Abstract
A chemically defined media consisting of carboxymethylcellulose (CMC) was developed to maximize the production of antibiotics, hexaene H-85 and azalomycine, by Streptomyces hygroscopicus CH-7. The production of antibiotics by filamentous organisms is often dependent on the morphology and size distribution of the pellet population within the culture. By adding the polymer to the fermentation medium, the growth was changed from a single large glob to small reproducible pellets, and wall growth was diminished to a minimum. Maximum concentrations of hexaene H-85 (146.7 mg/dm3) and azalomycine (188.6 mg/dm3) were reached at 3.0% and 1.0% (w/v) CMC, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Filamentous microorganisms such as Actinomycetales and filamentous fungi are used in several industrial fermentations. In particular, Streptomyces sp. is very important in antibiotic production [4]. To make the production of the antibiotic feasible, it is necessary to develop the optimum production, which includes, among the other conditions, chemically defined media. Product formation in submerged fermentations of filamentous microorganisms is dependent not only on the level of biomass present but also on the morphological profile of the culture [3]. The morphology of these microorganisms might vary from a free filamentous suspension to pellets, depending on the degree of aggregation. The formation of pellets in submerged fermentations begins as a result of the aggregation of spores and/or hyphae. The composition, structure, hydrophobicity, or charge of the cell wall as well as the presence of extracellular polymeric substances might influence aggregation between individual spores or hyphal elements. The most favorable form for metabolite production is a very small pellet. Dispersion of pellets of various filamentous organisms has been achieved by increasing inoculum size [1, 2, 5], changing the initial pH value of the medium, and adding aditives such as carboxymethylcellulose (CMC), chelators, Tween-80, or agar granules [1, 2, 5, 8]. None of these techniques had been examined with Streptomyces hygroscopicus. The experiments to be described were done to improve the growth antibiotics production (hexaene H-85 and azalomycine) by S. hygroscopicus CH-7, adding CMC in the chemically modified medium.
Materials and Methods
The strain S. hygroscopicus CH-7 isolated from a soil sample of Vojvodina produces, by fermentation, three antibiotics (polyene hexaene H-85, polyether nigericin, and macrodiolide azalomycine) and a proteases complex. The strain is protected by patents [6, 7].
Liquid Culturing
The basic, chemically defined fermentation medium used for growth in liquid culture contained the following (in g/dm3): glucose, 15; CaCO3, 2; NaCl, 2.5; yeast extract, 5; soya bean, 5; water up to 1 dm3; pH 6.8–7.0. Various amounts of CMC were added to the medium prior to autoclaving at concentrations shown in Table 1. The organism was cultivated in 1000-mL Erlenmayer flasks fixed on a rotary shaker (r = 2 cm) at 250 rpm and placed in a thermostated cabinet at 28°C for 7 days. All cultures were inoculated with 10% (v/v) culture, aged 24 h.
Measurement of Pellet Size
The pellet size was measured by using a Stereo Zoom 5 microscope (Leica) and a ruler with 1-mm graduations. Ten pellets of each sample were randomly chosen for measurement, and the average size was calculated.
Analytical Methods
Different solvents were used and tested for the extraction of the antibiotic from culture supernatant. The solvents for azalomycine and hexaene H-85 extraction were determined to be ethyl acetate and n-butanol, respectivelly [6].
The dry weight of the biomass in fermentation broth was determined using the following procedure. A 20-mL sample of the fermentation broth was placed in a plastic universal container and subjected to two centrifugation cycles at 3.500 rpm for 10 min. After the second cycle, the supernatant was removed and the solid residue was placed in a 105°C oven for 24 h before weighing.
Results and Discussion
Impact of CMC on Morphology
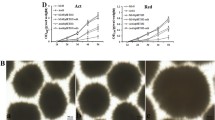
Table 1 shows that CMC extensively improved the cell morphology of S. hygroscopicus CH-7. Without CMC in a chemically defined medium, S. hygroscopicus grew in a large-pellet form (Fig. 1a). The addition of CMC to the fermentation medium resulted in many pellets of a smaller size (Fig. 1b–1e). The pellet size decreased and the units tended to be more and more uniform as the concentration of CMC was increased. Wall growth was diminished to slight or none in the flask containing 1.0–3.0% CMC. CMC caused an alteration in pellet structure.
Impact of CMC on Biomass and Antibiotics Production
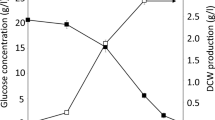
Figure 2 shows the positive effect of CMC on the biomass production. An increase in biomass production is accompanied by increasing the CMC concentration. Figure 3 shows the impact of CMC on antibiotics production in the shake flask cultivation. All data shown represent the averages of triplicate flasks. Hexaene H-85 was extracted witn n-buthanol and the concentration was determined by a change in absorbance (A) at λmax = 364 nm. Azalomycine was extracted by ethylacetate and the concentration was determined by a change in absorbance (A) at λmax = 252 nm. The maximum concentration of hexaene H-85 was reached at 3.0% CMC in the fermentation broth on the fourth day of fermentation (146.7 mg/dm3) and was higher compared to the control (94.58 mg/dm3). The maximum concentration of azalomycine was reached at 1.0% CMC in the fermentation broth in the fifth day of fermentation (188.6 mg/dm3) and was higher than that reached in the control (115.7 mg/dm3). The concentrations of 0.1% and 0.5% of CMC had no positive effect on antibiotics production, whereas 2.0%, 5.0%, and 7.0% concentrations of CMC changed the morphology of the microorganism and had a slight effect on antibiotic productions.
Because the pellet size decreased with an increasing dosage of CMC, it was clear that small pellets favor antibiotic production. CMC presumably reduced the agglomeration of spores and hyphea, resulting in more and smaller pellets.
References
Chen ZW, Ku CH, Weng CJ, Chen TL (1997) Effect of thickening agents on the penicillin fermentation. Appl Biochem Biotechnol 67:249–258
Domingues FC, Querioz JA, Cabral JMS, Fonseca LP (2000) The influence of culture conditions on mycelium structure and cellulose production by Trichoderma reesei Rut C-30. Enzyme Microb Technol 26:394–401
OĆleirigh C, Casez JT, Walsh PK, ÓShea DG (2005) Morphological engineerimg of Streptomyces hygroscopicus var. geldanus: regulation of pellet morphology through manipulation pf broth viscosity. Appl Microbiol Biotechnol. 68:305–310
Okami Y, Hotta K (1988) Search and discovery of new antibiotics. In: Goodfellow M, Williams ST, Mordarski M (eds) Actinomycetes in biotechnology. Academic Press, San Diego, pp 33–67
Vecht-Lifschitz SE, Magdassi S, Braun S (1990) Pellet formation and cellular aggregation in Streptomyces tendae. Biotechnol Bioeng 35:890–896
Vučetić J, Gojgić-Cvijović G, Karadžić I, Zdjelar K, Radovanović I, Đukanović Z (1990b) The process for production of polyether antibiotic. J Patents 3:810
Vučetić J, Karadžić I, Gojgić-Cvijović G, Zdjelar K, Vrvić M (1990c) The process for production of new polyene antibiotic. J Patents 3:810
Yang W, Hartwieg EA, Fang A, Demain AL (2003) Effects of carboxymethylcellulose and carboxypolymethylene on morphology of Aspergillus fumigatus NRRL 2346 and fumagillin production. Curr Microbiol 46:24–27
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ilić, S.B., Konstantinović, S.S., Veljković, V.B. et al. Impact of Carboxymethylcellulose on Morphology and Antibiotic Production by Streptomyces hygroscopicus . Curr Microbiol 57, 8–11 (2008). https://doi.org/10.1007/s00284-008-9143-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-008-9143-7