for 24 and 48 h with increasing concentrations of brevinin-1BYa, DC-brevinin-1BYa and [Ser18,Ser24]brevinin-1BYa (0.01–20 μM), in triplicate. Control cultures were treated with 0.1% DMSO. The effect of the peptides on cell viability was determined by measurement of ATP concentrations using a CellTiter-Glo Luminescent Cell Viability assay (Promega, Madison, WI, USA). Luminescent signals were measured using a GLOMAX Luminometer system.
HepG2 human hepatoma-derived cells (kindly provided by Prof. Haider Raza, United Arab Emirates University) were cultured as previously described (Bay and Cederbaum 2006). Cells (104 cells/well) were seeded into 96-well microtiter plates and grown to 90% confluence in minimum essential medium containing 10% fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin and 2 mM glutamine (100 μl) in an humidified atmosphere of 5% CO2 at 37°C. One hour before incubation, the medium in each well was replaced with minimum essential medium containing 5% fetal calf serum (100 μl). Peptides in the concentration range 0.1–20 μM were incubated with the cells at 37°C for 1 h in duplicate. Cytolytic activity was determined by the measurement of the release of lactate dehydrogenase into the medium by using a CytoTox96 nonradioactive cytotoxicity assay kit (Promega) according to the manufacturer’s protocol. The LC50 value was taken as the mean concentration of peptide producing 50% cell death in three independent experiments.
Results
Synthesis of DC-brevinin-1BYa
The synthesis of DC-brevinin-1BYa is illustrated schematically in Fig. 1. Microwave-assisted RCM of the linear [Agl18,Agl24] derivative resulted in formation of two components (peak 1 and peak 2) with the molecular mass of DC-brevinin-1BYa, which is consistent with the expected formation of cis and trans isomers. These peptides were readily separated by reverse-phase HPLC (Fig. 2). RCM of the Agl-derivative of human relaxin-3 A-chain also produced two DC derivatives, and it was shown that the earlier eluting component represented the cis isomer (Hossain et al. 2009). This is also the case with DC-insulin-like peptide 3 (Zhang et al. 2010) and DC-α-contotoxin (MacRaild et al. 2009) where the earlier-eluting peak corresponds to the cis form and the later-eluting peak is the trans. However, further studies are required to confirm that the more abundant peak 1 DC-brevinin-1BYa represents the cis- isomer. The conformational analysis and most of the biological studies were carried out with peak 1 material.
Estimation of hydrophobicity
Retention time on reverse-phase HPLC has been shown to provide a measure of the effective hydrophobicity of peptides with α-helical character (Tachi et al. 2002). Under the conditions of chromatography used, the retention times of the peptides were as follows: brevinin-1BYa 28.4 min, peak 1 DC-brevinin-1BYa 28.0 min, peak 2 DC-brevinin-1BYa 29.2 min and [Ser18,Ser24]brevinin-1BYa 26.1 min. It is concluded that replacement of the disulphide bond by an unsaturated dicarba isostere does not produce an appreciable change in the hydrophobicity of brevinin-1BYa, whereas elimination of the cysteine bridge produces a less hydrophobic peptide.
Conformational analysis
Secondary structure prediction using the AGADIR programme indicated that brevinin-1BYa has the propensity to adopt a helical conformation between residues 5–6 and 11–12 in the N-terminal to central region of the peptide. Consistent with this prediction, the circular dichroism spectrum of the peptide in a membrane-mimetic solvent (50% TFE/water) showed a typical α-helical pattern with double minima at 208 and 222 nm and a positive maximum at 192 nm (Fig. 3b). The α-helical contribution to the overall secondary structure was estimated to be at 26.6%, which corresponds to approximately 6–7 residues out of 24. The measured helicity of DC-brevinin-1BYa (22.4%) in this solvent is comparable to that of acyclic [Ser18,Ser24]-brevinin-1BYa (23.2%) but less than that of the native peptide. The overall CD spectrum of a peptide represents the combination of the individual spectra of each secondary structure found in the molecule. Consequently, a possible explanation for the reduced value is that the presence of turn structures modifies the value of the mean residue ellipticity at 222 nm, and consequently the estimation of the helical content. The spectrum of DC brevinin-1BYa displayed an inflection below 190 nm that was not observed in the spectrum of the native peptide, suggesting that the dicarba bond stabilizes one or more turn structures in the peptide (Perczel and Fasman 1992; Greenfield 1996). Consistent with the observations in TFE, the CD spectra in water indicated that brevinin-1BYa and the dicarba analogue were not in a complete random structure while the acyclic peptide produced a CD spectrum typical of a disordered conformation. Indeed, the degree of helicity of DC-brevinin-1BYa in water (8.0%) was slightly greater than that of brevinin-1BYa (6.5%) and appreciably greater than the helicity of [Ser18,Ser24]-brevinin-1BYa (3.4%). This suggests the presence of a nascent structuration in the cyclic part of the peptide.
Antimicrobial activities
The MIC values of a synthetic replicate of brevinin-1BYa and its derivatives against reference strains of bacteria are shown in Table 1. Replacement of the disulphide bond in brevinin-1BYa by an unsaturated dicarba bond resulted in a two-fold increase in potency against the Gram-positive bacterium S.aureus, the Gram-negative bacterium E. coli, and the opportunistic yeast pathogen C. albicans. The antimicrobial potencies of peak 1 and peak 2 DC-brevinin-1BYa were the same. In contrast, elimination of the cysteine bridge in the analogue [Ser18,Ser24]- brevinin-1BYa resulted in a two-fold decrease in activity against E. coli and a four-fold decrease against C. albicans. DC-brevinin-1BYa showed high potency against clinical isolates of MRSA with MIC values in the range 1–2 μM (Table 2). The peptide was bactericidal not bacteriostatic although the MBC values were higher for all strains (8 μM). DC-brevinin-1BYa also showed potent bactericidal activity against clinical isolates of MDRAB with MIC values in the range 4–8 μM and MBC values in the range 8–16 μM (Table 3).
Cytotoxicities
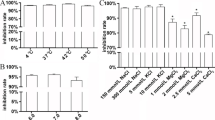
Both peak 1 and peak 2 DC-brevinin-1BYa showed increased haemolytic activity (LC50 = 4 μM) against human erythrocytes compared with the naturally occurring peptide (LC50 = 10 μM) (Table 1). Consistent with previous data (Pál et al. 2006), the acyclic peptide was appreciably less haemolytic (LC50 = 75 μM) than the cyclic derivatives. This trend towards greater cytolytic activity against human cells was also observed using HepG2 hepatoma-derived cells (LC50 = 4 vs. 6 μM for brevinin-1BYa) and MDA-MB-231 breast carcinoma cells (LC50 = 7 vs. 9 μM for brevinin-1BYa). The cytolytic activity of [Ser18,Ser24]-brevinin-1BYa against both cell lines was less than that of the cyclic peptides (Table 1). The concentration-dependence of cytotoxicity against MDA-MB-231 cells of the three peptides is shown in Fig. 4.
Effects of brevinin-1BYa, DC-brevinin-1BYa and [Ser18,Ser24]-brevinin-1BYa on the viability of MDA-MB-231 breast carcinoma cells. The left panels represent 24 h exposure, and the right panels represent 48 h exposure. All experiments were repeated at least three times. Data are mean (columns) and SEM (bars)
Discussion
The study has demonstrated that ring-closing metathesis (RCM) can be used to prepare an analogue of a naturally occurring antimicrobial peptide (brevinin-1BYa) in which the metabolically labile disulphide bridge is replaced by a non-reducible dicarba isostere. Brevinin-1 was first isolated from an extract of the skin of the Japanese pond frog R. brevipoda porsa (Morikawa et al. 1992), but subsequent work has shown that orthologous peptides are synthesized in the skins of a wide range of Eurasian and North American species belonging to the family Ranidae (Conlon 2011).
DC-brevinin-1BYa shows potent growth inhibitory activity against reference strains of Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria and against the opportunistic yeast pathogen C. albicans (Table 1). Of particular interest is the high potency of the peptide against two groups of clinically important pathogens for which antibiotic resistance has become a serious problem—methicillin-resistant S. aureus (MRSA) and multidrug-resistant Acinetobacter baumannii (MDRAB). Although resistance first appeared among nosocomial isolates (Perez et al. 2007; Martinez and Silley 2010), new strains of both MRSA (David and Daum 2010; Deleo et al. 2010) and MDRAB (Gootz and Marra 2008; Maragakis and Perl 2008) have emerged in the community causing infections in otherwise healthy people.
It is encouraging that DC-brevinin-1BYa shows increased antimicrobial potency compared with the naturally occurring peptide. In contrast, replacing the disulphide-bridged cysteine residues in the antimicrobial bacteriocin leucocin A with dicarba bonds generates an analogue that is approximately one-tenth as potent as the native peptide against Carnobacterium maltaromaticum (Derksen et al. 2006). However, the desired increased antimicrobial activity of DC-brevinin-1BYa is accompanied by increased cytotoxicity against human cells. The ability of the peptide to kill human hepatoma-derived and breast carcinoma-derived cells at low concentrations (LC50 < 7 μM) suggests the possibility of development into an anti-tumour agent, but this is offset by the even greater toxicity against human erythrocytes (LC50 = 4 μM). Unfortunately such high toxicity limits the potential of the peptide for development into a therapeutically valuable anti-infective drug, particularly for systemic use.
The antimicrobial activities of a peptide against microorganisms and the cytolytic activities against mammalian cells are determined by a complex interaction among cationicity, hydrophobicity, α-helicity and amphipathicity (Yeaman and Yount 2003; Conlon et al. 2007). Studies using model α-helical peptides have demonstrated that an increase in cationicity promotes interaction with the negatively charged phospholipids in the bacterial cell membrane and thereby increases antimicrobial potency whereas increases in hydrophobicity and the degree of α-helicity promotes hemolytic activity relative to antimicrobial activity (reviewed in Conlon et al. 2007). In the case of DC-brevinin-1BYa, replacement of the disulphide bridge with the isosteric dicarba bond produces no change in molecular charge, and a comparison of retention times on HPLC indicates that there has not been any appreciable change in hydrophobicity. The CD spectra are more difficult to interpret. Although there is a small increase in the degree of helicity of DC-brevinin-1BYa in water compared with brevinin-1BYa, the percent helicity in the membrane-mimetic solvent 50% TFE-water is less. The acyclic analogue appears to adopt a disordered conformation in water, and the degree of helicity in 50% TFE-water is similar to that of the dicarba derivative. The percent helicity of both DC-brevinin-1BYa and brevinin-1BYa in 50% TFE-water is lower than that reported for other members of the brevinin-1 family that have been studied. For example, it has been reported that the brevinin-1 peptide (FLGALFKVASKVLPSVKCAITKKC), termed gaegurin-5, adopts an extended α-helix spanning residues 3–20 with the strongly conserved Pro14 residue introducing a stable kink into the molecule (Park et al. 2002). Similarly, the helicity of the C-terminally α-amidated form of brevinin-1E (FLPLLAGLAANFLPKIFC KITRKC) in 2.5 mM SDS was reported to be as high as 74% (Kwon et al. 1998). As the CD spectrum of DC-brevinin-1BYa in 50% TFE exhibits features consistent with the presence of a turn structure, it is possible that the presence of turn conformations in the cyclic region of brevinin-1 peptides leads to under-estimation of the helical content of a peptide when calculated from the mean residue molar ellipticity at 220–222 nm (Greenfield 1996).
Two-dimensional proton NMR spectroscopy of the dicarba analogue of the A-chain of human relaxin demonstrated that the region around the dicarba bond does not adopt a single stable conformation in solution, rather there is a dynamic equilibrium among several conformers (Hossain et al. 2009). We propose, therefore, that there is a conformational equilibrium in DC-brevinin-1BYa involving a random conformation and one or more turn regions in the cyclic part of the peptide. The increased antimicrobial and cytotoxic activities of DC-brevinin-1BYa in vitro may arise from the stabilization of a particular turn conformation that possesses increased cell-penetrating ability.
In view of the appreciable toxicity of DC-brevinin-1BYa, there seemed little point in comparing its metabolic clearance rate in an animal model with the naturally occurring peptide. However, future studies using frog skin antimicrobial peptides with lower cytolytic activities against mammalian cells will investigate whether the dicarba bridge confers increased stability to reductases and resistance to proteolytic degradation in vivo. Brevinin-2GUb (GVIIDTLKGAAKTVAAELLRKAHCKLTNSC) from the Asian frog Hylarana güntheri represents a suitable candidate for further study as the peptide not only shows broad-spectrum antimicrobial activity and low haemolytic activity (LC50 = 700 μM) but potently stimulates insulin release and improves glucose tolerance in mice (Conlon et al. 2008).
Abbreviations
- CFU:
-
Colony forming units
- DC:
-
Dicarba
- DMF:
-
Dimethylformamide
- MDRAB:
-
Multidrug-resistant Acinetobacter baumannii
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- RCM:
-
Ring-closing metathesis
- TFA:
-
Trifluoroacetic acid
- TFE:
-
Trifluoroethanol
References
Auvynet C, Rosenstein Y (2009) Multifunctional host defense peptides: antimicrobial peptides, the small yet big players in innate and adaptive immunity. FEBS J 276:6497–6508
Barragán F, Moreno V, Marchán V (2009) Solid-phase synthesis and DNA binding studies of dichloroplatinum(ii) conjugates of dicarba analogues of octreotide as new anticancer drugs. Chem Commun 31:4705–4707
Bathgate RAD, Lin F, Hanson NF, Otvos L Jr, Guidolin A, Giannakis C, Bastiras S, Layfield S, Ferraro T, Ma S, Zhao C, Gundlach AL, Samuel CS, Tregear GW, Wade JD (2006) Relaxin-3: improved synthesis strategy and demonstration of its high-affinity interaction with the relaxin receptor LGR7 both in vitro and in vivo. Biochemistry 45:1043–1053
Bay J, Cederbaum AI (2006) Overexpression of CYP2E1 in mitochondria sensitizes HepG2 cells to the toxicity caused by depletion of glutathione. J Biol Chem 281:5128–5136
Clinical Laboratory and Standards Institute (2008a) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M07-A8. CLSI, Wayne
Clinical Laboratory and Standards Institute (2008b) Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard M27-A3. CLSI, Wayne
Conlon JM (2008) Reflections on a systematic nomenclature for antimicrobial peptides from the skins of frogs of the family Ranidae. Peptides 29:1815–1819
Conlon JM (2011) The contribution of skin antimicrobial peptides to the system of innate immunity in anurans. Cell Tissue Res 343:201–212
Conlon JM, Sonnevend A, Patel M, Davidson C, Nielsen PF, Pál T, Rollins-Smith LA (2003) Isolation of peptides of the brevinin-1 family with potent candidacidal activity from the skin secretions of the frog Rana boylii. J Pept Res 62:207–213
Conlon JM, Al-Ghaferi N, Abraham B, Leprince J (2007) Strategies for transformation of naturally-occurring amphibian antimicrobial peptides into therapeutically valuable anti-infective agents. Methods 42:349–357
Conlon JM, Power GJ, Abdel-Wahab YHA, Flatt PR, Jiansheng H, Coquet L, Leprince J, Jouenne T, Vaudry H (2008) A potent, non-toxic insulin-releasing peptide isolated from an extract of the skin of the Asian frog, Hylarana güntheri (Anura:Ranidae). Regul Pept 151:153–159
Conlon JM, Ahmed E, Condamine E (2009a) Antimicrobial properties of brevinin-2-related peptide and its analogs: efficacy against multidrug-resistant Acinetobacter baumannii. Chem Biol Drug Des 74:488–493
Conlon JM, Kolodziejek J, Nowotny N (2009b) Antimicrobial peptides from the skins of North American frogs. Biochim Biophys Acta 1788:1556–1563
David MZ, Daum RS (2010) Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 23:616–687
Deleo FR, Otto M, Kreiswirth BN, Chambers HF (2010) Community-associated methicillin-resistant Staphylococcus aureus. Lancet 375:1557–1568
Derksen DJ, Stymiest JL, Vederas JC (2006) Antimicrobial leucocin analogues with a disulfide bridge replaced by a carbocycle or by noncovalent interactions of allyl glycine residues. J Am Chem Soc 128:14252–14253
Diamond G, Beckloff N, Weinberg A, Kisich KO (2009) The roles of antimicrobial peptides in innate host defense. Curr Pharm Des 15:2377–2392
Forood B, Filiciano EJ, Niambiar KP (1993) Stabilization of α-helical structures in short peptides via end capping. Proc Natl Acad Sci USA 90:838–842
Frost DR (2010) Amphibian species of the world: an online reference. Version 5.4. American Museum of Natural History, New York, USA. http://www.research.amnh.org/herpetology/amphibia/index.php
Gootz TD, Marra A (2008) Acinetobacter baumannii: an emerging multidrug-resistant threat. Expert Rev Anti Infect Ther 6:309–325
Greenfield NJ (1996) Methods to estimate the conformation of proteins and polypeptides from circular dichroism data. Anal Biochem 235:1–10
Hossain MA, Rosengren KJ, Zhang S, Bathgate RA, Tregear GW, van Lierop BJ, Robinson AJ, Wade JD (2009) Solid phase synthesis and structural analysis of novel A-chain dicarba analogs of human relaxin-3 (INSL7) that exhibit full biological activity. Org Biomol Chem 7:1547–1553
Jacobsen Ø, Klaveness J, Rongved P (2010) Structural and pharmacological effects of ring-closing metathesis in peptides. Molecules 15:6638–6677
Kwon MY, Hong SY, Lee KH (1998) Structure-activity analysis of brevinin 1E amide, an antimicrobial peptide from Rana esculenta. Biochim Biophys Acta 1387:239–248
MacRaild CA, Illesinghe J, van Lierop BJ, Townsend AL, Chebib M, Livett BG, Robinson AJ, Norton RS (2009) Structure and activity of (2, 8)-dicarba-(3, 12)-cystino α-ImI, an α-conotoxin containing a nonreducible cystine analogue. J Med Chem 52:755–762
Maragakis LL, Perl TM (2008) Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 46:1254–1263
Martinez M, Silley P (2010) Antimicrobial drug resistance. Handb Exp Pharmacol 199:227–264
Mechkarska M, Ahmed E, Coquet L, Leprince J, Jouenne T, Vaudry H, King JD, Conlon JM (2010) Antimicrobial peptides with therapeutic potential from skin secretions of the Marsabit clawed frog Xenopus borealis (Pipidae). Comp Biochem Physiol C Toxicol Pharmacol 152:467–472
Mookherjee N, Hancock RE (2007) Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci 64:922–933
Morikawa N, Hagiwara K, Nakajima T (1992) Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem Biophys Res Commun 189:184–190
Muñoz V, Serrano L (1994) Elucidating the folding problem of helical peptides using empirical parameters. Nature Struct Biol 1:399–409
Nicolas P, El Amri C (2009) The dermaseptin superfamily: a gene-based combinatorial library of antimicrobial peptides. Biochim Biophys Acta 1788:1537–1550
Norrby SR, Nord CE, Finch R (2005) Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect Dis 5:115–119
Pál T, Abraham B, Sonnevend A, Jumaa P, Conlon JM (2006) Brevinin-1BYa: a naturally occurring peptide from frog skin with broad-spectrum antibacterial and antifungal properties. Int J Antimicrob Agents 27:525–529
Park SH, Kim HE, Kim CM, Yun HJ, Choi EC, Lee BJ (2002) Role of proline, cysteine and a disulphide bridge in the structure and activity of the anti-microbial peptide gaegurin 5. Biochem J 368:171–182
Perczel A, Fasman GD (1992) Quantitative analysis of cyclic beta-turn models. Protein Sci 1:378–395
Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA (2007) Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 51:3471–3484
Powers JP, Hancock RE (2003) The relationship between peptide structure and antibacterial activity. Peptides 24:1681–1691
Pukala TL, Bowie JH, Maselli VM, Musgrave IF, Tyler MJ (2006) Host-defence peptides from the glandular secretions of amphibians: structure and activity. Nat Prod Rep 23:368–393
Robinson AJ, Elaridi J, van Lierop BJ, Mujcinovic S, Jackson WR (2007) Microwave-assisted RCM for the synthesis of carbocyclic peptides. J Pept Sci 13:280–285
Robinson AJ, van Lierop BJ, Garland RD, Teoh E, Elaridi J, Illesinghe JP, Jackson WR (2009) Regioselective formation of interlocked dicarba-bridges in naturally occurring cyclic peptide toxins using olefin metathesis. Chem Commun 28:4293–4295
Stymiest JL, Mitchell BF, Wong S, Vederas JC (2003) Synthesis of biologically active dicarba analogues of the peptide hormone oxytocin using ring-closing metathesis. Org Lett 5:47–49
Tachi T, Epand RF, Epand RM, Matsuzaki K (2002) Position dependent hydrophobicity of the antimicrobial magainin peptide affects the mode of peptide-lipid interactions and selective toxicity. Biochemistry 41:10723–10731
Turton JF, Woodford N, Glover J, Yarde S, Kaufmann ME, Pitt TL (2006) Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J Clin Microbiol 44:2974–2976
Turton JF, Gabriel SN, Valderrey C, Kaufmann ME, Pitt TL (2007) Use of sequence-based typing and multiplex PCR to identify clonal lineages of outbreak strains of Acinetobacter baumannii. Clin Microbiol Infect 13:807–815
van Lierop BJ, Whelan AN, Andrikopoulos S, Mulder R, Jackson WR, Robinson AJ (2010) Methods for enhancing ring closing metathesis yield in peptides: synthesis of a dicarba human growth hormone fragment. Int J Pept Res Ther 16:133–144
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Zhang L, Falla TJ (2010) Potential therapeutic application of host defense peptides. Methods Mol Biol 618:303–327
Zhang S, Hughes RA, Bathgate RAD, Shabanpoora F, Hossain MA, Lin F, van Lierop BJ, Robinson AJ, Wade JD (2010) Role of the intra-A-chain disulfide bond of insulin-like peptide 3 in binding and activation of its receptor, RXFP2. Peptides 31:1730–1736
Acknowledgments
The authors thank Eman Ahmed, Milena Mechkarska, Kholoud Arafat and Annie Sunny (United Arab Emirates University) for technical assistance in this study. This work was supported by a Faculty Support Grant from the United Arab Emirates University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Membrane-active peptides: 455th WE-Heraeus-Seminar and AMP 2010 Workshop.
Rights and permissions
About this article
Cite this article
Hossain, M.A., Guilhaudis, L., Sonnevend, A. et al. Synthesis, conformational analysis and biological properties of a dicarba derivative of the antimicrobial peptide, brevinin-1BYa. Eur Biophys J 40, 555–564 (2011). https://doi.org/10.1007/s00249-011-0679-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-011-0679-2