Abstract
The ability of Burkholderia sp. VUN10013 to degrade anthracene in microcosms of two acidic Thai soils was studied. The addition of Burkholderia sp. VUN10013 (initial concentration of 105 cells g−1 dry soil) to autoclaved soil collected from the Plew District, Chanthaburi Province, Thailand, supplemented with anthracene (50 mg kg−1 dry soil) resulted in complete degradation of the added anthracene within 20 days. In contrast, under the same test conditions but using autoclaved soil collected from the Kitchagude District, Chanthaburi Province, Thailand, only approximately 46.3% of the added anthracene was degraded after 60 days of incubation. In nonautoclaved soils, without adding the VUN10013 inocula, 22.8 and 19.1% of the anthracene in Plew and Kitchagude soils, respectively, were degraded by indigenous bacteria after 60 days. In nonautoclaved soil inoculated with Burkholderia sp. VUN10013, the rate and extent of anthracene degradation were considerably better than those seen in autoclaved soils or in uninoculated nonautoclaved soils in that only 8.2 and 9.1% of anthracene remained in nonautoclaved Plew and Kitchagude soils, respectively, after 10 days of incubation. The results showed that the indigenous microorganisms in the pristine acidic soils have limited ability to degrade anthracene. Inoculation with the anthracene-degrading Burkholderia sp. VUN10013 significantly enhanced anthracene degradation in such acidic soils. The indigenous microorganisms greatly assisted the VUN10013 inoculum in anthracene degradation, especially in the more acidic Kitchagude soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence of polycyclic aromatic hydrocarbons (PAHs) in the environment can exert adverse effects on many living organisms, mainly through their mutagenic and carcinogenic properties [17, 18]. Thus, there is considerable interest in efforts to remediate sites contaminated with PAHs. Bioaugmentation, the introduction of competent microorganisms, is an in situ cost-effective soil bioremediation method that can enhance pollutant removal from contaminated sites [7]. However, the success of bioaugmentation in soil is dependent on various environmental factors; among these, the soil pH is especially critical [4, 13, 14]. Extremes in pH would be expected to have a negative influence on hydrocarbon biodegradation [11].
Acidic soil is a common occurrence in many regions of southeast Asia such as Thailand, Malaysia, Philippines, and Indonesia. Extensive aquaculture and rice production have led to the development of acid sulfate soil. Subsequent activities by iron-oxidizing bacteria combined with those by sulfate-reducing bacteria under anaerobic conditions resulted in the production of sulfuric acid that rendered the soil acidic, with pH values lower than 4.0 in many instances [5, 6, 8, 15]. Exacerbating the problem of acidity, the eastern Thai provinces of Rayong and Chonburi are known to have sites contaminated with petroleum hydrocarbons due to the presence of petrochemical industries and sea transportation of crude or refined oil products [3]. The presence of petroleum hydrocarbon contamination in acidic soil may pose a unique problem in efforts to remediate such sites.
In the literature, most of the bioaugmentation studies with PAH-degrading bacteria have been done in soil with pH near neutrality [9]. Little is known about how effective bioaugmentation can be in PAH-contaminated acidic soil. In this study, we tested the potential of bioaugmentation in two typical acidic soils from eastern Thailand using anthracene as the model PAH test compound. Burkholderia sp. VUN10013 was used as the inoculum. Strain VUN10013 was previously isolated from PAH-contaminated soil at an abandoned factory site located near Port Melbourne, Victoria, Australia, in the same study as described by Boonchan et al. [2]. This bacterial strain is able to use phenanthrene and anthracene as sole carbon and energy sources [15].
Materials and Methods
Soils
Soils from uncontaminated local sites, with no previous history of PAH contamination, were obtained from a hilly area at Kitchagude District and Plew District, Chanthaburi Province, Thailand. These soils were separately sieved to a particle size of 1.0 mm before use. The physical and chemical characteristics of the two soils were analyzed by Land Development Regional Office 2 located in Chonburi Province, Thailand.
Preparation of Media and PAH Stock Solutions
The basal salt medium (BSM) contained per liter: 0.4 g K2HPO4, 0.4 g KH2PO4, 0.4 g (NH4)2SO4, 0.3 g NaCl, 5 ml of trace element solution, 5 ml of vitamin solution, and 5 ml of magnesium/calcium solution [2]. Solutions of vitamins, trace elements, and calcium/magnesium were filter-sterilized (0.22 μm, Sartorious AG, Germany) and added to autoclaved BSM. Stock solution of each PAH compound was prepared in dimethylformamide (DMF). In PAH degradation experiments, the stock solutions of individual PAHs were added to BSM to give the following final quantities per liter: 250 mg for phenanthrene; 100 mg for naphthalene; 50 mg for fluorene, anthracene, pyrene, fluoranthene, and chrysene; and 25 mg for benzo[a]pyrene.
Bacterial Strain, Culture Condition, and Preparation of Bacterial Inocula
Burkholderia sp. VUN10013 was grown and subcultured as described by Somtrakoon et al. [15]. Bacterial inocula for soil microcosm were prepared by growing VUN10013 at 30°C with shaking at 175 rpm in BSM supplemented with anthracene (50 mg l−1) (Sigma-Aldrich Chemie GmbH, Steinhein, Germany) until late exponential phase (4–5 days). Then 5 ml of this culture were transferred to 45 ml of nutrient broth and incubation continued for 24 h. Cells were harvested by centrifugation (4,500g for 5 min) at 4°C and washed twice with sterilized BSM. The cell pellets were resuspended in BSM and this cell suspension was used as the inoculum.
Biodegradation of Individual PAHs in Liquid Media
Experiments to assess the ability of strain VUN10013 to degrade individual PAHs were performed in 50-ml serum bottles that were sealed with Teflon-lined caps. The PAHs selected for testing were benzo[a]pyrene, chrysene, fluoranthene, fluorene, naphthalene, and pyrene. Anthracene and phenanthrene, previously shown to be utilized by VUN10013 as the sole carbon source [15], were tested also as controls. Before inoculation, 0.1 ml of the individual PAH stock solution described above was added to the BSM (10 ml). The phenanthrene-grown bacterial inoculum (∼1 ml) was then added to the medium to give an initial cell population of approximately 103 cells ml−1. Abiotic controls included sterile medium containing only PAH. As another control, BSM containing a particular PAH compound was inoculated with autoclaved cells to achieve an initial bacterial population equivalent to approximately 104 cells ml−1. The experiment was conducted in triplicate while all controls were done in duplicate. The cultures were incubated in the dark at 30°C with shaking at 175 rpm. Bacterial growth was followed by measuring total cell protein concentrations according to the method of Lowry et al. [12] using bovine serum albumin as the standard.
Biodegradation of anthracene in liquid broth was studied further using protocol described above for individual PAH degradation, except that anthracene was added to BSM to final concentrations of 50, 100, and 150 mg L−1. The media were inoculated with anthracene-grown VUN10013 cells and the culture incubated as described above.
Anthracene Degradation in Soil
Autoclaved and nonautoclaved soils were subdivided into 100-g (dry weight) lots in 750-ml jars. These soils were spiked with anthracene (50 mg kg−1 dry soil) prepared in dichloromethane (DCM). After thorough mixing, the solvent was allowed to evaporate inside a fume hood at room temperature (27 ± 1°C). The soil in each jar was inoculated with the Burkholderia sp. VUN10013 cell suspension to give an initial population of 105 cells g−1 dry soil. Uninoculated control soils contained anthracene without bacterial inoculation. All soil microcosms were supplemented periodically with sterile BSM to maintain the water-holding capacity of the soil at approximately 60%. Incubation was performed in the dark at room temperature (27 ± 1°C). Triplicate 1-g samples of dry soil from each jar were collected every 10 days for the analysis of the remaining anthracene by gas chromatography–mass spectroscopy (GC-MS).
PAH Extraction and Analytical Procedures
Extraction of PAHs from liquid media and soil samples was performed according to Somtrakoon et al. [15]. PAH concentrations in the DCM extracts and standards were measured using a gas chromatograph (Agilent Technology, 6890N Network GC System) equipped with a mass spectroscopic detector (Agilent Technology, 5973 Network Mass Selective Detector). Separation was achieved using a DB1 capillary column (30 m × 25 mm, i.d. = 25 μm). GC conditions were previously described by Somtrakoon et al. [15].
Data Analysis
The percentage of remaining anthracene was expressed as the mean ± standard deviation (SD). One-way analysis of variance was used to test for statistical significance among treatments. Subsequent multiple comparisons of means were performed using the Duncan comparison method. Statistical significance was accepted at P < 0.05.
Results and Discussion
The acidic soil in parts of eastern Thailand is contaminated with petroleum hydrocarbons because of petrochemical industries and sea transportation of crude oil and oil-refined products. The combination of petroleum contamination and soil acidity poses unique challenges in the bioremediation of such soils. There is little information on the effectiveness of bioaugmentation with a competent microbial inoculum to degrade petroleum compounds in acidic soil. In this study we examined anthracene degradation in two acidic Thai soils by a PAH-degrading bacterium Burkholderia sp. VUN10013. This bacterial strain is able to utilize phenanthrene or anthracene as the sole carbon source and can degrade pyrene and fluoranthene by cometabolism [15].
In initial experiments we examined the ability of VUN10013 to utilize several other high- and low-molecular-weight (MW) PAH compounds as the sole carbon source. The results showed that in addition to phenanthrene and anthracene, VUN10013 could utilize lower-MW PAHs fluorene and naphthalene as the sole carbon source. This was judged by the disappearance of each of these test PAHs, concomitant with accumulation of cell biomass in liquid media (data not shown). High-MW PAHs such as benzo[a]pyrene, chrysene, fluoranthene, and pyrene were not utilized by VUN10013, as judged by the lack of PAH disappearance and cell biomass accumulation in the liquid media (data not shown). Anthracene (up to 150 mg L−1) was utilized efficiently by anthracene-grown VUN10013 inoculum. For example, at 150 mg L−1 anthracene, about 90% of the anthracene was degraded in 10 days. During this period, the total cell protein content increased from 7.1 to 67.9 μg ml−1. Anthracene-grown VUN10013 inoculum was used in subsequent soil studies.
The soils used in this study were relatively acidic. The pH values of the Plew and Kitchagude soils were 4.9 and 4.3, respectively. Other characteristics of the two soils are shown in Table 1. Three different experimental treatments were used to investigate anthracene degradation in the two acidic Thai soils. In the first treatment, the disappearance of 50 mg anthracene kg−1 dry soil in autoclaved soil inoculated with 105 cells per g dry soil of anthracene-grown Burkholderia sp. VUN10013 cells was investigated. In the second treatment, the degradation of anthracene by indigenous microorganisms and Burkholderia sp. VUN10013 cells inoculated into nonautoclaved soil was determined. In the third treatment, the uninoculated nonautoclaved microcosms were used to assess the ability of indigenous soil microbial population to degrade anthracene. The uninoculated microcosms containing autoclaved soils were used to examine abiotic losses of anthracene.
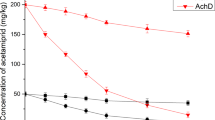
Effective degradation of PAHs in soil requires that a competent microbial population exists in soil. If the number of competent microbial cells is too low, then bioaugmentation with known PAH degraders may be an option to enhance PAH degradation. This is well illustrated in the two acidic Thai soils used in this study. As seen in Figure 1C and D, in nonautoclaved Plew or Kitchagude soils only about 20% of the spiked anthracene was degraded after 60 days. Thus, the indigenous microorganisms in the two acidic Thai soils had very limited ability to degrade anthracene. Addition of the VUN10013 inoculum led to the degradation of more than 90% of the spiked anthracene after 10 days. The number of indigenous anthracene-degrading microorganisms in the Plew and Kitchagude soils was probably too low. Thus, bioaugmentation with the anthracene-grown VUN10013 cells was effective in dramatically enhancing anthracene degradation.
Interestingly, in nonautoclaved Kitchagude soil inoculated with VUN10013 cells, anthracene was effectively degraded within 10 days as in the Plew soil (Fig. 1D). This is likely a result of the combined action of VUN10013 cells and indigenous anthracene-degrading cells to degrade anthracene. In the autoclaved Kitchagude soil, the indigenous anthracene degraders were killed which led to slower degradation of anthracene by VUN10013 cells alone (Fig. 1B). Yu et al. [16] also reported that the combined action of an introduced enriched consortium and indigenous microorganisms could degrade fluoranthene and phenanthrene to a greater extent than either one alone. Our results showed that the presence of indigenous microorganisms in both Plew and Kitchagude soils could improve anthracene degradation in the acidic soils by Burkholderia sp. VUN10013. This effect was more pronounced in the more acidic Kitchagude soil (Fig. 1B). The precise mechanism(s) by which indigenous microorganisms assist with anthracene degradation by VUN10013 in the acidic soils is not known. We hypothesize that indigenous microorganisms in both soils help to increase the bioavailability of anthracene to Burkholderia sp. VUN10013, for example, through the production of biosurfactants. This needs to be examined in future studies.
In autoclaved Plew soil, anthracene was effectively degraded by VUN10013 cells such that less than 2% remained after 20 days (Fig. 1A). However, in autoclaved Kitchagude soil, anthracene degradation by the VUN10013 inoculum was sluggish and only about 50% was degraded after 60 days (Fig. 1B). One reason for the superior degradation of anthracene in autoclaved Plew soil relative to Kitchagude soil may be due to the difference in organic contents of these soils. In the Kitchagude soil with a higher organic content (3.66%), the VUN10013 inoculum may preferentially utilize other available carbon sources instead of anthracene; this may result in slower anthracene degradation than that in the Plew soil which has a much lower organic content (0.74%). Another likely reason may be that the Kitchagude soil had a lower pH (4.3) compared with the Plew soil (pH 4.9). A pH near neutrality is generally thought to favor PAH degradation by bacteria [1], and a pH of 4.9 may be near the bottom range for effective anthracene degradation by VUN10013 cells. Further decreases in pH led to reduced efficiency in anthracene degradation as seen in the Kitchagude soil. The results from autoclaved soil suggest that Plew soil was more suitable for the activity of Burkholderia sp. VUN10013 than the Kitchagude soil.
Soil pH is a significant parameter that affects microbial biomass buildup, microbial diversity, and microbial activity [1]. Several studies have attempted to stimulate the degradation of PAH in acidic soil. For example, Uyttebroek et al. [16] obtained a pyrene-utilizing bacterial culture from an extremely acidic PAH-contaminated soil (pH 2.4) by enrichment at pH 3. This enrichment culture grew on pyrene at pH 2 and also mineralized 77% of the supplied [14C]pyrene at pH 3 after 5 days of incubation. Hwang and Cutright [10] reported that addition of a phenanthrene- and pyrene-degrading consortium could remove phenanthrene from soils (pH 3.56). For phenanthrene, 90 and 96% of the initial concentration was degraded in freshly spiked and aged soils, respectively, at day 32. Our results indicated that Burkholderia sp. VUN10013, which was previously isolated from a slightly acidic soil (pH 5.3) from Australia, could effectively degrade anthracene in acidic Thai soils.
In summary, the present study demonstrates the ability of Burkholderia sp. VUN10013 to effectively degrade anthracene in acidic Thai soils which are freshly contaminated with this PAH. This shows good promise for using Burkholderia sp. VUN10013 for PAH remediation. Further studies are needed to see if this approach can work with other PAH contaminants and under different soil conditions.
References
Blagodatskaya EV, Anderson T (1999) Adaptive responses of soil microbial communities under experimental acid stress in controlled laboratory studies. Appl Soil Ecol 11:207–216
Boonchan S, Britz ML, Stanley GA (1998) Surfactant-enhanced biodegradation of high molecular weight polycyclic aromatic hydrocarbons by Stenotrophomonas maltropilla. Biotechnol Bioeng 59:481–494
Boonyatumanond R, Wattayakorn G, Togo A, Takada H (2006) Distribution and origins of polycyclic aromatic hydrocarbons in riverine, estuarine, and marine sediments in Thailand. Mar Pollut Bull 52:942–956
Bouchez T, Patureau D, Dabert P, Jureschko S, Doré J, Delgenè S, Moletta R, Wangner M (2000) Ecological study of a bioaugmentation failure. Environ Microbiol 2:179–190
Chouychai W, Thongkukiatkul A, Upatham S, Lee H, Pokethitiyook P, Kruatrachue M (2007) Phytotoxicity assay of crop plants to phenanthrene and pyrene contaminants in acidic soil. Environ Toxicol 22:597–604
Cramb RA (2005) Farmers’ strategies for managing acid upland soils in Southeast Asia: an evolutionary perspective. Agric Ecosys Environ 106:69–87
Fantroussi SE, Agathos SN (2005) Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr Opin Microbiol 8:268–275
Golez NV (1995) Formation of acid sulfate soil and its implications to brackish water ponds. Aquacult Eng 14:297–316
Hamamura H, Olsun SH, Ward DM, Inskey WP (2005) Diversity and functional analysis of bacterial communities associated with natural hydrocarbons seeps in acidic soils at Rainbow Spring, Yellow Stone National Park. Appl Environ Microbiol 71:5943–5950
Hwang S, Cutright TJ (2002) Biodegradation of aged pyrene and phenanthrene in a natural soil. Chemosphere 47:891–899
Leahy JG, Colwell RR (1990) Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54:305–331
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Neale SP, Shah Z, Adams WA (1997) Changes in microbial biomass and nitrogen turnover in acidic organic soils following limiting. Soil Biol Biochem 29:1463–1474
Providenti MA, Lee H, Trevors JT (1993) Selected factors limiting the microbial degradation of recalcitrant compounds. J Ind Microbiol 12:379–395
Somtrakoon K, Suanjit S, Prayad P, Kruatrachue M, Lee H, Upatham S (2008) Phenanthrene stimulates the degradation of pyrene and fluoranthene by Burkholderia sp. VUN10013. World J Microbiol Biotechnol 24:523–531
Uyttebroek M, Vermeir S, Wattiau P, Ryngaert A, Springael D (2007) Characterization of cultures enriched from acidic polycyclic aromatic hydrocarbon-contaminated soil for growth on pyrene at low pH. Appl Environ Microbiol 73:3159–3164
Yu SH, Ke L, Wong YS, Tam NFY (2005) Degradation of polycyclic aromatic hydrocarbons (PAHs) by a bacterial consortium enriched from mangrove sediments. Environ Int 31:149–154
Yuan SY, Wei SH, Chang BV (2000) Biodegradation of polycyclic aromatic hydrocarbons by a mixed culture. Chemosphere 41:1463–1468
Acknowledgments
The authors acknowledge financial support from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0127/2545) to student (K. Somtrakoon) and advisor (S. Upatham). We thank the Post-Graduate Education, Training and Research Program in Environmental Science, Technology and Management for permission to use the GC-MS instrument and the Land Development Regional Office 2 (Chonburi, Thailand) for soil analysis.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Somtrakoon, K., Suanjit, S., Pokethitiyook, P. et al. Enhanced Biodegradation of Anthracene in Acidic Soil by Inoculated Burkholderia sp. VUN10013. Curr Microbiol 57, 102–106 (2008). https://doi.org/10.1007/s00284-008-9157-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-008-9157-1