Abstract
In the rhizosphere, exopolymers are also known to be useful to improve the moisture-holding capacity. The ability of the isolates from coastal sand dunes to produce exopolymers was determined. Among which the isolate, showing very high production of exopolysaccharide (EPS), Microbacterium arborescens––AGSB, a facultative alkalophile was further studied for exopolymer production. The isolate a gram-positive non-spore forming, slender rod, catalase positive, oxidase negative, showed growth in 12% sodium chloride. The culture was found to produce exopolymer which showed good aggregation of sand which has an important role in the stabilization of sand dunes. The exopolymer was further analysed. The cold isopropanol precipitation of dialysed supernatants grown in polypeptone yeast extract glucose broth produced partially soluble EPSs with glucose as the sole carbon source. Chemical analysis of the EPS revealed the presence of rhamnose, fucose, arabinose, mannose, galactose and glucose. On optimization of growth parameters (sucrose as carbon source and glycine as nitrogen source), the polymer was found to be a heteropolysaccharide containing mannose as the major component. It was interesting to note that the chemical composition of the exopolymers produced from both unoptimized and optimized culture conditions of Microbacterium arborescens––AGSB is different from those of other species from the same genera. This study shows that marine coastal environments such as coastal sand dunes, are a previously unexplored habitat for EPS-producing bacteria, and that these molecules might be involved in ecological roles protecting the cells against dessication especially in nutrient-limited environments such as the coastal sand dunes more so in the extreme conditions of pH. Such polysaccharides may help the bacteria to adhere to solid substrates and survive during the nutrient limitations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal sand dunes are a stressed and extreme environment in terms of the nutrient availability, fluctuations in temperature and low moisture content [1]. The vegetation found here is adapted to this habitat by developing various mechanisms to sustain the stress condition [2]. Diverse microorganisms are associated with this vegetation and are found to produce secondary metabolites, which aid in their survival, such as exopolysaccharides (EPSs). EPSs from bacteria are a complex mixture of high molecular polymers (MW > 10,000) produced by microorganisms. EPSs are involved in the formation of microbial aggregates and adhesion to surfaces. Bacteria have been shown to cement soil particles together by forming polysaccharide substances. In their native state, polysaccharides are easily degraded by other microorganisms but they appear to be protected from such degradation soon after they are firmly bound within an aggregate or incorporated into a clay lattice. The production of binding material of a polysaccharide nature by bacteria would cause sand particles to adhere and build up an aggregate. They can protect the cells from the harsh external environment and provide with energy and carbon source in decreasing nutrient-limiting conditions. Furthermore, EPSs significantly influence the surface physicochemical properties which are of considerable importance in governing bacterial flocculation and adhesion. The origin of EPSs is very complex, and their components and content heavily depend on many factors, such as bacterial group, cultivation time, substrate, ion strength, growth state etc. [3]. EPS facilitates nutrient and water retention, cell/substrate adhesion, cell–cell signaling and protection of individual cells from chemical degradation or attack [4]. Among the exopolymers, EPSs have also found applications in many industrial sectors [5, 6]. In our study on coastal sand dune bacteria, we isolated alkalophilic bacteria associated with sand dune vegetation; among the alkalophilic isolates screened for EPS, an isolate MIRA 15 was found to produce large amounts of EPS as compared to the other isolates. We report here our studies on EPS produced by this facultative alkalophilic bacteria, identified as Microbacterium arborescens––AGSB found predominantly associated with the rhizosphere of Ipomoea pes caprae, a plant growing on coastal sand dunes and forming sand aggregation. It is envisaged that the EPS-producing bacteria found in the dunes may be together aiding in the stabilization of dunes by forming sand aggregates.
Materials and Methods
Screening of Bacterial Cultures for EPS Production
Bacterial cultures were grown in 50 ml of PPYG broth in 250-ml flask. The flasks were incubated at room temperature on a rotary shaker at 160 rpm for 48 h. The culture broths were centrifuged at 8,000 rpm at 4°C for 10 min and EPS was estimated using the phenol–sulphuric acid method [7].
Microorganism
The bacterium Microbacterium arborescens––AGSB was isolated from the rhizosphere of Ipomoea pes caprae, a plant growing on coastal sand dunes.
Media
Polypeptone yeast extract glucose (PPYG) agar, pH 10 was used for isolating alkalophiles. This basal culture medium for EPS production contained peptone, 5 g/l; yeast extract, 1.5 g/l; disodium hydrogen phosphate, 1.5 g/l; sodium chloride, 1.5 g/l; magnesium chloride, 0.1 g/l; glucose, 10%; sodium carbonate, 10%; Agar, 15 g/l.
Aggregation of Soil by Microbacterium arborescens––AGSB
The EPS producing, Microbacterium arborescens culture was grown in PPYG broth (pH 10.5) and a 10% inoculum was inoculated in 1 kg of sandy soil, agricultural soil and mine reject soil and left undisturbed for 45 days. A control was also maintained for each. After incubation period of over a month, the soils were sieved in sieves with different mesh sizes to check for aggregate formation.
Culture Conditions
In all experiments for EPS production, plates and flasks were incubated for 2 days at 28°C, and EPS was obtained during stationery phase of growth. Flasks (500 ml) containing 100 ml of PPYG medium were inoculated with a loopful of the primary bacterial culture and incubated at 160 rpm on shaker. Optimal growth conditions were determined in 1-l flask containing 200 ml of approximate medium. Cultures were incubated for 48 h and cell growth monitored on a colorimeter (Elico) as increase in turbidity.
Growth Kinetics and Optimization of Growth Parameters
Forty-eight hour old culture was inoculated in PPYG medium, pH 10 and incubated on shaker at room temperature (RT). At regular intervals, the turbidity and absorbance was determined at 510 and 600 nms on a colorimeter, and the culture broth samples were centrifuged at 8,000 x g for 10 min. EPS present in the supernatants was estimated by a Phenol–sulphuric acid method [7].The effects of various culture conditions and nutrients, such as carbon sources, nitrogen source, yeast extract, rate of agitation and size of inoculum on EPS production were studied, and a medium for maximum EPS production was defined.
EPS Isolation
Bacterial exopolymer was extracted following the method of [2, 8, 9].
Chemical Analysis of EPS
Lyophilized EPS was hydrolysed with 2 N HCl for 2 h at 100°C in ampoules flushed with Nitrogen before sealing [10]. Total carbohydrates [7], Uronic acids (Galacturonic acid) [11], Proteins [12], Pyruvates [13] were estimated in the hydrolysate. Inorganic content of the polymer was estimated by gravimetric method, and the pre-weighed EPS sample was ashed at 400°C for 4 h and cooled to room temperature. The resulting ash was quantified as the inorganic content of the EPS. Sugars were analysed as their alditol acetate by GLC [8] on a Chrom pack model CP 9002 on Sil-88: Middleburg, a capillary column (L = 25 m, i.d. = 0.32 mm, df = 0.12 μm) with a flame ionization detector (FID) being used.
Results
Aggregation of Soil by Microbacterium arborescens
After 45 days, aggregates were observed in the sandy soil inoculated with Microbacterium as compared to the uninoculated control (Fig. 1). The particle size distribution curve depicted the aggregate formation in the sandy soil inoculated with Microbacterium as compared to uninoculated control. The graph represents the particle size distribution varying from 75 μ onwards for the control soil whereas in the case of sandy soil inoculated with Microbacterium, the particle size distribution starts from 250 μ onwards indicating the size of the sand aggregates formed.
Growth Kinetics and Optimization of Growth Parameters
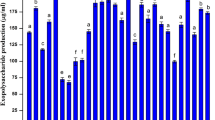
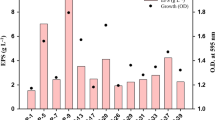
The strain Microbacterium arborescens––AGSB, a gram-positive, non-sporing, and rod-shaped bacterium grown on PPYG medium for 48 h showed maximum EPS at 24 h (Fig. 2; Table 1). After 24 h, the turbidity did not increase, however, sugar concentration in supernatant after dialysis was found to be maximum. The culture was further grown in poly peptone yeast extract glucose medium, with different carbon sources such as glucose, fructose, maltose, galactose, xylose and sucrose. Sucrose was found to produce maximum amount of EPS with 1% being optimum. Among the different nitrogen sources used, peptone, ammonium chloride, glycine, ammonium sulphate, sodium nitrate and urea, 1.5% glycine was found to produce maximum amount of EPS. Further 0.3% yeast extract concentration was found to produce maximum amount of EPS at rpm of 160 with 5% inoculum. The optimized medium was defined as sucrose (1%), glycine (1.5%), yeast extract (0.3%). The cultures inoculated in the PPYG culture broth gave a yield of 86 mg/l of polymer, while the optimized medium gave a polymer yield of three-fold, i.e. 280 mg/l as estimated in the dialysed supernatant.
Characterization of the EPS
The cold isopropanol precipitation of dialysed supernatants grown in PPYG produced a single polymer (PP1) while the optimized/defined medium was found to produce two polymers (OP1 & OP2) which are partially soluble in distilled water and 1 N NaOH but completely soluble in 2 N HCl under heat (boiling water for 30 min). The chemical composition (Table 2) showed that the polymers had pyruvates and uronic acids, although concentration of the latter was higher in OP1 and OP2 polymers while proteins fraction was higher in PP1 polymer as compared to OP1 and OP2 polymers. GC analysis of polymers revealed the presence of unusually high amount of mannose (58–74%) in OP1 & OP2 (Fig. 3b & c) (Table 3) while PP1 (Fig. 3a) had only 14% mannose present. Further rhamnose was found in large amounts in PP1 polymer (56%), OP1 (9%) and OP2.
Discussion
Aggregation and aggregate stability are of fundamental importance to soil profile characteristics and in determining agricultural capacity. The soils when inoculated with culture broth and incubated showed the formation of aggregates of varying sizes. On sieving these aggregates, it was observed that the aggregates recovered from sand ranged in size from about 1.5–3 cm in length and 0.7–2 cm in breadth. There is strong evidence that soil polysaccharide contribute to soil aggregate stability [14]. Bacteria have been shown to cement soil particles together by forming polysaccharide substances. There is strong evidence that microbial polysaccharides contribute to soil aggregate stability, and Forster [15] has stressed the importance of bacteria in the aggregation of dune sand. After a 45-day period of incubation, aggregate formation was observed in the sandy soil, but the agricultural soils and mine reject soils did not show good aggregate formation, and these aggregates were also found to be fragile as compared to the strong binding shown by the Microbacterium culture towards sand.
The total yield of EPS produced by Microbacterium arborescens––AGSB was greatly influenced by the components in the medium with the composition of the EPS being influenced by the carbon source present in the medium. Sucrose was the most efficient carbon source for EPS production, and its concentration had a marked effect on EPS yield. Therefore, increasing the sucrose concentration resulted in increased EPS production; the maximum EPS production occurred with 10 g of sucrose per litre in the medium. It is reported that marine strain Hahella chejuensis produced the highest EPS yield in batch culture when grown on sucrose [16], but increase in EPS production was not correlated with growth, which remained almost unchanged. Such production of EPS, with little or no growth, occurring is reported [17]. The isolate Microbacterium arborescens––AGSB from rhizosphere of coastal sand dune vegetation was found to produce 86 mg/l of exopolymer in PPYG broth medium, while on optimization of growth parameters, it produced 286 mg/l of exopolymers. The authors [4] in their studies on EPS-producing Microbacterium kitamiense sp. nov., isolated from the wastewater of a sugar-beet factory reported that these organisms produced both insoluble and soluble EPS. The authors [18] also reported that strains of Microbacterium sp. isolated from soil and rhizosphere of saline soils produced the most abundant EPS as against the Bacillus species.
The polymers differed in their chemical composition as shown by colorimetric and GC analysis. Polymer PP1 had high levels of proteins while the polymer synthesized by Microbacterium arborescens in optimized medium, OP1, had lower amounts of proteins. Another important distinguishing feature between both EPSs was the higher concentration of neutral sugars in polymer OP1. This suggests that the polymer OP1 is not only a polysaccharide but a glycoprotein. This is further supported by detection of significant surfactant activity and the fact that this polymer was not completely soluble in distilled water. It is interesting to note that the chemical composition of EPS from both polymers of Microbacterium arborescens is different from other species of the same genera. Non-sugar components including sulphate and protein make up a relatively smaller portion of EPS on a per-weight basis. However, they may be extremely important to the tertiary structure and physical properties of the EPS.
The EPS from PPYG broth medium had approximately 6% uronic acid (Galacturonic acid) while the EPSs from the optimized PPYG medium had 24% and 35% uronic acids (Galacturonic acid). Exopolymers produced by marine bacteria generally contain 20–50% of the polysaccharide as uronic acid [19]. Bouchotroch et al. [20] on their studies on EPS from Halomonas reported that in general the carbohydrate content of the polymers was low. Uronic acids contain an acidic carboxyl group that is ionisable at seawater pH. This contributes a negative charge to the overall polymer [21]. Matsuyama et al. [4] showed that a new Microbacterium species, M. kitamiense produced both soluble and insoluble EPS. Analysis of these EPSs showed that they contained neither protein nor uronic acids, significantly differing from the EPS produced by Microbacterium MC3B-10. Similarly, polymer produced by Bacillus sp. MC6B-22 contained amino sugars and uronic acids. In contrast, [22] other researchers have reported the synthesis of a sulphated heteropolysaccharide composed exclusively of mannose and glucose in a Bacillus thermoantarcticus strain. Morales et al. [9] reported that Microbacterium MC3B-10 polymer is not a polysaccharide but a glycoprotein in nature as it had high levels of protein (36%) and lower concentration of neutral sugars. The sugar composition of the EPS examined in this study showed that pentoses (ribose and xylose), hexoses (rhamnose, fucose, galactose, mannose and glucose) were present with glucose, galactose and mannose being the most abundant monosaccharides. These sugars are typically found in bacterial EPS [23]. The calculated molar ratio (rhamnose:fucose:arabinose:mannose:galactose: glucose) based on the percent recovery for PP1 polymer (PPYG) was 34:4:1:8:3:6 while for OP1 & OP2 (optimizedmedium) was12.5:1.5:1:80:27.5:12.5 (rhamnose:fucose:arabinose:mannsoe:galactose:glucose) and 10:9:1:2:410:50:50 (ramnose:fucose:ribose:arabinose:mannose:galactose:glucose), respectively. The presence of the sugar mannose as a major component is common in other exopolymers, [21, 24, 25].
Mancuso et al. [25] in their studies on chemical characterization of EPSs from antarctic marine bacteria reported that mannose represented the most abundant neutral sugar in the EPS. A study [21] examined the exopolymers produced by another Antarctic marine isolate, Pseudomonas haloplanktis TAC 125, and showed the polysaccharide component to consist of mannose with traces of glucose. The sugar constituents of soluble EPSs of Microbacterium kitami C2T were 14% (w/w) rhamnose, 19% mannose, 25% galactose and 42% glucose, while those of insoluble EPSs were 27% rhamnose, 13% mannose, 9% galactose and 51% glucose [4].
This study suggests the fact that marine coastal sand dune environments are a previously unexplored habitats for EPS-producing bacteria, and that these molecules might be involved in ecological roles protecting the cells against dessication especially in nutrient-limited environments like the coastal sand dunes more so in the extreme conditions of pH. Further, the EPS-producing bacteria may be contributing to the cementation of the sand dunes.
References
Desai KN, Untawale AG (2002) Sand dune vegetation of Goa; Conservation and Management. Botanical Society of Goa, Goa
Decho AW, Lopez GR (1993) Exopolymer microenvironments of microbial flora. Limnol Oceanog 38:1633–1645
Read RR, Costerton JW (1987) Purification and characterization of adhesive exoplysaccharides from Pseudomonas putida and Pseudomonas fuorescens. Can J Microbiol 33:1080–1090
Matsuyama H, Kawasaki K, Yumoto I, Shida O (1999) Microbacterium kitamiense sp. nov., a new polysaccharide-producing bacterium isolated from the wastewater of a sugar-beet factory. Int J Syst Bacteriol 49:1353–1357
Guezennec J (2002) Deep sea hydrothermal vents: a new source of innovative bacterial exopolysaccharides of biotechnological interest? J Indust Microbiol Biotechnol 29:204–208
Wotton RS (2004) The ubiquity and many roles of exopolymers (EPS) in aquatic systems. Sci Mar 68:13–21
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Bhosle NB, Sawant SS, Garg A, Wagh AB (1995) Isolation and partial chemical analysis of exopolysaccharides from a marine fouling diatom Navicula subinfecta. Bot Marina 38:103–110
Decho AW (1990) Microbial exopolymer secretions in ocean environment, their roles in food webs and marine processes. Oceanog Mar Biol Annu Rev 28:73–153
Morales BOO, Santiago-García JL, Chan-Bacab MJ, Moppert X, Miranda-Tello E, Fardeau ML, Carrero JC, Bartolo-Pérez P, Valadéz-González A, Guezennec J (2007) Characterization of extracellular polymers synthesized by tropical intertidal biofilm bacteria. J Appl Microbiol 102:254–264
Filisetti-cozzi TM, Nicholas CC (1991) Measurements of uronic acid without interference from neutral sugar. Anal Chem 197:157–162
Lowry OH, Nira J, Rosenbrough A, Farr L, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Slonecker JH, Orentas DG (1962) Pyruvic acid, a unique component of an exocellular bacterial polysaccharide. Nature 194:478–479
Martin JP (1971) Decomposition and binding action of polysaccharides in soil. Soil Biol Chem 3:33–41
Forster SM (1979) Microbial aggregation of sand in an embryo dune system. Soil Biol Chem 11:537–543
Ko SH, Lee HS, Park SH, Lee HK (2000) Optimal conditions for the production of exopolysaccharide by marine microorganism Hahella chenjuensis. Biotechnol Bioproc Eng 5:181–185
Cerning J, Renard C, Thibault JF, Bouillanne C, Landon M, Desmazeaud M, Toposirovic L (1994) Carbon source requirements for exopolysaccharide production by Lactobacillus casei CG11 and partial structure analysis of the polymer. Appl Environ Microbiol 60:3914–3919
Ashraf M, Berge O, Azam F, Heulin T (1999) Bacterial productivity of salt affected sites: I diversity of exopolysaccharide producing bacteria isolated from the rhizosphere of wheat (Triticum aesticum L.) grown in normal and saline Pakistani soils. Pak J Biol Sc 2:201–206
Kennedy AFD, Sutherland IW (1987) Analysis of bacterial exopolysaccharides. Biotechnol Appl Biochem 9:12–19
Bouchotroch S, Quesada E, Izquierdo I, Rodroguez M, Bejar V (2000) Bacterial exopolysaccharides produced by newly discovered bacteria belonging to the genus Halomonas, isolated from hypersaline habitats in Morocco. J Ind Microbiol Biotechnol 24:338–374
Corsaro MM, Lanzetta R, Parrilli E, Parrilli M, Tutino ML, Ummarino S (2004) Influence of growth temperature on lipid and phosphate contents of surface polysaccharides from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC 125. J Bacteriol 186:29–34
Manca MC, Lama L, Improta R, Esposito E, Gambacorta A, Nicolaus B (1996) Chemical composition of two exopolysaccharides from Bacillus thermoantarcticus. Appl Environ Microbiol 62:3265–3269
Kenne L, Lindberg B (1983) Bacterial Polysaccharides. In: Aspinall GO (ed) The polysaccharides. Academic Press, New York, pp 287–363
Anton J, Meseguer I, Valera FR (1988) Production of an extracellular polysaccharide by Haloferax mediterranei. Appl Environ Microbiol 54:2381–2386
Mancuso NCA, Guezennec J, Bowman JP (2005) Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: A Review. Mar Biotechnol 7:253–271
Acknowledgements
The authors thank the Ministry of Earth Sciences for the financial assistance provided for this research study. The authors further acknowledge the help rendered by Dr N B Bhosle, National Institute of Oceanography, Goa for the Gas chromatographic analysis of the polymers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Godinho, A.L., Bhosle, S. Sand Aggregation by Exopolysaccharide-Producing Microbacterium arborescens––AGSB. Curr Microbiol 58, 616–621 (2009). https://doi.org/10.1007/s00284-009-9400-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9400-4